Abstract
This paper describes the He non-thermal plasma treatment of liquid impregnated cotton fabrics. The cotton fabrics were soaked in either H2O, D2O or ethanol after which they were placed in an atmospheric pressure parallel-plate DBD reactor. The influence of the used liquid in combination with the plasma exposure time was studied using OES, FTIR, XPS and SEM. The addition of (deuterated) water was found to aid more efficiently in the incorporation of polar functional groups onto the cotton surface in comparison to a pure He plasma treatment. The presence of H and OH species in the discharge also caused extensive etching of the surface, which led to the formation of microcraters. The impregnation with ethanol was responsible for the formation of a plasma-based thin film on top of the cotton substrate, which was characterized by a high content of C–C bonds and a smooth surface morphology. These results show that the soaking of cotton fabric prior to plasma exposure can help to more effectively alter its surface properties compared to a dry plasma treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-thermal plasma treatments using oxygen-rich discharge gases are known to introduce a variety of polar oxygen-containing functional groups onto the surface of cellulosic materials including –OH, C=O and O–C=O groups, which can greatly enhance the surface wettability, dyeability and adhesion of biopolymer coatings such as chitosan. Several papers have already investigated the effect of atmospheric pressure non-thermal (or cold) plasma treatment on the properties of cotton fabrics (Aileni et al. 2019; Haji et al. 2013, 2016, 2017, 2019; Patino et al. 2011; Rajasekaran and Muthuraman 2019; Sun and Stylios 2004; Wang et al. 2019). In particular, non-thermal plasma activation in the presence of water in liquid or vapor phase is a well-known technique to incorporate functional groups onto a polymer surface. In this context, underwater plasma treatments have been studied sporadically to fabricate substrates with homogeneous monofunctional surfaces (Joshi et al. 2009b), to induce direct functionalization, stimulate cross-linked layer formation and/or to graft or deposit mono-functional layers (Friedrich et al. 2008; Joshi et al. 2008, 2009a, 2013; Khlyustova et al. 2015). Despite these promising results, the presence of moisture during a plasma surface treatment is often still viewed upon as an impurity (Ananth and Mark 2008). As a result, to date, only few studies have looked at the intentional addition of liquids/vapors to discharge systems used for surface modification (Acsente et al. 2016; De Geyter et al. 2013; Van Deynse et al. 2014, 2017). Moreover, most of these studies only focused on the addition of low amounts of vaporized water to the discharge gas (Acsente et al. 2016; De Geyter et al. 2013; Van Deynse et al. 2014). However, more recent studies have shown that, upon the addition of a liquid or vapor to the plasma discharge, the steady-state wettability of the plasma-exposed surface can be effectively altered, opening up new possibilities for enhanced surface modification of different substrates (Acsente et al. 2016; De Geyter et al. 2013; Van Deynse et al. 2014, 2017). Within our research facilities, research in recent years has been focused on the impregnation of porous polymeric materials (e.g. cotton, filter paper) with precursor solutions to obtain polymer-like coatings by means of in situ plasma polymerization (Molina et al. 2014, 2017). Whereas for plasma polymerization where precursors are applied in the gas phase, the coating properties are modulated by the precursor flow rate, system pressure, discharge power and plasma treatment time (Yasuda and Hirotsu 1978), plasma polymerization ignited onto a liquid-impregnated substrate is usually modulated by tuning the concentration of the precursor present in the liquid (Molina et al. 2014). However, to the best of our knowledge, the direct functionalization of a porous polymeric substrate by non-polymerizable liquid impregnation plasma has not been studied in detail until now. For this reason, in this study, cotton fabrics were therefore impregnated in either H2O, D2O or ethanol (EtOH) prior to performing plasma surface treatments. Helium (He) was chosen as discharge gas since a global model of He/H2O plasma chemistry in a parallel plate DBD reported the abundance of H2O+, water cluster ions, atomic and molecular metastable oxygen, OH(A) metastables and different ground-state neutrals such as H,O, H2, O2, OH, HO2 and H2O2 in He/H2O plasmas, with varying densities depending on the concentration of water vapor in the feeding gas (Liu et al. 2010). By first impregnating the cotton samples in water, the hypothesis is that the local concentration of water molecules at the substrate-plasma interface can be greatly enhanced, which may have a positive effect on the plasma surface modification process. Moreover, by replacing normal water with its deuterated form, better insights can be acquired on the role that H and OH species play in the surface modification processes. Finally, impregnation with EtOH is also examined as it can also introduce additional OH species in the plasma, which may in turn enhance the plasma surface treatment effects. Analysis of the active plasma species present in the plasma gas phase was done by optical emission spectroscopy (OES), while the plasma-induced effects on the cotton fabrics were analyzed by X-ray photoelectron spectroscopy (XPS), Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) and scanning electron microscopy (SEM).
Experimental
Materials
Cotton fabrics (plain weave, woven textiles, bleached without optical brightener with a 180 g/m2 weight, article 210 from EMPA) were used as substrates. Concentrated detergent for pre-treating textiles (Tanaterge® Advanced) was acquired from Tanatex. Cotton was first conditioned by washing the fabric for 45 min at 60 °C, under constant stirring at 250 rpm. The washing solution was prepared using Tanaterge® as detergent at 2 g/L concentration and washing was carried out at a 1/25 (w/v) load/wash solution ratio. Subsequently, the fabric was rinsed by placing it into deionized water for 20 min. Finally, the cotton fabric was dried and stored in a conditioned room at 67% relative humidity (RH) at a temperature of 24 °C. Deionized water, deuterium oxide (D2O) with D2 ≥ 99.96% provided by Eurosi-top and EtOH of absolute degree provided by Merck were used as impregnation liquids.
Impregnation and plasma treatment
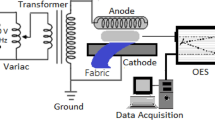
A DBD reactor operating at atmospheric pressure was used in this work, similar to the one reported in previous work (Jovančić et al. 2016; Molina et al. 2013a) and is schematically depicted in Fig. 1. A gas mass flow controller (Bronkhorst, the Netherlands) was used to introduce helium gas at a fixed rate of 5 standard liters per minute (5 slm) in the reactor chamber between the upper and lower electrode, which were both covered with a dielectric material (borosilicate glass). The gas gap between the two dielectrics was maintained at 12 mm and the volume between the electrodes was approximately ~ 0.1 L. A 16-kHz AC signal was generated with a GF-855 function generator (Promax, Spain) connected to a linear amplifier AG-1012 (T&C Power Conversion, USA). A matching network and two transformers (HR-Diemen S.A., Spain) were also connected to the amplifier output to increase the output voltage up to approximately 20 kV peak-to-peak. The incident power in the plasma reactor was kept constant at 30 W.
Cotton fabrics (3 × 3 cm2, ~ 0.16 gr) were introduced into the plasma reactor by placing these on the lower dielectric plate and 100 µl of the impregnation liquid (H2O, D2O or EtOH) was placed onto the cotton substrates (see Fig. 1). The liquid weight with respect to the weight of the cotton samples used in the experiments is equal to 0.5–0.7 gr liquid/1 gr cotton. Since the water absorption capacity of bleached cotton is ~ 2 gr water/1 gr cotton, plasma treatment was performed immediately after liquid impregnation in order to form a thin film of liquid on the cotton substrates before equilibrium of the liquid absorption capacity is reached (Hsieh et al. 1996). Subsequently, a plasma treatment in helium was carried out for a fixed period of 300 s. A control sample was also prepared by performing the helium plasma treatment step on a cotton sample without any impregnation. In addition, a single cycle of consecutive cotton impregnation and posterior He plasma treatment (300 s) was also repeated 3 and 6 times.
OES analysis
OES analysis was used to characterize the plasma active species inside the He discharge during surface modification. Diagnostics of the plasma gas was carried out with a quartz optic fiber connected to a Black Comet spectrometer (Stellarnet, USA) with concave gratings. Spectra were recorded in the UV–Vis wavelength range 190–850 nm (spectral resolution of 0.5 nm) with an integration time of 4 s and a solid angle of ~ 0.9 steradians. No UV emission was measured below ≈ 280 nm, since the DBD plasma reactor is made of borosilicate glass. The exact location of the quartz optic fiber in the DBD reactor can be seen in Fig. 1.
Attenuated Total Reflectance FTIR (ATR-FTIR) measurements
ATR-FTIR spectra of untreated and plasma-modified cotton fabrics were collected using a Nicolet Avatar 360 spectrometer equipped with a Smart iTR ATR sampling accessory (Thermo Scientific, USA). Spectra were obtained with an average of 32 scans using a resolution of 4 cm−1. An advanced ATR correction algorithm (OMNIC 7.3 from Thermo Electron Corporation) was used to correct for band intensity distortion, peaks shifts and polarization effects. Corrected ATR-FTIR spectra were found highly comparable to their transmission equivalents (Molina et al. 2013b). The background signal was extracted by means of linear backgrounds performed between 4000–3665 cm−1, 3665–2987 cm−1, 2987–2634 cm−1, 2634–1800 cm−1, 1800–1492 cm−1, 1492–1186 cm−1 and 1186–830 cm−1. Spectra were normalized to the C–O stretching vibration peak of cellulose located at 1031 cm−1.
XPS analysis
The surface chemical composition of the untreated and plasma-modified cotton fabrics was analyzed using a PHI 5000 Versaprobe II XPS device equipped with an Al Kα X-ray source (hν = 1486.6 eV) operated at 25 W. XPS analysis was conducted on samples, which have been placed in the XPS introduction machine, within 1 month after plasma modification. All measurements were conducted in a vacuum of at least 10−6 Pa and the photoelectrons were detected with a hemispherical analyzer positioned at an angle of 45° with respect to the normal of the sample surface. Survey scans and individual high resolution C1s spectra were recorded with a pass energy of 187.85 eV (eV step = 0.8 eV) and 23.5 eV (eV step = 0.1 eV) respectively. Binding energies were referenced to the C1s photoelectron peak position for C–O species at 286.5 eV (Molina et al. 2017) or C–C species at 285.0 eV in case of the EtOH impregnated sample (Gorjanc et al. 2010). Surface elemental composition was determined after a linear background subtraction from the area of the different photoemission peaks using the corresponding sensitivity factors of the XPS machine (Castle 1984). In this paper, the presented XPS results are the average of 3 different measurement points on a single cotton sample.
SEM imaging
Possible plasma-induced changes in the surface morphology of the cotton samples were recorded using a Hitachi S-3500N SEM device operated at 5 kV. Prior to SEM imaging, a Quorum Q150RS gold sputter coater was used to deposit a gold coating of a thickness of approximately 20 nm on the samples to minimize charging.
Results
OES
OES is a very powerful method to identify active species present in a plasma. The identification of said species is of great relevance in this work, as it can provide further insights into the occurring plasma/surface reactions. The UV–Vis emission spectra obtained during the He plasma treatment of cotton and cotton impregnated with different liquids (water, D2O and EtOH) are presented in Fig. 2. The emission spectra recorded during the He plasma treatment of the non-impregnated cotton sample (Fig. 2a, b) present different emission lines characteristic for atomic He (501.5 nm, 587.5 nm, 667.8 nm, 706.5 nm, 728.1 nm). In addition, emission bands due to N2+ (391.4 nm, 427.8 nm, 470 nm), N2 (315.9 nm, 337.1 nm, 357.6 nm, 380.4 nm), OH radicals (OH·: 308.9 nm, 287 nm) and emission lines due to H atoms (Hα line at 656.3 nm and Hβ line at 486.1 nm) can also be observed in the OES spectra. The presence of these species can be mainly attributed to residual humid air remaining in the plasma reactor. The observed plasma species are in excellent agreement with earlier published OES results of helium discharges operating at atmospheric pressure (Asandulesa et al. 2010; Bierstedt et al. 2015). It is also important to mention that during He plasma treatment of the non-impregnated cotton sample the plasma is operating in a diffuse regime independent of the used plasma treatment time.
Figure 2 does however reveals small differences in the OES spectra as a function of plasma treatment time for the He plasma treatment: at long plasma treatment time (300 s), an emission line due to atomic oxygen (777.4 nm) appears which might suggest that possible water evaporation from the non-impregnated cotton substrates occurs at longer plasma treatment times. At the short plasma treatment time (30 s), the emission spectrum during the He plasma treatment of cotton impregnated with water is mainly composed of emission lines/bands corresponding to He, H and OH·, the latter 2 mainly originating from the dissociation of H2O used for the impregnation of the cotton samples. Furthermore, in contrast to the OES spectrum obtained during the plasma treatment of pure cotton, only a single, very small band attributed to N2+ species could be distinguished. However, at long plasma treatment times (300 s) (Fig. 2b), the emission spectrum during the He plasma treatment of cotton impregnated with water again resembles that of pure He, indicating that within 300 s, most of the impregnated water had been evaporated from the cotton substrate.
Emission spectra recorded during the plasma treatment of cotton impregnated with D2O resemble these of cotton impregnated with H2O. Emission bands due to N2 and N2+ species are however more pronounced in case of the D2O impregnated sample when compared to the H2O impregnated cotton. In addition, a small additional OD· emission band at 287 nm could also be observed resulting from the dissociation of D2O used to impregnate the cotton sample. It is also important to mention that when cotton is impregnated with H2O or D2O, two different plasma regimes can be observed as a function of plasma treatment time which can be clearly differentiated in the OES spectra by examining the intensity of the He line at 706.5 nm. As the presence of filaments decreases the overall plasma light emission and thus also the intensity of the He line at 706.5 nm compared to the diffuse plasma regime, the following can be concluded: at short treatment times (30 s) in case of the H2O and D2O impregnated samples, the plasma is operating in the filamentary regime due to the presence of high amounts of H2O or D2O. In contrast, at longer treatment times (300 s), most of the liquids are evaporated and the plasma again operates in the diffuse mode similar as in case of the He treatment of the non-impregnated sample.
During the He plasma treatment of cotton impregnated with EtOH, new emission bands attributed to carbon species (CN, CH and C2) could be observed at short plasma treatment times (30 s). These emission bands correspond to the CN violet system (360–390 nm), CH (430, 390 and 314 nm) and the C2 Swan system (a3∏u − d3∏g) consisting of various vibrational transitions (450–470 nm (Δν = − 1), 480–520 nm (Δν = 0) and 520–570 nm (Δν = 1)). This is in accordance with literature and could be attributed to the dissociation of EtOH used for impregnation (Ikegami et al. 2004). In case of EtOH impregnation, emission bands due to carbon and nitrogen species resulting from ethanol dissociation could still be clearly distinguished after 300 s, whereas the atomic oxygen line was no longer recorded. These results provide a strong indication that the ethanol reforming had not yet been completed after 300 s (Yanguas-Gil et al. 2004).
ATR-FTIR
Besides investigating the plasma gas phase, the effects of impregnation with different liquids (H2O, D2O and EtOH) and posterior He plasma treatment (300 s) on the chemical composition of the cotton substrates were also investigated by means of ATR-FTIR.
First of all, the ATR-FTIR spectra of untreated and He plasma-treated cotton samples are presented in Fig. 3a. The FTIR spectrum of untreated cotton shows multiple bands characteristic for cellulose (Chung et al. 2004; Garside and Wyeth 2003): a very broad OH stretching peak in the region 3750–2995 cm−1, a broad peak in the region 3000–2800 cm−1 attributed to CH stretching vibrations, a smaller but rather broad peak at ≈ 1635 cm−1 which is usually assigned to the OH bending mode of adsorbed water, a peak at 1430 cm−1 due to CH and C–OH bending vibrations, a broad peak in the region 1400–1300 cm−1 due to CH and CH2 bending vibrations, a peak in the region 1160–1108 cm−1 due to C–O–C stretching and a peak in the region 1030–998 cm−1 due to C–O stretching vibrations. Figure 3a reveals no significant differences in the ATR-FTIR spectra of non-impregnated cotton samples after performing multiple consecutive He plasma treatments. This is in excellent agreement with earlier published results showing no differences in ATR-FTIR spectra of polymer films as a result of plasma surface treatment (Morent et al. 2008). Indeed, as plasma active species are only able to interact with the top few nanometers of a material, the penetration depth of ATR-FTIR is too large to detect the surface chemical changes induced by plasma treatment (Morent et al. 2008).
ATR-FTIR spectra obtained on non-impregnated (a) and H2O (b), D2O (c) and EtOH (d) impregnated cotton samples after 1, 3 and 6 cycles of consecutive impregnation/300 s He plasma treatment. UT stands for the untreated cotton sample. Enlarged carboxylate region (1800–1500 cm−1) obtained for cotton samples impregnated with H2O (e) and EtOH (f)
The ATR-FTIR spectra of cotton samples undergoing consecutive water impregnation and He plasma treatments are shown in Fig. 3b. In contrast to the non-impregnated cotton sample, differences in ATR-FTIR spectra could be observed between the untreated and plasma-modified samples: with an increasing number of impregnation/plasma treatment cycles, the broad peak at 1635 cm−1 gradually increases. A more detailed investigation of this wavenumber region (see Fig. 3e) indicates that a new band attributed to carboxylate (O–C=O−) stretching vibrations appears at 1720 cm−1 which gains in intensity as a function of treatment cycles. In addition, Fig. 3e also reveals that the peak at 1635 cm−1 also increases as a function of impregnation/plasma treatment cycles. As the broad OH band in the region 3750–2295 cm−1 decreases with plasma treatment cycles, this increase at 1635 cm−1 could thus not be attributed to higher amounts of adsorbed water, but is most likely the result of the generation of carbonyl groups (C=O) as a consequence of the plasma-induced cotton oxidation process. This seems to be corroborated by the ATR-FTIR spectrum corresponding to untreated cotton immersed in D2O for 24 h and dried at room temperature (sample UT D2O). The UT D2O ATR-FTIR spectrum highly resembles the ATR-FTIR spectrum of untreated cotton (see Fig. 3b, e), but a new band in the region 2650–2250 cm−1 associated to the OD stretching peak of D2O can be observed in the former sample (see Fig. 3b). It could therefore be concluded that the intensity of the peak at 1635 cm−1 should not only be attributed to the OH bending mode of adsorbed water but the possible signal formation of carbonyl groups should also be taking into account. These carbonyl groups could be formed in the untreated cotton samples as a consequence of the bleaching process carried out to the cotton prior to its use in this work.
Similar trends are observed when D2O is used as impregnation liquid (Fig. 3c). The ATR-FTIR spectra also show an increasing peak at 1635 cm−1 and at 1720 cm−1, proving the formation of carbonyl and carboxylate groups on the plasma-modified cotton samples. Similar as for the water impregnated samples, both peaks also increase with increasing treatment cycles, suggesting a higher incorporation of carbonyl and carboxylate groups with increasing impregnation/plasma treatment cycles. However, because of the different position of the OH stretching peak of H2O (3750–2995 cm−1) and the OD stretching peak of D2O (2650–2250 cm−1), a new band corresponding to the absorbed D2O in the cotton substrate also appears. Additionally, the H–O–H bending band of water (1638 cm−1) also shifts towards lower wavenumbers for D–O–D (1205 cm−1) (Belhadj et al. 2015; Liu et al. 2016). Nonetheless, the intensity of this band is typically much lower compared to the OD stretching band, making it difficult to clearly differentiate the D–O–D peak in the presented ATR-FTIR spectra. Additionally, it also seems that there is a correlation between the decrease in the band associated to the OH stretching peak (3750–2995 cm−1) of cellulose and the increase in the carbonyl and carboxylate groups as a consequence of a plasma-induced oxidation process and an interchange of OH groups present in the cotton substrates with OD groups. Therefore, when replacing H2O by D2O, it is again suggested that the increased peak at 1635 cm−1 cannot be attributed solely to adsorbed D2O and should thus be partially attributed to carbonyl groups formed because of cellulose oxidation (C=O). Comparing the ATR-FTIR spectra of He plasma-treated cotton with and without liquid impregnation, it seems that the presence of H2O or D2O favors the oxidation of cellulose because of the diffusion and formation of oxygen and nitrogen reactive species (ROS, RNS) in the adsorbed liquids (H2O2, OH·, O2−·, ONOO− and NO·) (Brisset and Pawlat 2016; Gorbanev et al. 2016; Ikawa et al. 2010; Tani et al. 2012).
The ATR-FTIR spectra corresponding to consecutive ethanol (EtOH) impregnation and He plasma treatments of cotton substrates (up to 6 impregnation/plasma treatments) are depicted in Fig. 3D. In this particular case, a small increase in absorbance of the region corresponding to CH stretching modes (3000–2800 cm−1) is observed as a function of impregnation/plasma treatment cycles, suggesting the incorporation of hydrocarbon groups, which is in agreement with the obtained OES spectra showing the presence of excited carbon species in the plasma. Additionally, an increase in the band at 1720 cm−1 is observed whereas the band at 1635 cm−1 only marginally increases (see Fig. 3f), suggesting that also in this case, plasma-assisted oxidation processes occur resulting in the incorporation of mainly carboxylate groups.
XPS
Since the analysis depth of ATR-FTIR is typically between 600 and 1000 nm, crucial information is lost from the uppermost polymer layers, which is particularly important in case of plasma surface modification, as non-thermal plasmas are known to primarily influence the first 50 nm of a material (Holländer et al. 1999; Tatoulian et al. 1995; Wang et al. 2008). In contrast, XPS analyses only the first 3–10 nm of a material surface, making it a very suitable technique to track plasma-induced surface changes (Castle 1984; Cools et al. 2015). Therefore, the changes in the surface chemical composition of the cotton substrates have also been studied by XPS in this work.
The atomic surface chemical composition of the He plasma-treated cotton samples and the cotton samples first impregnated with different liquids (water, D2O and EtOH) prior to plasma modification are presented in Table 1. Consecutive liquid impregnation and He plasma treatment steps of cotton substrates did not significantly change the surface elemental composition and for this reason, only results of first impregnation and He plasma treatment were mentioned in Table 1. This latter reveals that the He plasma treatment of cotton substrates does not significantly change the atomic surface chemical composition with respect to the untreated one. This result is quite surprising as plasma-induced surface oxidation was expected, since OH radicals and atomic oxygen were observed in the OES spectra during He plasma treatment (Fig. 2). It is also important to mention that the obtained O/C ratio for the untreated cellulose sample is considerably lower than the expected O/C ratio for pure cellulose (O/C = 0.83), which can be attributed to surface non-cellulosic components, which naturally occur on cotton (Kolarova et al. 2013) and which have not been removed from the samples by the performed washing step. Table 1 also reveals that if the cotton sample was first impregnated with H2O, no significant difference in oxygen content could again be observed. On the other hand, a small increase in oxygen content was observable on the D2O impregnated sample. Moreover, when cotton was impregnated with EtOH, a strong increase in carbon content combined with a large decrease in oxygen content was observed, which was highly unexpected.
High-resolution spectra of the C1s envelopes were also recorded to obtain additional insights into the chemical changes induced on the cotton substrates and the fitted C1s peaks are depicted in Fig. 4 for a single cycle of impregnation and plasma treatment.
As shown in Fig. 4, the C1s spectrum of the untreated cotton sample is deconvoluted into 4 distinct peaks, the largest peak being the one at 286.5 ± 0.1 eV, corresponding to aliphatic C–O–/C–OH functionalities, as expected for cellulose. Two shoulder bands at higher binding energies are also observed that can be attributed to O–C–O/C=O functionalities (287.7 ± 0.1 eV), which are present in the chemical structure of cellulose and O–C=O functionalities (289.1 ± 0.1 eV) most likely originating from the bleaching process carried out on the cotton samples (Molina et al. 2017; Patino et al. 2011). Moreover, a peak at 285.0 ± 0.1 eV needs to be included as well to obtain a good fit of the C1s peak: this peak probably originates from some non-cellulosic components such as waxes, proteins or pectin, which typically cover natural cotton fibers (Topalovic et al. 2007). Figure 4 also reveals that after performing the different plasma modification procedures, similar XPS fitting peaks as the ones used for the untreated cotton sample can still be applied to deconvolute the high resolution C1s spectra of the plasma-modified samples, although the area below each individual peak seems to vary depending on the performed plasma modification step. From these fitted spectra, relative concentrations of the different carbon bonds present at the surface of the cellulose samples can be determined and these quantitative results are presented in Table 2 for all samples under study using a single cycle of impregnation and plasma treatment.
This table clearly reveals that the He plasma treated cotton has a surface chemical composition which closely resembles the surface chemical composition of the untreated cotton sample, which is consistent with the results shown in Table 1. As such, it can thus be concluded that the performed helium plasma treatment does not induce a significant surface oxidation, although reactive oxygen plasma species are present in the discharge. Most likely, a combination of functionalization and etching simultaneously occurs leading to an unaffected total oxygen content after He plasma treatment. Table 2 also shows that when performing the plasma treatment on the H2O impregnated sample, a more pronounced increase in oxygen-containing surface functional groups is obtained: a considerable increase in the amount of C–O, C=O/O–C–O and O–C=O groups can be seen upon plasma treatment. This increase in C–O, C=O/O–C–O and O–C=O groups is even more pronounced when the cotton sample is first impregnated in D2O, which is in agreement with the O/C ratio results given in Table 1. The XPS results in this section thus confirm the incorporation of C=O and O–C=O groups on the cotton surface, which was also seen in the ATR-FTIR spectra shown in the previous section and additionally also reveal the incorporation of C–O functional groups on the cotton surface. Taking into account the considerably large penetration depth of FTIR (≈0.6–1 µm), it can also be concluded that the C=O and O–C=O functional groups are not only incorporated at the surface of the cotton samples, but also in the subsurface region.
When examining the samples impregnated with ethanol prior to plasma treatment, a completely different trend can be observed: a strong decrease in the oxygen-containing functional groups occurs in combination with a large increase in the amount of C–C groups. This peculiar result suggests the formation of a thin oxygenated hydrocarbon coating on the cotton sample, which will be further confirmed by SEM imaging performed in the following section.
SEM
Possible surface damage induced by the performed plasma treatments was also investigated by SEM and the obtained SEM images are shown in Fig. 5.
A single He plasma treatment is found to have a minimal impact on the surface morphology of the cotton sample, as a similar physical appearance compared to the untreated cotton was recorded (not shown for reader clarity). In contrast, a more profound impact on the surface integrity is observed with increasing repetitions of He plasma treatments. After 6 He plasma treatments, the cotton surface exhibits some surface damage in the form of microcraters due to plasma-induced etching processes (Karahan and Özdoğan 2008). Similar morphological changes have been reported when performing corona plasma treatments of cotton (Mihailović et al. 2011).
On the other hand, when cotton is first impregnated either with H2O or D2O and subsequently exposed to the He plasma, profound topographic changes could be observed even after one treatment cycle. In addition, by increasing the number of impregnation/plasma treatments, the observed surface damage significantly increases and after 6 impregnation/plasma treatment cycles, structures similar to particles appear on the cotton fibers, proving the excessive damage to the cotton fibers because of the high plasma exposure times. It is suggested that the presence of H2O or D2O on the cotton surface may induce many streamers/filaments during plasma treatment which could in turn be responsible for the excessive damage promoted on the cotton surface when compared to the He treatment without impregnation. This hypothesis was further confirmed by the OES results, which revealed that the He plasma on the H2O and D2O impregnated cotton samples operated in the filamentary mode at treatment times below 300 s.
When EtOH is used as impregnation liquid prior to the He plasma treatment, it could be observed that the cotton surface is covered with a flat coating, which is in agreement with the conclusions drawn from XPS analysis. In the case of ethanol impregnation, a plasma deposition process thus takes place instead of a plasma activation process. The film-forming behavior of EtOH-enriched plasmas has already been described before (Van Deynse et al. 2017), thereby further confirming the results obtained in this work. It is also important to mention that the presence of this film does not produce any significant visual color change of the cotton substrates.
Conclusions
In this study, cotton fibers were impregnated with H2O, D2O or EtOH prior to an atmospheric pressure He plasma treatment in a parallel-plate DBD set-up. OES analysis showed an increase in H atoms and OH radicals during the plasma treatment of H2O impregnated cotton samples, as was to be expected. Replacing water with its deuterated counterpart resulted in higher concentrations of N metastable species, which showed the indirect influence of free H species in the plasma. Ethanol plasma induced the formation of a variety of CN, C and CO species, even after longer treatment times, indicating that the presence of carbon induced a completely different plasma chemistry. Chemical analysis of the cotton samples revealed that the impregnation with (deuterated) water resulted in a more efficient plasma-induced oxidation of the cotton compared to the dry plasma treatment, while the presence of ethanol led to the formation of a thin oxygenated hydrocarbon film on top of the cotton sample. SEM analysis showed the profound etching of the cotton fibers as a result of the presence of the (deuterated) water, while for the EtOH impregnated samples, a smooth morphology could be distinguished, further indicating the presence of the thin film. Overall, it can be concluded that the impregnation of cotton fabrics with a variety of liquids can induce a more efficient surface modification compared to a plasma treatment as such.
References
Acsente T, Ionita MD, Teodorescu M, Marascu V, Dinescu G (2016) Surface modification of polymethylmethacrylate foils using an atmospheric pressure plasma jet in presence of water vapors. Thin Solid Films 614:25–30. https://doi.org/10.1016/j.tsf.2015.12.037
Aileni RM, Albici S, Chiriac L, Subtirica A, Dinca LC (2019) Aspects of the hydrophobic effect sustainability obtained in plasma for cotton fabrics. Ind Textila 70:223–228. https://doi.org/10.35530/IT.070.03.1475
Ananth NB, Mark JK (2008) Repetitively pulsed atmospheric pressure discharge treatment of rough polymer surfaces: II. Treatment of micro-beads in He/NH3/H2O and He/O2/H2O mixtures. Plasma Sources Sci Technol 17:035025. https://doi.org/10.1088/0963-0252/17/3/035025
Asandulesa M, Topala I, Dumitrascu N (2010) Effect of helium DBD plasma treatment on the surface of wood samples. Holzforschung 64:223–227. https://doi.org/10.1515/hf.2010.025
Belhadj H, Hakki A, Robertson PK, Bahnemann DW (2015) In situ ATR-FTIR study of H2O and D2O adsorption on TiO2 under UV irradiation. Phys Chem Chem Phys 17:22940–22946. https://doi.org/10.1039/C5CP03947A
Bierstedt A, Panne U, Rurack K, Riedel J (2015) Characterization of two modes in a dielectric barrier discharge probe by optical emission spectroscopy and time-of-flight mass spectrometry. J Anal At Spectrom 30:2496–2506. https://doi.org/10.1039/c5ja00332f
Brisset J-L, Pawlat J (2016) Chemical effects of air plasma species on aqueous solutes in direct and delayed exposure modes: discharge, post-discharge and plasma activated water. Plasma Chem Plasma Process 36:355–381. https://doi.org/10.1007/s11090-015-9653-6
Castle JE (1984) Practical surface analysis by Auger and X-ray photoelectron spectroscopy. D. Briggs and M. P. Seah (Editors). John Wiley and Sons Ltd, Chichester, 1983, 533 pp. Surf Interface Anal 6:302. https://doi.org/10.1002/sia.740060611
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr Polym 58:417–420. https://doi.org/10.1016/j.carbpol.2004.08.005
Cools P, De Geyter N, Morent R (2015) Plasma modified textiles for biomedical applications. Intech. https://doi.org/10.5772/59770
De Geyter N, Sarani A, Jacobs T, Nikiforov AY, Desmet T, Dubruel P (2013) Surface modification of poly-epsilon-caprolactone with an atmospheric pressure plasma jet. Plasma Chem Plasma Process 33:165–175. https://doi.org/10.1007/s11090-012-9419-3
Friedrich JF, Mix R, Schulze RD, Meyer-Plath A, Joshi R, Wettmarshausen S (2008) New plasma techniques for polymer surface modification with monotype functional groups. Plasma Process Polym 5:407–423. https://doi.org/10.1002/ppap.200700145
Garside P, Wyeth P (2003) Identification of cellulosic fibres by FTIR spectroscopy—thread and single fibre analysis by attenuated total reflectance. Stud Conserv 48:269–275. https://doi.org/10.1179/sic.2003.48.4.269
Gorbanev Y, O’Connell D, Chechik V (2016) Non-thermal plasma in contact with water: the origin of species. Chem Eur J 22:3496–3505. https://doi.org/10.1002/chem.201503771
Gorjanc M, Bukošek V, Gorenšek M, Mozetič M (2010) CF4 plasma and silver functionalized cotton. Text Res J 80:2204–2213. https://doi.org/10.1177/0040517510376268
Haji A (2017) Improved natural dyeing of cotton by plasma treatment and chitosan coating; optimization by response surface methodology. Cell Chem Technol 51:975–982
Haji A (2019) Dyeing of cotton fabric with natural dyes improved by mordants and plasma treatment. Prog Color Color Coat 12:191–201
Haji A, Barani H, Qavamnia SS (2013) In situ synthesis of silver nanoparticles onto cotton fibres modified with plasma treatment and acrylic acid grafting. Micro Nano Lett 8:315–318
Haji A, Qavamnia SS, Bizhaem FK (2016) Salt free neutral dyeing of cotton with anionic dyes using plasma and chitosan treatments. Ind Text 67:109–113
Holländer A, Wilken R, Behnisch J (1999) Subsurface chemistry in the plasma treatment of polymers. Surf Coat Technol 116:788–791. https://doi.org/10.1016/S0257-8972(99)00297-2
Hsieh Y-L, Thompson J, Miller A (1996) Water wetting and retention of cotton assemblies as affected by alkaline and bleaching treatments. Text Res J 66:456–464. https://doi.org/10.1177/004051759606600707
Ikawa S, Kitano K, Hamaguchi S (2010) Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Process Polym 7:33–42. https://doi.org/10.1002/ppap.200900090
Ikegami T, Nakanishi F, Uchiyama M, Ebihara K (2004) Optical measurement in carbon nanotubes formation by pulsed laser ablation. Thin Solid Films 457:7–11. https://doi.org/10.1016/j.tsf.2003.12.033
Joshi R, Schulze RD, Meyer-Plath A, Friedrich JF (2008) Selective surface modification of poly(propylene) with OH and COOH groups using liquid-plasma systems. Plasma Process Polym 5:695–707. https://doi.org/10.1002/ppap.200700175
Joshi R, Friedrich JF, Wagner M (2009a) Study of carboxylic functionalization of polypropylene surface using the underwater plasma technique. Eur Phys J D 54:249–258. https://doi.org/10.1140/epjd/e2009-00088-6
Joshi R, Schulze RD, Meyer-Plath A, Wagner MH, Friedrich JF (2009b) Selective surface modification of polypropylene using underwater plasma technique or underwater capillary discharge. Plasma Process Polym 6:S218–S222. https://doi.org/10.1002/ppap.200930601
Joshi R, Friedrich J, Krishna-Subramanian S (2013) Surface modification of ultra-high molecular weight polyethylene membranes using underwater plasma polymerization. Plasma Chem Plasma Process 33:921–940. https://doi.org/10.1007/s11090-013-9476-2
Jovančić P, Vílchez A, Molina R (2016) Synthesis of thermo-sensitive hydrogels from free radical copolymerization of NIPAAm with MBA initiated by atmospheric plasma treatment. Plasma Process Polym 13:752–760. https://doi.org/10.1002/ppap.201500194
Karahan H, Özdoğan E (2008) Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fiber Polym 9:21–26
Khlyustova A, Galmiz O, Zahoran M, Brablec A, Černak M (2015) Underwater discharge plasma-induced coating of poly(acrylic acid) on polypropylene fiber. J Mater Sci 50:3504–3509. https://doi.org/10.1007/s10853-015-8913-4
Kolarova K, Vosmanska V, Rimpelova S, Svorcik V (2013) Effect of plasma treatment on cellulose fiber. Cellulose 20:953–961. https://doi.org/10.1007/s10570-013-9863-0
Liu DX, Bruggeman P, Iza F, Rong MZ, Kong MG (2010) Global model of low-temperature atmospheric-pressure He + H2O plasmas. Plasma Sources Sci Technol 19:025018. https://doi.org/10.1088/0963-0252/19/2/025018
Liu H, Wang Y, Bowman JM (2016) Quantum local monomer IR spectrum of liquid D2O at 300 K from 0 to 4000 cm−1 is in near-quantitative agreement with experiment. J. Phys. Chem. B 120:2824–2828. https://doi.org/10.1021/acs.jpcb.6b01722
Mihailović D et al (2011) Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 18:811–825. https://doi.org/10.1007/s10570-011-9510-6
Molina R, Ligero C, Jovančić P, Bertran E (2013a) In situ polymerization of aqueous solutions of NIPAAm initiated by atmospheric plasma treatment. Plasma Process Polym 10:506–516. https://doi.org/10.1002/ppap.201200121
Molina R, Sole I, Vilchez A, Bertran E, Solans C, Esquena J (2013b) Surface functionalization of macroporous polymeric materials by treatment with air low temperature. Plasma J Nanosci Nanotechnol 13:2819–2825. https://doi.org/10.1166/jnn.2013.7369
Molina R, Gómez M, Kan C-W, Bertran E (2014) Hydrophilic–oleophobic coatings on cellulosic materials by plasma assisted polymerization in liquid phase and fluorosurfactant complexation. Cellulose 21:729–739. https://doi.org/10.1007/s10570-013-0131-0
Molina R, Teixidó JM, Kan C-W, Jovančić P (2017) Hydrophobic coatings on cotton obtained by in situ plasma polymerization of a fluorinated monomer in ethanol solutions. ACS Appl Mater Interfaces 9:5513–5521. https://doi.org/10.1021/acsami.6b15812
Morent R, De Geyter N, Leys C, Gengernbre L, Payen E (2008) Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf Interface Anal 40:597–600. https://doi.org/10.1002/Sia.2619
Patino A, Canal C, Rodríguez C, Caballero G, Navarro A, Canal JM (2011) Surface and bulk cotton fibre modifications: plasma and cationization. Influence on dyeing with reactive dye. Cellulose 18:1073–1083. https://doi.org/10.1007/s10570-011-9554-7
Rajasekaran P, Muthuraman B (2019) Effect of plasma pre-treatment on the conductivity of polypyrrole-coated cotton fabric. Fiber Polym 20:2114–2119. https://doi.org/10.1007/s12221-019-1177-x
Sun D, Stylios G (2004) Effect of low temperature plasma treatment on the scouring and dyeing of natural fabrics. Text Res J 74:751–756. https://doi.org/10.1177/004051750407400901
Tani A, Ono Y, Fukui S, Ikawa S, Kitano K (2012) Free radicals induced in aqueous solution by non-contact atmospheric-pressure cold plasma. Appl Phys Lett 100:254103. https://doi.org/10.1063/1.4729889
Tatoulian M, Arefi-Khonsari F, Mabille-Rouger I, Amouroux J, Gheorgiu M, Bouchier D (1995) Role of helium plasma pretreatment in the stability of the wettability, adhesion, and mechanical properties of ammonia plasma-treated polymers. Application to the Al-polypropylene system. J Adhes Sci Technol 9:923–934. https://doi.org/10.1163/156856195X00798
Topalovic T, Nierstrasz VA, Bautista L, Jocic D, Navarro A, Warmoeskerken M (2007) Analysis of the effects of catalytic bleaching on cotton. Cellulose 14:385–400. https://doi.org/10.1007/s10570-007-9120-5
Van Deynse A, De Geyter N, Leys C, Morent R (2014) Influence of water vapor addition on the surface modification of polyethylene in an argon dielectric barrier discharge. Plasma Process Polym 11:117–125. https://doi.org/10.1002/ppap.201300088
Van Deynse A, Morent R, Leys C, De Geyter N (2017) Influence of ethanol vapor addition on the surface modification of polyethylene in a dielectric barrier discharge. Appl Surf Sci 419:847–859. https://doi.org/10.1016/j.apsusc.2017.05.111
Wang C, Liu Y, Xu H, Ren Y, Qiu Y (2008) Influence of atmospheric pressure plasma treatment time on penetration depth of surface modification into fabric. Appl Surf Sci 254:2499–2505. https://doi.org/10.1016/j.apsusc.2007.09.074
Wang X et al (2019) The application of atmospheric plasma for cotton fabric desizing. Fiber Polym 20:2334–2341. https://doi.org/10.1007/s12221-019-9330-0
Yanguas-Gil A, Hueso JL, Cotrino J, Caballero A, Gonzalez-Elipe AR (2004) Reforming of ethanol in a microwave surface-wave plasma discharge. Appl Phys Lett 85:4004–4006. https://doi.org/10.1063/1.1808875
Yasuda H, Hirotsu T (1978) Critical evaluation of conditions of plasma polymerization. J Polym Sci Pol Chem 16:743–759. https://doi.org/10.1002/pol.1978.170160403
Acknowledgments
This work received financial support from the MAT2016-79866-R project (AEI/FEDER, UE). P. Cools would like to acknowledge the Special Research Fund of Ghent University for funding his post-doctoral mandate (BOF17/PDO/023). The authors would also like to thank the contribution of the scanning electron microscopy service of the Institute of Marine Sciences (ICM-CSIC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molina, R., Bitar, R., Cools, P. et al. Effect of liquid impregnation on DBD atmospheric pressure plasma treatment of cotton. Cellulose 27, 7847–7859 (2020). https://doi.org/10.1007/s10570-020-03306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03306-3