Abstract
In this paper, a facile and efficient approach to robust and durable superhydrophobic cotton fabric was presented via in situ CuO deposition and stearic acid (STA) coating. The combined effects of both rough structure and low surface energy endowed cotton fabric (Cot) with superhydrophobicity, water repellency, and self-cleaning property. Moreover, the as-prepared fabric (Cot–CuO–STA) could keep its robust superhydrophobicity under harsh environmental conditions of acidic, alkaline and salt solutions, high temperature, mechanical abrasion and washing. Importantly, the obtained Cot–CuO–STA with WCA of 156.5° had great potential in oil/water separation with high separation efficiency of up to 98.7% for various oils (dichloromethane, trichloromethane, soybean oil, and n-heptane). Further, fascinating permeate flux (more than 1800 L.m−2.h−1) and remarkable recyclability made Cot–CuO–STA a promising application in oil-contaminated water treatment and marine spilt oil cleanup.
Graphic abstract
Robust and durable superhydrophobic cotton fabric was fabricated for oil/water separation via a facile and efficient route. The resultant fabric exhibited remarkable separation efficiency for different kinds of oils, fascinating permeate flux, and excellent recyclability.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inspired by amazing organisms in nature, such as lotus leaves (Yang et al. 2018; Farhadi et al. 2011) and gecko feet (Pokroy et al. 2009), superhydrophobic materials with low surface energy and hierarchical micro/nanostructure have attracted attention and interest because of their practical applications in self-cleaning (Yang et al. 2019; Gelebart et al. 2017), anticorrosion (Lvov et al. 2016; Zhao et al. 2017), antifouling (Qing et al. 2019; Fisher et al. 2018), antiicing (Liu et al. 2014; Li et al. 2019) and oil/water separation (Wang et al. 2015; Liu et al. 2019a, b, c). To date, numerous methods have been exploited to obtain artificial superhydrophobic surfaces, such as sol–gel method (Lin et al. 2019; Liu et al. 2018), chemical vapor deposition (Cheng et al. 2019; Zhang et al. 2011), template processing (Chen et al. 2018; Wang et al. 2015), plasma etching (Liu et al. 2019a, b, c; Cai et al. 2019), electrospinning (Lu et al. 2009; Zhu et al. 2018), dip-coating (Ge et al. 2020; Zhang et al. 2019a, b; Dong et al. 2019), spraying method (Peng et al. 2018; Foorginezhad et al. 2019) and thiol-ene click chemistry (Xue et al. 2019; Yang et al. 2020).
Although great success has been achieved in the fabrication of superhydrophobic surfaces, low mechanical and bonding strength still impede their practical applications in industry and engineering fields (Jia et al. 2018; Zhang et al. 2019a, b). Most superhydrophobic surfaces have low resistance, especially exposing them to surroundings of corrosive substances, high temperature, strong light, abrasion and washing. These harsh conditions may destroy the superhydrophobicity of the material surface, hindering its applications. Therefore, much effort has been spent to improve the durability of the artificial superhydrophobic surface in recent years. Hou et al. (2018) successfully prepared POSS-based superhydrophobic fabrics by thiol-ene click chemistry, which displayed excellent durability to corrosive liquids, UV irradiation, high temperature, washing and mechanical abrasion. Zhou et al. (2017a, b) prepared superhydrophobic cotton fabric with good resistance to UV radiation, high temperature, organic solvent, and mechanical abrasion by modifying phytic acid-metal complexes and PDMS on textile substrates. Chen et al. (2015) obtained a versatile material with flame retardant, self-healing and superhydrophobic properties by impregnating cotton fabric with APP/bPEI/F-POSS. However, challenges remain to exist for facile and green preparation strategy of robust and durable superhydrophobic surface. That is realizable for industrial applications, especially in terms of mild processing conditions and large-scale production.
In this work, a facile, efficient approach to robust and durable superhydrophobic cotton fabric was proposed via in situ CuO deposition and stearic acid (STA) coating. CuO micro/nano particles have the advantages of safety, low cost, biocompatibility, photocatalytic, and antibacterial activity. They have been often applied to prepare multifunctional textiles (Rezaie et al. 2018; El-Nahhal et al. 2018; Perelshtein et al. 2009). Moreover, the deposition of CuO micro/nano particles on fabric can improve the stability of coated surface. Therefore, it is promising to endow cotton fabric (Cot) with super-hydrophobicity and excellent resistance based on the synergistic effect between CuO micro/nano particles and STA, inducing a robust hierarchical structure and low surface energy. In this context, the morphology, wetting property, chemical structure, environmental durability, antifouling, self-cleaning, and oil/water separation performances of the superhydrophobic cotton fabric were investigated systematically. The obtained superhydrophobic cotton fabric displayed remarkable stability and durability for acidic, alkaline or saline solutions, mechanical abrasion and washing. Therefore, this robust and durable superhydrophobic cotton fabric with facile fabrication has wide application prospects.
Experiment section
Materials
Cotton fabric (136 g/m2, yarn fineness of 30 tex) and soybean oil were purchased from local market. Copper acetate (Cu(CH3COO)2), stearic acid (CH3(CH2)16COOH), sodium hydroxide (NaOH), Sudan III, trichloromethane (CHCl3), ethanol, n-heptane (C7H16) and ichloromethane (CH2Cl2) were purchased from Aladdin Industrial Corporation (Shanghai, China). All chemical reagents were analytical-grade and used without further purification.
Sample preparation
Superhydrophobic cotton fabric was fabricated via in situ CuO deposition and stearic acid (STA) coating. Briefly, clean cotton fabric (5 cm × 5 cm) was immersed into CuAc solution (0.5 ~ 3wt.%) to react at 70 °C for 30 min, subsequently heating up to 100 °C for another 30 min reaction. Then, the fabric was washed with deionized water and dried at 60 °C for 1 h to obtain CuO-deposited fabric (Cot–CuO). After that, the Cot–CuO was coated using 1 wt.% of STA solution for 5 min using a dip-coating method and then dried at 60 °C, each cycle for 30 min. Finally, the superhydrophobic cotton fabric (Cot–CuO–STA) was obtained. For comparison, cotton fabric was directly immersed into STA solution to obtain STA-coated fabric (Cot–STA) as control sample.
Characterization
Surface morphology of the samples was characterized by Field Emission Scanning Electron Microscopy (FESEM, S-4800, Hitachi, Japan) at an accelerating voltage of 15 kV. Chemical structure of the samples was analyzed using Fourier Transform Infrared Spectrometer (FTIR, Nicolet 5700, Thermo Electron Corp., USA) ranging from 4000 to 400 cm−1 at a resolution of 2 cm−1 and X-ray Photoelectron Spectrometer (XPS, Thermo Fisher Scientific, USA) with Al K-Alpha monochrome X-ray source. Crystal structure of the samples was characterized by X-ray Diffractometry (XRD, Rigaku Corp. D/max-2400). Water contact angles (WCA) were measured by optical contact angle apparatus (JC2000C1, China) at room temperature with 5µL water droplets, and the average values were obtained by measuring each sample at five different positions. Tensile strength of the samples (15 × 5 cm2) was tested using Instron 5967 testing machine (Instron Corp., USA) at a rate of 100 mm/min according to ASTMD 5035-2006 standard method.
Stability tests
Acidic, alkaline, and NaCl solutions (0.9, 2.5 and 10 wt.%) were used to investigate the chemical stability of the Cot–CuO–STA, respectively. The fabric was immersed into different solutions, taken out at desired time interval, and then washed with deionized water and dried at 60 °C for WCA measurement.
High temperature stability was determined by placing in a drying oven at 100–200 °C for 2 h. For mechanical stability test, the fabric weighing 200 g was placed face-down to 800 or 1500 mesh sandpaper, respectively, and dragged back and forth (10 cm for a cycle). The WCA was determined after different abrasion cycles. Washing resistance was tested according to AATC 61-2006(2A) standard method. Samples (15 cm × 5 cm) were washed in stainless steel washing tank containing detergent with a concentration of 0.15% (w/v) at 49 °C with rotating speed of 40 rpm. After 45 min, the sample was taken out, then washed and dried. One machine washing cycle is equivalent to five home washings.
Measurement of oil/water separation
Dichloromethane, trichloromethane, soybean oil and n-heptane were used to evaluate the oil/water separation efficiency of the Cot–CuO–STA. Oil or organic solvents colored with Sudan III were mixed with water with volume ratio of 1:1, then the mixture (200 mL) was poured into a sand core funnel through the fabric. The separated oil and water were collected, respectively. The separation efficiency (η) was calculated according to the following Eq. (1).
where M0 and M1 are the weight of water before and after separation, respectively.
The permeate flux (J, L m−2 h−1) was calculated according to Eq. (2).
where V is permeate volume, S is effective area of the fabric, and t is permeate time (h).
Results and discussion
Fabrication and characterization
The preparation process of the superhydrophobic cotton fabric was presented in Scheme 1. The cotton fabric was first modified with CuO micro/nanoparticles via in situ chemical precipitation method, and then coated with STA solution by dip-coating process. CuO micro/nanoparticles successfully anchored on the fabric surface confirmed by FESEM images (Fig. 1). The neat microfibers in fabric have smooth surfaces (Fig. 1a). After deposits of CuO, the microfibers exhibit roughness at the micro/nanoscale due to the formed CuO micro-particles (Fig. 1b). CuO particles are densely embedded and randomly oriented, generating a rough microstructure in fabric surface. After STA coating, the fabric displays a stacked sheet structure (Fig. 1d). These surface heterogeneities contribute to the superhydrophobic surface, which can entrap air (Liu et al. 18,19,c). The Cot–CuO–STA has a WCA of 156.5°, indicating the superhydrophobic surface (inset in Fig. 1d). By contrast, the Cot–STA was demonstrated to be superhydrophilic as shown in Fig. 1c, due to the formation of few lamellar structures even immersing fabric in STA solution for seven times (Fig. S1). The results demonstrated that CuO deposition could facilitate the coating of STA on the fabric surface.
Theoretically, the surface factors of fabric such as density, roughness and surface energy can influence the superhydrophobic behavior of fabric surface. Thus, the effect of CuAc concentrations (five times coating with STA) and coating times (2 wt.% of CuAc) on the WCA values of Cot–CuO–STA was investigated. As CuAc concentrations increased from 0.5 to 3 wt.%, CuO particle densities increased, and CuO particles densely anchored on the fabric surface (Fig. S2). Fig. S3 shows the effect of CuAc concentrations on the surface morphologies of Cot–CuO–STA with STA coating for five times. It can be found that stacked sheet structure generated and gradually increased with the increases of CuAc concentrations, which might be due to the increment in density and roughness of fabric surface generated by CuO particles, promoting STA coating. Consequently, the WCA of Cot–CuO–STA slightly increased from 154.5° to 156.9° (Fig. 2a). And, the WCA of Cot–CuO–STA improved to 157.5° from 152.7° with increasing the coating times from one to seven times (Fig. 2b), attributed to more compact lamellar structures (Fig. S4). The results indicated that CuO deposition was beneficial for the coating of STA on the fabric surface, and the coexistence of micro/nanostructure and low surface energy is key for creating a superhydrophobic surface, like natural lotus leaves with wax and nano-papillae (Zhou et al. 2017a, b; Nanda et al. 2019). Figure 2c shows the contact, deformation and departure processes of water droplet on the Cot–CuO–STA surface within 5 s. It can be seen that water droplet contacted with the surface could detach from the substrate even though severe deformation, revealing a self-cleaning surface.
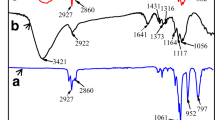
The wetting property of the modified fabric derived from not only surface structure but also its chemical composition. Figure 3a shows the crystal phases of Cot, Cot–CuO, Cot–STA and Cot–CuO–STA, respectively. Cot and Cot–STA show diffraction peaks of cellulose crystal at 2θ = 14.9°, 16.6°, and 22.9° (Riaz et al. 2019). New characteristic peaks appeared at 2θ = 35.8°, 38.9°, 48.7°, 58.2°, 61.5°, 66.2° and 68.1° after deposition process in Cot–CuO and Cot–CuO–STA, which were demonstrated as the crystal phase of CuO (Ethiraj et al. 2012). Figure 3b shows FTIR spectra of different samples. It can be seen that the absorption peaks of cellulose fabric before and after CuO deposition are the same, except the absorption band at around 598 cm−1 attributing to Cu–O stretching vibration (Kliche and Popovic 1990). In addition, C–H stretching vibration at 2902 cm−1 has split into –CH3 and –CH2 asymmetrical and symmetrical stretching vibrations of the long chain aliphatic groups of STA at 2915 cm−1 and 2850 cm−1, respectively (Kong et al. 2015). The results verified the modification of cellulose with CuO and STA. Further, the surface chemical composition was determined by XPS spectra and shown in Fig. 3c–f. For Cot and Cot–STA, only C and O peaks were found (Fig. 3c). After CuO deposition, new peak at 935 eV appeared, attributed to Cu 2p. Figure 3e–f show that the Cu 2p peak has two peaks attributed to Cu 2p3/2 at 933.7 eV and Cu 2p1/2 at 954.0 eV, respectively (Ren et al. 2018). The C1s peak of Cot can be divided into two components at 284.7 eV and 286.2 eV, which belong to C–C and C–O bonds, respectively (Fig. 3d) (Yan et al. 2019). Moreover, the C1s peak of Cot–STA was composed of two peaks corresponding to C–C (284.3 eV) and C=O (287.6 eV), respectively. After CuO deposition and STA coating, the peak weakened. Thus, the combination of CuO particle and STA coating not only improved the roughness of cotton fabric but also decreased its surface energy, mainly contributing to the superhydrophobic surface. Further, the effect of surface treatment on the mechanical strength and air permeability was investigated and shown in Fig. S5. Superhydrophobic treatment did not affect the mechanical properties of the fabric keeping their tensile strength of 11 MPa and elongation at break of 21 ~ 23%, expectedly had a negative effect on its breathability with mild reduction of air permeability.
During practical application, harsh environmental conditions may destroy the super-hydrophobicity of material surface, hindering its applications. Thus, the durability of the Cot-CuO-STA was investigated, and the results were presented in Fig. 4. After immersing the fabric in acidic and basic solutions for 3 days, the WCA was almost constant and higher than 153° at pH 5 ~ 13, indicating excellent superhydrophobicity (Fig. 4a). Even immersion for 7 days, the WCA was still in the range of 141°– 154°. Although, the strong acidic (pH=1) demonstrated a negative effect on the fabric surface due to corrosion (Fig. 4b), the obtained superhydrophobic surface in this work displayed remarkable resistance against acidic and alkaline conditions. Additionally, when the fabric was immersed into mild (0.9, 2.5 wt.%) or strong (10 wt.%) salt solutions for 7 days, WCA was in the range 146°–152° (Fig. 4c). The reduction of WCA with prolonging immersion days was due to salt corrosion (Tang et al. 2019). Meanwhile, the fabric maintained its original water repellency with WCA more than 156° even though exposing under high temperature for 2 h (Fig. 4d). All the results demonstrated the high chemical stability of Cot–CuO–STA.
In order to assess the fabric robustness against mechanical force, sandpaper abrasion and mechanical washing tests were carried out. Cot–CuO–STA subjected to 200 g weight was dragged back and forth for 10 cm as a cycle, as shown in Fig. 5c. It was found that from Fig. 5a the WCA was 151° even after 20 abrasion cycles with a 1500 mesh sandpaper. Although the WCA slightly declined after 20 abrasion cycles with 800 mesh sandpaper, the surface still kept outstanding water repellency (WCA = 146°). The surface morphology and rough structure of the fabric maintained after sandpaper abrasion (inset in Fig. 5a). Also, superhydrophobic property of Cot–CuO–STA was maintained with a WCA of 151° after 10 washing cycles (Fig. 5b). Although the WCA slightly decreased to 148° after 20 washing cycles, the surface still remained excellent water repellency. The results showed that the Cot–CuO–STA has remarkable mechanical resistance. Excellent durability, especially under rigorous environments such as acidic, basic, salt solutions, high temperature and mechanical damage, is crucial for material to expand its application fields.
Antifouling and self-cleaning performances
The water repellency of Cot–CuO–STA is also verified through a silver mirror phenomenon due to the air layer between the fabric surface and liquid (Fig. 6a), and a water jet spraying experiment (Fig. 6b). As soon as the waterspout contacts the surface, bounces immediately. Further, the antifouling and self-cleaning performances of the Cot–CuO–STA were investigated by using methylene blue (MB) solution and blush Y powders as pollutants. As shown in Fig. 7a, when MB solution directly poured onto the surface of Cot–CuO–STA, the solution flowed directly to the bottom. While the fabric surface maintained clean and dry. Furthermore, when the water was fallen on the.
polluted surface by blush Y powders, the fabric was not wetted, penetrated and stained by blush Y (Fig. 7b). Meanwhile, the blush Y powders well swilled out by the water falling on the fabric surface. These results strongly indicated the remarkable antifouling and self-cleaning performances of the obtained Cot-CuO-STA.
Oil/water separation
Moreover, the super-repellency of the Cot–CuO–STA was demonstrated by assessing the adsorption of water and oil colored using MB and Sudan III, respectively. As shown in Fig. 8a, when the two kinds of colored liquids dropped onto the Cot, the droplets were quickly soaked and penetrated the cotton fabric due to its amphiphilic. In contrast, the Cot–CuO–STA exhibits superhydrophobicity and superlipophilicty, and effectively repels colored water droplets resulting in the formation of round-liquid balls on the interface (Fig. 8b). This excellent water-repellency demonstrates its promising oil/water separation performance. As shown in Fig. 8c, the separation of oil/water mixture can be performed by using simple and available equipment. Colored oil can permeate through the Cot–CuO–STA rapidly and drop in the conical flask due to its affinity with Cot–CuO–STA surface. At the same time, water is retained above the fabric owing to its superhydrophobicity.
Further, the effect of separation cycles on the oil/water separation efficiency of Cot-CuO-STA was evaluated and shown in Fig. 8d. In this study, various oils including trichloromethane, dichloromethane, n-hexane and soybean oil were selected as heavy oil and light oil, respectively. It is clearly that the separation efficiency of Cot–CuO–STA for different kinds of oils was all as high as 98.7%. Although the separation efficiency showed a slight decline after 10 cycles, the efficiencies for all cycles still kept above 97%. Also, fast spreading of oil and easy saturation are crucial features of the separating membrane to promote the rapid oil/water separation. Thus, the permeate flux of the Cot–CuO–STA for oil/water mixture was investigated by using trichloromethane and dichloromethane as representatives. The Cot–CuO–STA displayed a fascinating permeate flux (more than1800 L m−2 h−1) for both trichloromethane and dichloromethane, shown in Fig. 9. After repeated separation for 10 cycles, the permeate flux of trichloromethane was still 1666 L m−2 h−1, maintaining 92% of its initial permeate flux, while 1745 L m−2 h−1 of dichloromethane, 97% of its initial permeate flux. These results indicated that the obtained Cot–CuO–STA has outstanding oil/water separation performance and recyclability, exhibiting promising applications in oil-contaminated water treatment and marine spilt oil cleanup.
Conclusion
A facile and scalable approach to robust and durable superhydrophobic cotton fabric was proposed by the combination of CuO deposition and stearic acid coating. The in situ CuO deposition endowed the cotton fabric with a hierarchically rough structure, which enables and binds stearic acid on the fabric surface. The obtained fabric (Cot–CuO–STA) exhibited a remarkable superhydrophobicity with WCA of 156.5°, a water repellency and a self-cleaning property. Moreover, the Cot–CuO–STA displayed robust resistance to acid, alkali, salt, high temperature, mechanical abrasion and washing. Importantly, the resultant Cot–CuO–STA can be applied for oil/water separation with separation efficiency all as high as 98.7% for different kinds of oils. Further, fascinating permeate flux (more than1800 L.m−2.h−1) and remarkable recyclability made Cot–CuO–STA a promising application in oil-contaminated water treatment and marine spilt oil cleanup.
References
Cai J, Wang T, Hao W, Ling H, Hang T, Chung YW, Li M (2019) Fabrication of superamphiphobic Cu surfaces using hierarchical surface morphology and fluorocarbon attachment facilitated by plasma activation. Appl Surf Sci 464:140–145
Chen S, Li X, Li Y, Sun J (2015) Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton fabric. ACS Nano 9(4):4070–4076
Chen Y, Meng J, Zhu Z, Zhang F, Wang L, Gu Z, Jiang L, Wang S (2018) Controlled growth of patterned conducting polymer microsuckers on superhydrophobic micropillar-structured templates. Adv Funct Mater 28:1800240
Cheng Y, Zhu T, Li S, Huang J, Mao J, Yang H, Gao S, Chen Z, Lai Y (2019) A novel strategy for fabricating robust superhydrophobic fabrics by environmentally-friendly enzyme etching. Chem Eng J 355:290–298
Dong XL, Gao SW, Huang JY, Li SH, Zhu TX, Cheng Y, Zhao Y, Chen Z, Lai YK (2019) A self-roughened and biodegradable superhydrophobic coating with UV shielding, solar induced self-healing and versatile oil-water separation ability. J Mater Chem A 7:2122–2128
El-Nahhal IM, Elmanama AA, Amara N, Qodih FS, Selmane M, Chehimi MM (2018) The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater Chem Phys 215:221–228
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7(1):70
Farhadi S, Farzaneh M, Kulinich SA (2011) Anti-icing performance of superhydrophobic surfaces. Appl Surf Sci 257(14):6264–6269
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360:739–742
Foorginezhad S, Zerafat MM (2019) Fabrication of superhydrophobic coatings with self-cleaning properties on cotton fabric based on octa vinyl polyhedral oligomeric silsesquioxane/polydimethylsiloxane (OV-POSS/PDMS) nanocomposite. J Colloid Interface Sci 540:78–87
Gao SW, Dong XL, Huang JY, Li SH, Li YW, Chen Z, Lai YK (2018) Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem Eng J 333:621–629
Ge MZ, Cao CY, Liang FH, Liu R, Zhang Y, Zhang W, Zhu TX, Yi B, Tang YX, Lai YK (2020) A ‘‘PDMS-in-water’’ emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horiz 5:65–73
Gelebart AH, Mulder DJ, Varga M, Konya A, Vantomme G, Meijer EW, Selinger RLB, Broer DJ (2017) Making waves in a photoactive polymer film. Nature 546:632
Hou K, Zeng Y, Zhou C, Chen J, Wen X, Xu S, Cheng J, Pi P (2018) Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem Eng J 332:150–159
Jia S, Chen H, Luo S, Qing Y, Deng S, Yan N, Wu Y (2018) One-step approach to prepare superhydrophobic wood with enhanced mechanical and chemical durability: driving of alkali. Appl Surf Sci 455:115–122
Kliche G, Popovic ZV (1990) Far-infrared spectroscopic investigations on CuO. Phys Rev B 42(16):10060
Kong L, Chen X, Yu L, Wu Z, Zhang P (2015) Superhydrophobic cuprous oxide nanostructures on phosphor-copper meshes and their oil–water separation and oil spill cleanup. ACS Appl Mater Inter 7(4):2616–2625
Li Q, Guo Z (2019) Lubricant-infused slippery surfaces: Facile fabrication, unique liquid repellence and antireflective properties. J Colloid Interface Sci 536:507–515
Lin D, Zeng X, Li H, Lai X, Wu T (2019) One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J Colloid Interface Sci 533:198–206
Liu G, Wang W, Yu D (2019a) Robust and self-healing superhydrophobic cotton fabric via UV induced click chemistry for oil/water separation. Cellulose 26(5):3529–3541
Liu R, Dai J, Ma L, Chen J, Shi X, Du Y, Li Z, Deng H (2019b) Low-temperature plasma treatment-assisted layer-by-layer self-assembly for the modification of nanofibrous mats. J Colloid Interface Sci 540:535–543
Liu Y, Fu K, Liu J, Tian Y, Zhang H, Wang R, Zhang B, Zhang H, Zhou F, Zhang Q (2019c) Design and preparation of a multi-fluorination organic superhydrophobic coating with high mechanical robustness and icing delay ability. Appl Surf Sci 497:143663
Liu Y, Moevius L, Xu X, Qian T, Yeomans JM, Wang Z (2014) Pancake bouncing on superhydrophobic surfaces. Nat Phys 10:515
Liu Z, Lin Z, Zhou L, Yang Z, Chen D, Zhang C (2018) High-performance planar perovskite solar cells using low temperature, solution–combustion-based ickel oxide hole transporting layer with efficiency exceeding 20%. Adv Energy Mater 8:1703432
Lu X, Wang C, Wei Y (2009) One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small 5(21):2349–2370
Lvov Y, Wang W, Zhang L, Fakhrullin R (2016) Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv Mater 28:1227–1250
Nanda D, Sahoo A, Kumar A, Bhushan B (2019) Facile approach to develop durable and reusable superhydrophobic/superoleophilic coatings for steel mesh surfaces. J Colloid Interface Sci 535:50–57
Peng C, Chen Z, Tiwari MK (2018) All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat Mater 17:355
Perelshtein I, Applerot G, Perkas N, Wehrschuetz-Sigl E, Hasmann A, Guebitz G, Gedanken A (2009) CuO–cotton nanocomposite: formation, morphology, and antibacterial activity. Surf Coat Tech 204(1–2):54–57
Pokroy B, Kang S, Mahadevan L, Aizenberg J (2009) Self-organization of a mesoscale bristle into ordered, hierarchical helical assemblies. Science 323:237–240
Qing Y, Long C, An K, Hu C, Liu C (2019) Sandpaper as template for a robust superhydrophobic surface with self-cleaning and anti-snow/icing performances. J Colloid Interface Sci 548:224–232
Ren T, Yang M, Wang K, Zhang Y, He J (2018) CuO nanoparticles-containing highly transparent and superhydrophobic coatings with extremely low bacterial adhesion and excellent bactericidal property. ACS Appl Mater Inter 10(30):25717–25725
Rezaie AB, Montazer M, Rad MM (2018) Environmentally friendly low cost approach for nano copper oxide functionalization of cotton designed for antibacterial and photocatalytic applications. J Clean Prod 204:425–436
Riaz S, Ashraf M, Hussain T, Hussain MT, Younus A (2019) Fabrication of robust multifaceted textiles by application of functionalized TiO2 nanoparticles. Colloid Surface A 581:123799
Tang S, Zhang Y, Sana H, Hu J (2019) Hydrophobic surface contained Ca and/or Ce myristate fabricated on AZ31 by one-step electrodeposition for corrosion protection in NaCl. Appl Surf Sci 496:143627
Wang B, Liang W, Guo Z, Liu W (2015) Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem Soc Rev 44:336–361
Xue C, Fan Q, Guo X, An Q, Jia S (2019) Fabrication of superhydrophobic cotton fabrics by grafting of POSS-based polymers on fibers. Appl Surf Sci 465:241–248
Yan B, Zhou Q, Zhu X, Guo J, Mia MS, Yan X, Chen G, Xing T (2019) A superhydrophobic bionic coating on silk fabric with flame retardancy and UV shielding ability. Appl Surf Sci 483:929–939
Yang M, Liu W, Jiang C, Xie Y, Shi H, Zhang F, Wang Z (2019) Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloid Surface A 570:172–181
Yang M, Liu W, Liang L, Jiang C, Liu C, Xie Y, Shi H, Zhang F, Pi K (2020) A mild strategy to construct superhydrophobic cotton with dual self-cleaning and oil–water separation abilities based on TiO2 and POSS via thiol-ene click reaction. Cellulose pp 1–11
Yang Y, Li X, Zheng X, Chen Z, Zhou Q, Chen Y (2018) 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv Mater 309:1704912
Zhang J, Seeger S (2011) Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv Funct Mater 21:4699–4704
Zhang S, Liu H, Yang S, Yang S, Shi X, Zhang D, Shan C, Mi L, Liu C, Shen C, Guo Z (2019a) Ultrasensitive and highly compressible piezoresistive sensor based on polyurethane sponge coated with a cracked cellulose nanofibril/silver nanowire layer. ACS Appl Mater Inter 11:10922–10932
Zhang X, Liu Z, Li Y, Wang C, Zhu Y, Wang H, Wang J (2019b) Robust superhydrophobic epoxy composite coating prepared by dual interfacial enhancement. Chem Eng J 371:276–285
Zhao Y, Xing C, Zhang Z, Yu L (2017) Superhydrophobic polyaniline/polystyrene micro/nanostructures as anticorrosion coatings. React Funct Polym 119:95–104
Zhou C, Chen Z, Yang H, Hou K, Zeng X, Zheng Y, Cheng J (2017a) Nature-inspired strategy toward superhydrophobic fabrics for versatile oil/water separation. ACS Appl Mater Inter 9(10):9184–9194
Zhou H, Wang H, Niu H, Zhao Y, Xu Z, Lin T (2017b) A waterborne coating system for preparing robust, self-healing, superamphiphobic surfaces. Adv Funct Mater 27(14):1604261
Zhu Z, Liu Y, Hou H, Shi W, Qu F, Cui F, Wang W (2018) Dual-bioinspired design for constructing membranes with superhydrophobicity for direct contact membrane distillation. Environ Sci Technol 52:3027–3036
Acknowledgments
The work was financially supported by the Public Technology Research Plan of Zhejiang Province (LGF18E030003) and the Fundamental Research Funds of Zhejiang Sci-Tech University (2019Q008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, R., Yu, Y., Adkhamjon, G. et al. Bio-inspired cotton fabric with superhydrophobicity for high-efficiency self-cleaning and oil/water separation. Cellulose 27, 7283–7296 (2020). https://doi.org/10.1007/s10570-020-03281-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03281-9