Abstract
An underwater diaphragm discharge generated at atmospheric pressure by high voltage pulses was used for plasma polymerization of acrylic acid and simultaneously for the deposition of polymer coating on polypropylene multifilament fibers. The deposition process was monitored by Fourier transform infrared spectroscopy in combination with scanning electron microscopy. Possible schemes of polymerization are suggested also.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyolefins, like polypropylene (PP) or polyethylene, are widely used in many technological fields due to their suitable bulk properties, easy processability, and low manufacturing cost. While maintaining the bulk properties of polymers, the plasma modification of their surface properties can tailor polymers for specific use [1]. Many industrial applications of PP films also require good adhesion to the second surface for example, its adhesion of inks during printing, painting, coatings, lamination etc. Generally, the presence of polar groups and the morphology of the surface play a very important role for obtaining good adhesion. However, PP films/fibers have limitation to their adhesion properties due to their non-polar nature and low surface tension.
Many researchers are interested in the production of thin organic layers containing carboxylic groups on PP by various methods. The polymerization of acrylic acid solution on the PP substrate after electron beam bombardment or gas discharge treatment have been studied in [2, 3] while the photografting acrylic acid was also used for better adhesion between PP and polyethylene films [4–6].
The underwater electrical discharge makes it possible to generate in liquid phase the non-equilibrium, low-temperature plasmas with chemical active species. These species such as H, OH radicals and hydrated electrons induce in aqueous solutions various chemical reactions which result in surface treatment of both natural and synthetic polymer materials. There are several reported experimental arrangements based on the underwater discharge that are suggested for various applications such as wastewater treatment [7–9], oxidative degradation [10], surface modification [11], etc.
The diaphragm discharge which has been used in our experiments represents peculiar type of underwater electrical discharges. As discussed in [12–15], the discharge ignition is preceded by the generation of steam and steam-gas bubbles in the electrolyte bulk. The process is caused by the local overheating of the solution. As it is known [13], the particular mechanism of bubble growth and electric breakdown depends on the electrode geometry and primarily on the diameter of the overlapping cross section of the capillary or diaphragm. It is generally agreed that in this case the generation of a bubble results in the overlapping of the transporting channel and the termination of the electrolysis current. If potential difference is large enough, electric breakdown occurs. Data of [14] allows to specify the assumed scenario of the underwater discharge formation in the case of a thin capillary. The generation of a bubble in the form of a ring growing from the side of the walls caused by the local solution overheating will really result in the progressive narrowing of the transporting channel along the capillary axis and the increase of the voltage drop in this part. As in the previous case, with the voltage drop being great enough, there occurs the electric breakdown of the bubble. However, the complete overlapping of the capillary before the breakdown is not necessary. It is easy to verify that, in this case, the breakdown conditions will depend on the full voltage in the cell and the system’s geometry. The experimental data for capillary discharges are not in contradiction with the described situation [15].
Finally, the underwater and glow discharge electrolysis treatments of polymers show more variability and efficiency because of the additive’s broad variety which can be used to improve the polymer surface modification process. The UV radiation, electrons, and free radicals generated by discharge penetrate into liquid and induce polymer surface modification [16].
In this paper, we suggest to use the pulsed HV diaphragm discharge for surface treatment of PP fibers. Note that contrary to invariable DC voltage, the pulsed DC power supply has several advantages. Namely, the pulsed DC voltage overcomes big problem with heating of temperature samples such as PP which always appears while using a power supply with steady DC. It also means that the consumed energy is low. Diagnostic methods used in experiments monitor properties of treated samples as well as the state of plasma.
Materials and methods
Materials
PP multifilament fibers used in experiments are produced by KrampeHarex, Germany. The acrylic acid, purity of 99.5 %, was obtained by Acros Organics. The distilled water solution of acrylic acid prepared at various concentrations in the range of 0.1–3.3 M L−1 was used as liquid environment for the discharge.
The rest of acrylic acid which remains on the surface of PP fibers after the plasma treatment was always washed in acetone. The acetone was used because the poly(acrylic acid) unlike the acrylic acid that does not dissolve in it.
Experimental setup
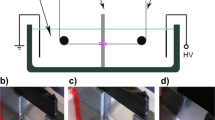
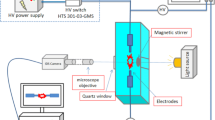
The underwater plasma reactor setup shown in Fig. 1 consists of two electrodes submerged in electrochemical cell separated by a dielectric wall with diaphragm. The core of the underwater assembly, the diaphragm with the inner diameter of 2.5 mm, separates as a barrier the two filled chambers solution apart. The PP fiber passes through diaphragm with a speed of 10 cm min−1. The DC pulses of 100 Hz was applied with a peak-to-peak voltage of maximum 25 kV, pulse duration of 3 μs, and a rise time of around 0.2 μs. The typical voltage waveform of pulsed diaphragm discharge in aqueous solution is shown in Fig. 2. Experiments were carried out in two versions. First, the work solution was filled in both chambers of electrochemical cell (volume of solution was 500 mL) while in the second case the tap water is used in one chamber of cell and the second chamber was filled by acrylic acid solution. The fiber passed through aqueous solution into the plasma zone and thereafter continued into the work solution again. The mean contact time of fiber and plasma was about 30 s. According to [17] the polymerization of acrylic acid caused by DC corona discharge occurs after 12 min. In order to observe the polymerization in the acrylic acid based solution, the last one was treated by plasma during 20 min. This time was supposed to be enough for polymerization to take place.
Since the contact time of fiber and working solution in plasma zone is short, the mechanism of grafting effect of treated solution on the treated fiber has been studied. The PP fibers were treated by underwater plasma discharge generated in distilled water and then the treated fibers were immersed in the poly(acrylic acid) solution. The grafting was carried out for 15 min at a room temperature.
Instrumentals
FTIR-ATR spectroscopy and scanning electron microscopy were used to characterize the efficiency of coating effect.
FTIR spectra were acquired on a VERTEX 80 spectrometer by Brucker Optics with ATR reflectance attachment in the range of 4000–450 cm−1 with a spectral resolution of 4 cm−1. The samples for IR were prepared in the KBr pellets.
Treated and untreated fibers were evaluated using the scanning electron microscope TS 5136MM by TESCAN. The surface morphology of fibers treated under different conditions was investigated. Before SEM observation, samples were dried and coated with gold.
Results and discussion
The acrylic acid coating
It was supposed that PP fiber passed through acrylic acid solution is coated by thin layer with functional groups. Then, the acrylic acid is polymerized in the plasma zone under the radical mechanism (see the Scheme 1) as reported in [11].
The IR spectra of untreated PP fibers as well as the spectra of fibers treated in acrylic acid solutions of various concentrations are shown in Fig. 3. All spectra are normalized to the absorption peak at 2922 cm−1, which represents the CH2 group vibration of the main PP polymer chain. All spectra in Fig. 3 exhibit the broad band around 3200–3600 cm−1 that can be assigned to O–H elastic vibrations. Also all spectra show four large peaks in the wave number range 3000–2800 cm−1: the peak at 2955 and 2873 cm−1 can be attributed to CH3 asymmetric and symmetric stretching vibrations, respectively while the peaks at 2922 and 2843 cm−1 are due to CH2 asymmetric and symmetric stretching vibrations, respectively. IR spectra also show two intensive peaks at 1460 and 1378 cm−1. The peak 1460 cm−1 is caused by CH3 asymmetric deformation vibration or CH2 scissor vibrations, while the peak at 1378 cm−1 is due to CH3 symmetric deformation vibration. All spectra contain numerous small peaks in wave number range of 1200–750 cm−1. The peak at 1167 cm−1 can be attributed to C–C asymmetric stretching, CH3 asymmetric rocking and C–H wagging vibrations, while the peak at 998 cm−1 is due to CH3 asymmetric rocking vibrations. The peak at 840 cm−1 is attributed to CH2 rocking vibrations [18, 19]. As it is shown in Fig. 3, the (c) curve has an additional peak at 1554 cm−1 (shown by pointer). This band can be assigned to –COOH group (antisymmetric vibrations). According to [20] the peak position depends on adsorption process.
Figure 4 depicts SEM photographs of untreated and treated fibers in acrylic acid solution. The untreated PP fiber surface is smooth (Fig. 4a) while after treatment in 0.2 M acrylic acid one can see that the surface is slightly rough and a film of poly(acrylic acid) is deposited on the surface of the fiber (Fig. 4b). In the first case, the OH groups which were formed in solution by underwater diaphragm discharge react with PP chain and form polar groups on the surface. The molecules of poly(acrylic acid) were attached to surface via these polar groups. However, there were differences in the surface morphology for fibers treated in 0.2 M and 0.4 M solution of acrylic acid (Fig. 4c). In the case of high concentration of acrylic acid, the fiber surface is smooth like a surface of untreated fiber. It can be explained in two ways: (i) the deposited film covered the fiber fully or (ii) there is no film on the surface. According to our data of IR spectra, the second hypothesis is correct. However, one can assume that the thin film of poly(acrylic acid) was deposited on the PP surface but the selected method of monitoring is not enough for surface analysis. One can suggest that the concentration of 0.2 M of acrylic acid is more convenient for the formation of uniform surfaces on the PP fibers. On the other hand according to [21, 22] there is condition of concentration. In the existence of acrylic acid solution, the polymerization on surface usually competes with homopolymerization occurring in acrylic acid solution. When the monomer concentration is low, the polymerization on surface has more chance to occur than homopolymerization. However, at high monomer concentration, homopolymerization becomes more dominant than polymerization on the surface that occurs only in a confined region.
The activation of PP in water
Therefore to highlight chemical changes on the surface, one must subtract a reference sample spectrum from treated one. Figure 5 presents the subtraction of ATR-IR spectra measured for PP fibers in acrylic acid and PP-untreated samples (used as reference). As a result, two new IR bands appear. The first band is rather broad. It stretched between 3300 and 3530 cm−1, while being centered at about 3400 cm−1. It is attributed to OH elastic vibrations. Band 2 consists of 4 resolved peaks at 1440, 1554, 1641, and 1714 cm−1, which attributed to C=O and COOH groups of poly(acrylic acid) [18, 23]. It should be noted that the same results were obtained for all concentrations till 1 M. Thus, one can deduce that adhesion of poly(acrylic acid) on the PP fiber occurs at the concentration of acid less than 1 M.
Grafting effect
Data of ATR spectra and SEM photographs morphology after grafting shoewd that the results are the same as data which obtained at direct of PP fiber and acrylic acid in plasma zone. The ATR spectra also have a band, that attributed to OH elastic vibrations, and peaks, that attributed to C=O and COOH groups of poly(acrylic acid). At high concentration of acrylic acid there is no grafting effect at the surface. It can be explained that at large concentration of acid, the polymerization process by plasma action does not occur.
Based on results mentioned above one can suggest a mechanism of interaction of PP surface and poly(acrylic acid). The ignition of electrical discharge inside water generates formation of chemically active species such as H, OH radicals, solvated electrons, singlet oxygen, etc. These active species may react with polymer molecules. And, hence, the breaking of molecular bands on the surface occurs and bonding of polar OH groups (mainly C–O–H, C=O, and C–O) as seen in the Scheme 2 takes place. Authors of [22] suggest that acrylic acid/poly(acrylic acid) grafted to activated PP by radical mechanism with the participation of primary carbon atom (Scheme 3). It was supposed that COOH group attached to PP surface only in random order and formed thin polymer film, which has irregular structure [11]. The grafting mechanism of acetylsalicylic acid to the PP monofilament fibers in plasma-solution system was assumed [24]. The connection occurs via methyl group substitution by carboxyl one. Taking into consideration the conditions of plasma ignition and plasma treatment, the most probable mechanism of grafting poly(acrylic acid) can be shown in Scheme 4. The appearance of peak at 3200–3600 cm−1 before and after grafting poly(acrylic acid) can be explained.
Conclusions
Using pulsed underwater discharge enables treating of PP multifilament fibers and formation of polar groups on the surface. It allows to graft poly(acrylic acid) to PP fibers. As shown by the experimental results, the grafting effect of poly(acrylic acid) on the activated PP surface is the same as at direct coating of surface with poly(acrylic acid). The big advantage of treatment over grafting is the time needed for adhesion. In case of using only one chamber with chemicals, the results obtained are practically the same. Hence, in order to decrease consumption of chemicals the usage of second method for activation of PP fiber surface is better.
References
Inagaki N (1996) Plasma surface modification and plasma polymerization. Tecnomic Publishing, Lancaster
Poncin-Epaillard F, Chevet B, Brosse JC (1994) Modification of isotactic polypropylene by a cold plasma or an electron beam and grafting of the acrylic acid onto these activated polymers. J Appl Polym Sci 53:1291–1306
Sellin N, Sinezio J, Campos C (2003) Surface composition analysis of PP films treated by corona discharge. Mater Res 6:163–166
Dogue ILJ, Mermilliod N, Boiron G, Staveris S (1995) Improvement of polypropylene film adhesion in multilayers by various chemical surface modifications. Int J Adhes Adhes 15:205–210
Dogue ILJ, Forch R, Mermilliod N (1995) Grafting of acrylic acid onto polypropylene—comparison of two pretreatments: γ-irradiation and argon plasma. Nucl Instr Methods Phys Res B 105:164–167
Winnik FM, Morneau A, Mika AM, Childs RF, Roig A, Molins E (1998) Polyacrylic acid pore-filled microporous membranes and their use in membrane-mediated synthesis of nanocrystalline ferrihydrite. Can J Chem 76:10–17
Gao JZ (2006) A novel technique for wasterwater treatment by contact glow discharge electrolysis. Pak J Biol Sci 9:323–329
Khlustova AV, Maximov AI, Subbotkina IN (2010) The electrical discharge action on the wastewater for cleaning. High Temp Mater Proc 14:185–191
Dors M, Metel E, Mizeraczyk J (2007) Phenol degradation in water by pulsed streamer corona discharge and Fenton reaction. Int J Plasma Environ Sci Technol 1:76–81
Lu QF, Yu J, Gao JZ (2006) Degradation of 2,4-dichlorophenol by using glow discharge electrolysis. J Hazard Mater 136:526–531
Joshi R, Schulze RD, Meyer-Plath A, Friedrich JF (2008) Selective surface modification of poly(propylene) with OH and COOH groups using liquid-plasma systems. Plasma Process Polym 5:695–707
Maximov A (2007) Physics, chemistry and application of the AC diaphragm discharge and related discharges in electrolyte solutions. Contrib Plasma Phys 47:111–118
Teslenko VS, Drozhzhin AP, Sankin GN (2006) The autocyclic ring breakdown in electrolyte with induced collapse of bubbles. Lett J Technol Phys 32:24–31 (in Russian)
Khlyustova AV, Manakhov AM, Maksimov AI (2009) A scenario of development of low-voltage “underwater” discharge. Surf Eng Appl Electrochem 45:485–488
Nikiforov AY, Leys C (2007) Influence of capillary geometry and applied voltage on hydrogen peroxide and OH radical formation in AC underwater electrical discharges. Plasma Sources Sci Technol 16:273–280
Yang P, Deng JY, Tai YW (2003) Confined photo-catalytic oxidation: a fast surface hydrophilic modification method for polymeric materials. Polymer 44:7157–7164
Malik MA, Ahmed M, Rehman E, Naheed R, Ghaffar A (2003) Synthesis of superabsorbent copolymers by pulsed corona discharges in water. Plasma Polym 8:271–279
Siaratta V, Vohrer U, Hegemann D, Muller M, Oehr C (2003) Plasma functionalization of polypropylene with acrylic acid. Surf Coat Technol 174–175:805–810
Morent R, De Geyter N, Leys C, Gengembre L, Payen E (2008) Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf Interface Anal 40:597–600
Kirwan LJ, Fawell PD, van Brounswijk W (2003) In situ FTIR-ATR examination of poly(acrylic acid) adsorbed onto hematite at low pH. Langmuir 19:5802–5807
Choi HS, Kim YS, Zhang Y, Tang S, Myung SW, Shin BS (2004) Plasma-induced graft co-polymerization of acrylic acid onto polyurethane surface. Surf Coat Technol 182:55–64
Gornukhina OV, Shikova TG, Ageeva TA, Titov VA, Golubchikov OA (2008) Post-plasma graft co-polymerization of polypropylene and acrylic acid. Chem Chem Technol 51:75–79 (in Russian)
Alavi MHS, Habibi M, Amrollahi R, Afshar Taromi F (2011) A study of plasma polymerization of acrylic acid using APF plasma focus device. J Fusion Energy 30:184–189
Golubchikov OA, Gornukhina OV, Vershinina IA, Ageeva TA, Titov VA (2007) The polypropylene materials of medical purpose modificated by acetylsalicylic acid. Chem Chem Technol 50:65–68 (in Russian)
Acknowledgements
This research has been supported by the Project R&D center for low-cost plasma and nanotechnology surface modifications CZ.1.05/2.1.00/03.0086 funded by European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khlyustova, A., Galmiz, O., Zahoran, M. et al. Underwater discharge plasma-induced coating of poly(acrylic acid) on polypropylene fiber. J Mater Sci 50, 3504–3509 (2015). https://doi.org/10.1007/s10853-015-8913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8913-4