Abstract
There is a need for durable flame retardant treatments for cotton fabric in order to reduce the risk associated with fires. Many current industrial treatments make use of toxic halogenated organic flame retardants or utilize formaldehyde-evolving chemistry. A facile two-step process is described to coat cotton fabric based on a spontaneous crosslinking reaction between branched polyethyleneimine (PEI) and hexachlorocyclotriphosphazene (HCCP). A coating produced from solutions of 10 wt% PEI and 5 wt% HCCP endows the cotton fabric with a high limiting oxygen index (33.8%), self-extinguishing behavior in open flame testing, and an 85% reduction in peak heat release rate. This treated fabric also maintains self-extinguishing behavior after a simulated washing test. This unique combination of properties is the result of a strongly networked coating that intumesces during burning. The simplicity of this treatment and its formaldehyde-free chemistry make it a good option for replacing organo-halogen and formaldehyde-evolving treatments.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is an important natural textile fiber that is primarily cellulose (88.0–96.5%). It is used in the production of clothing and furniture upholstery, both residentially and industrially, due to its excellent hydrophilicity, air permeability, and comfort (Gordon 2006). Despite their various benefits, these cotton textile products are highly flammable, presenting a fire risk to both human life and property (Alongi and Malucelli 2015). Improving the flame retardancy of cotton is crucial. Various methodologies have been explored to modify the combustion behavior of cotton textiles. Despite their high efficiency, traditional halogenated flame retardants (bromine and chlorine-based) are gradually being removed from the marketplace due to toxicity concerns (Morgan 2019).
Of the halogen-free flame retardants available, various phosphorus-, nitrogen-, silicon-, and boron-based compounds are most widely used on cotton fabric. Proban, a well-known durable phosphorus-based flame retardant from the 1950s, was developed based on tetrakis(hydroxymethyl) phosphonium-urea condensate and ammonia cure processing (Horrocks 1986; Weil and Levchik 2008). Unfortunately, the Proban treatment results in formaldehyde emission, which is harmful to humans. Many researchers have been developing formaldehyde-free and environmentally-benign flame retardants for cotton textiles using various finishing chemistries: phosphonate (Liu et al. 2012), triazine (Li et al. 2015), phosphoramidate (Nguyen et al. 2013; Zhao et al. 2017), ammonium salts of inorganic/organic phosphonic acid (Chen et al. 2015; Wang et al. 2018; Xu et al. 2019), and boron-nitrogen compounds (Chan et al. 2018; Tawiah et al. 2019). Treatments have been deposited using a pad-dry-cure technique or sol–gel methodology based on phosphorus- and silicon-containing compounds (Castellano et al. 2019; Jiang et al. 2019; Nie et al. 2019). Environmentally benign layer-by-layer assembly of flame retardant coatings on cotton fabric have also been studied (Laufer et al. 2012; Li et al. 2019; Liu et al. 2018a, b; Pan et al. 2014).
With a structure comprised of alternating phosphorus and nitrogen atoms, cyclophosphazenes can impart flame retardant behavior to cellulosic materials (Fontenot et al. 2015). Some cyclophosphazene derivatives have been applied on cotton by UV-grafting (Edwards et al. 2015; Mayer-Gall et al. 2015) and sol–gel technology (Dutkiewicz et al. 2018; Wang et al. 2016). Despite their promising influence on cotton, these halogen-free and formaldehyde-free flame retardant systems often require complicated synthetic procedures, complex processing, or multiple cycles of immersion. Zope et al. reported an effective water-based flame retardant spray coating for cotton based on the spontaneous crosslinking reaction between para-phenylenediamine and tetrakis(hydroxymethyl)phosphonium chloride (Zope et al. 2017), but this was not a formaldehyde-free system for finishing cotton textiles. More recently, some cyclomatrix-type polyphosphazenes based on the reaction of hexachlorocyclotriphosphazene (HCCP) and dihydric phenol/amine have been used as additive flame retardants for polymers (Qiu et al. 2017; Wen et al. 2016; Yang et al. 2018).
In the present work, a crosslinked coating based on branched polyethyleneimine (PEI) and HCCP was deposited as a formaldehyde-free flame retardant coating for cotton fabric. Exposing cotton to an 8 wt% solution of PEI, followed by a 4 wt% solution of HCCP, generates a poly(PEI-co-HCCP) coating that adds 18 wt% to the weight of the cotton. This crosslinked coating increases the limiting oxygen index (LOI) value of cotton more than 50% and imparts self-extinguishing behavior in vertical flame testing. Additionally, the coating appears to limit the evolution of volatile small molecules. The combination of simplicity, effective flame suppression and formaldehyde-free process make this unique coating an interesting alternative to currently used cotton treatments.
Materials and methods
Chemicals
Hexachlorocyclotriphosphazene (HCCP, 98%) and branched polyethyleneimine (PEI, Mw ~ 25,000 g mol−1) were purchased from Sigma-Aldrich (St. Louis, MO). Bleached and desized cotton print cloth, with an approximate weight of 100 g m−2, was purchased from Test fabrics, Inc (West Pittston, PA). Chloroform (CHCl3, GR ACS, Ethanol stabilized) was provided by Millipore Sigma (Billerica, MA). All solutions were prepared with chloroform. All reagents mentioned above were utilized as received without further purification.
Preparation and deposition of poly(PEI-co-HCCP) coating
Mixing of PEI and HCCP results in a spontaneous precipitation polymerization to form poly(PEI-co-HCCP) by the crosslinking reaction between NH2 and P-Cl in polar organic solvent (CHCl3, CH3CN, etc.) (Köhler et al. 2014). Utilizing this chemistry, poly(PEI-co-HCCP) coatings were deposited on cotton fabric by a convenient two-step procedure. Prior to coating, cotton fabric was rinsed with NaOH aqueous solution (adjusting the pH value of distilled water to 10.0 with 1M NaOH solution) and then air dried in an oven at 70 °C for 4 h. Equal weight solutions of PEI (x wt%) and HCCP (y wt%) were prepared separately. Cotton samples were dipped into a PEI solution for 10 min, followed by wringing out excess solution and hanging to dry in a 70 °C oven for 10 min. Afterwards, the fabric was immersed in HCCP solution for 10 min, followed by wringing and drying (70 °C) for 10 min. Finally, the coated fabric was washed with distilled water for 1 min and dried at 70 °C overnight. The as-coated cotton fabric is denoted as Cotton-PxHy. Cotton fabric coated with only 10 wt% PEI (Cotton-P10) was prepared as a control sample together with untreated cotton. The coating procedure is illustrated in Fig. 1. A similar two-step procedure was used to deposit the coatings of 10 wt% PEI and 10 wt% PEI-5 wt% HCCP on silicon wafers (wafer-P10 and wafer-P10H5) to characterize the crosslinked chemical structure. The poly(PEI-co-HCCP) coating (P6H3) was also deposited on a 75 mm × 75 mm × 1 mm glass slide (Vistavision, VWR International, Radnor, PA) following the same procedure used for fabric. A razor blade was then used to scrape the poly(PEI-co-HCCP) coating into a pan for thermogravimetric analysis.
Characterization and measurements
Infrared spectra of polished silicon wafers (University Wafer, Boston, MA) with different coatings were recorded using an Alpha Platinum ATR-FTIR spectrometer (Bruker, Billerica, MA) using 32 scans in the mid-infrared region from 4000 to 500 cm−1. Thermal stability of each uncoated and coated cotton sample (approximately 10 mg) was evaluated using a Q50 TGA apparatus (TA Instruments, USA). Fabric samples were heated from ambient temperature up to 700 °C, with a sample purge flow of 60 mL s−1 nitrogen or air (with a balance purge flow of 40 mL s−1 nitrogen), under a controlled heating ramp of 10 °C min−1. To avoid the influence of adsorbed water, samples were dried in the TGA by heating from ambient temperature to 100 °C. Each sample was then cooled down to ambient temperature prior to running the official analysis. Flammability and combustibility were evaluated by limiting oxygen index (LOI), vertical flame and microscale combustion calorimetry (MCC) tests. LOI values of the fabric (135 mm × 52 mm) were measured based on ASTM D-2863-09, using an oxygen index tester (Motis COI, Motis technology CO., LTD, China). Vertical flame testing was conducted on a model VC-2 vertical flame cabinet (Govmark, Farmingdale, NY). According to ASTM D6413-08, 300 mm × 76 mm samples were vertically hung in a metal clamp and exposed to a flame with 38 mm height from a Bunsen burner for 12 s to obtain the afterflame time, afterglow time and char length. All fabric samples were run in triplicate for MCC according to ASTM D7309. During testing, each sample was heated at a 1 °C s−1 rate from 100 to 700 °C in a nitrogen atmosphere together with a combustion of degradation products in a combustor at 900 °C in the presence of oxygen. Heat release and other combustion parameters were calculated based on the oxygen consumption. Selected samples were washed with AATCC 1993 standard nonphosphate detergent and continuously stirred in a suitable beaker at 49 °C for 45 min according to a slightly modified version of AATCC TM 61-2013 Test 2A (i.e. no steel balls were included in the wash). After washing a given number of times, samples were again tested in the vertical flame to evaluate wash durability.
Surface morphologies of the fabric and char were observed with scanning electron microscopy (SEM, HITACHI, SU8010, Japan). In an effort to prevent charging, the samples were sputter-coated using an Au–Pd target (MSP1S, SHINKKU VD) before testing. Thermogravimetric–Fourier transform infrared spectroscopy (TG–FTIR) testing was performed on a Q50 TGA device (TA Instruments, USA), which was connected on an IS50 FTIR spectrometer (Thermo Fisher Scientific, USA). The insulating pipe and gas cell were both maintained at 250 °C to avoid secondary reactions and the condensation of volatile gases. Each sample (approximately 15 mg) was tested from 40 to 700 °C, with a 20 °C min−1 rate under nitrogen atmosphere, together with series recording of infrared spectra between 650 and 4000 cm−1.
Results and discussion
Infrared spectroscopy of poly(PEI-co-HCCP) coatings
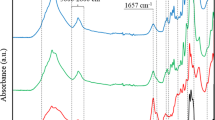
Poly(PEI-co-HCCP) crosslinked coatings, deposited on silicon wafers, were analyzed with FTIR spectroscopy. An untreated wafer, wafer-P10 and HCCP were tested as control samples. The broad absorption peaks around 3247 cm−1 in the wafer-P10 and wafer-P10H5 spectra belong to the N–H stretching vibration for NH2 and NH groups, as shown in Fig. 2. In both wafer-P10 and wafer-P10H5 spectra, the aliphatic C–H stretching vibrations (2940 cm−1, 2820 cm−1), the N–H bending vibrations (1575 cm−1), and C–H bending vibrations (1463 cm−1) from CH2, CH, and amino groups in branched PEI are observed (Kasprzak et al. 2015). The characteristic absorptions of P–N and P=N groups from the phosphazene skeleton are centered at 871 cm−1 and 1171 cm−1 in the spectrum of the PEI-HCCP coating. A new absorption peak at 1108 cm−1 can be assigned to the exocyclic P–N bonds formed by the crosslinking reaction between NH2 and P–Cl (Köhler et al. 2014), which is close to the absorption peak of tertiary amine in the branched structure of PEI (Wafer-P10).
Thermal stability
The thermal stability of cotton fabric was evaluated by thermogravimetric analysis under nitrogen, as shown in Fig. 3. The initial decomposition temperature (T5%), maximum weight loss rate (Rmax), maximum-rate decomposition temperature (Tmax), and residue at 700 °C are summarized in Table 1. Uncoated cotton displays a typical one-step decomposition curve, and the T5% and Tmax values are found to be 327 °C and 368 °C, respectively. The main decomposition range, from 330 to 400 °C, results from the depolymerization of cellulose through trans-glycosylation reactions (Shafizadeh et al. 1982). As shown in Fig. S1, poly(PEI-co-HCCP) shows lower T5% (304 °C) and Tmax (333 °C) than cotton, while the char yield is as high as 66.0 wt%. Thus, Cotton-P6H3 shows a reduction of about 50 °C in both T5% and Tmax, which can be ascribed to the lower thermal stability of poly(PEI-co-HCCP). As expected, the Rmax for cotton-P6H3 was decreased from 26.9 to 18.8 wt% min−1, and the char yield increased significantly from 9.4 to 34.9 wt%. The decline in the Rmax value of cotton-P6H3 indicates that stable char formed during the initial decomposition limits further degradation of the cotton, resulting in a higher char yield after heating. Furthermore, with increasing PEI and HCCP concentration, the decomposition of cotton is further reduced (Rmax values of Cotton-P8H4 and Cotton-P10H5 are reduced to 16.8 and 9.5 wt% min−1, respectively) and there is increased char residue at 700 °C. Fig. S2 and Table S1 provide the TGA (and DTG) curves and relevant data for fabric samples obtained from testing under an oxidizing atmosphere. Similar to the behavior under a nitrogen atmosphere, the poly(PEI-co-HCCP) coating changes the thermal decomposition process and improves the formation of char.
Flame retardancy
The flame retardancy of cotton was characterized by the limiting oxygen index, vertical flame testing and microscale combustion calorimetry. The images of coated and uncoated cotton after vertical flame testing are shown in Fig. 4, while all of the flame retardancy results are summarized in Table 2. The LOI value of uncoated cotton is only 18.7%. During the vertical flame test, cotton is completely consumed, with an average afterflame time of 6 s. Because of the crosslinking reaction between PEI and HCCP mentioned above, the weight gain of the coated cotton increases from 15 to 23 wt% with increasing concentration of PEI and HCCP in the coating solution. As expected, LOI value of the coated fabric increases to 25.3%, 28.5%, and 33.8% for Cotton-P6H3, Cotton-P8H4, and Cotton-P10H5, respectively.
Similar with LOI, the results of vertical flame tests show a strong relationship with the weight gain of coated cotton. Cotton-P6H3 cannot self-extinguish and the flame spreads to the edge of the fabric, leaving a char of 30 cm and a residue of 40.0 ± 0.3 wt%. The increased PEI and HCCP concentration resulted in a steady increase of the weight gain and flame retardancy of cotton fabric. Figure 4 reveals that Cotton-P8H4 and Cotton-P10H5 do exhibit self-extinguishing behavior in the vertical flame test. Both coated cotton samples completely stopped flame propagation in less than 6 s. These coated fabric pieces easily pass ASTM D6413, with high char residue (95.0 ± 0.3 wt%) and low char length (10.0 ± 0.4 cm). As a result of Cotton-P10H5 exhibiting the best flame retardancy based on the LOI and vertical flame testing, Cotton-P10 was evaluated as a control sample. Figure 4 shows that coating with 10 wt% PEI cannot endow cotton with flame retardancy in the absence of HCCP. The fabric is almost completely destroyed, leaving only a brittle char layer. After a wash-fastness test (at 49 °C for 45 min), Cotton-P10H5 was again tested in the vertical flame. This washed fabric still self-extinguishes, although a second washing appears to damage the coating, so there is more work to be done.
SEM images of uncoated cotton and Cotton-P10H5 (best flame retardant sample) were taken to observe the surface morphologies before and after burning (Fig. 5). Before burning, in comparison to uncoated cotton with its smooth surface (Fig. 5a1, a2), the individual fibers of Cotton-P10H5 are wrapped by the thick crosslinked coating (Fig. 5b1, b2). After burning, the fabric construction and single fibers of uncoated cotton are destroyed completely, leaving charred fibrous remains (Fig. 5a3, a4). With the introduction of the poly(PEI-co-HCCP) coating, it is clear that the structure and shape of the fabric are remarkably well-maintained (Fig. 5b3, b4). The occurrence of the bubbles on the surface of the afterburn residues are due to the entrapment of the decomposition gas from the coating and cotton during combustion. The “bubble-containing surface morphology” was also observed in other work and believed to be evidence of an intumescent action (Haile et al. 2015). This intumescent protective char layer keeps the cotton matrix from being damaged by fire and results in self-extinguishing behavior during the vertical flame test.
Microscale combustion calorimetry is another useful technique for evaluating the flame retardancy of polymers (Lyon and Walters 2002). Figure 6 overlays the heat release rate (HRR) curves for cotton samples, while parameters such as temperature at peak HRR (Tp) and total heat release (THR) are summarized in Table 2. The peak HRR values of Cotton-P6H3, Cotton-P8H4, and Cotton-P10H5 decline from 375.2 (for uncoated cotton) to 71.4, 60.3, and 56.8 W g−1, respectively, indicating an excellent suppression effect of the poly(PEI-co-HCCP) coating. Moreover, the Tp values of coated cotton gradually declines with increasing concentration of PEI and HCCP. This can be ascribed to the earlier decomposition for higher solids in the coating solutions, which promote stable char earlier and thus inhibit heat release. The poly(PEI-co-HCCP) complex significantly reduces THR values (over 51.4%), as shown in Table 2, but these values change very little with increasing coating weight. As shown in Fig. 6, the HRR trends of coated fabric are very similar. It can be concluded that the poly(PEI-co-HCCP) coating significantly suppresses the release of flammable volatiles from cotton during pyrolysis, which demonstrates an effective condensed phase mechanism. The P6H3 coating is able to reduce the heat release value of cotton substantially, while increasing coating weight only results in minimal improvement. Thus, it appears that the inhibition effects for the heat release of cotton is not strongly influenced by the concentration of PEI and HCCP.
Evolved volatiles analysis
Figure 7a, b show the 3D diagrams of gaseous volatiles of cotton and Cotton-P10H5, respectively. Peaks belonging to ether groups (C–O–C; 1083 cm−1), carbonyl groups (C=O; 1747 cm−1), carbon monoxide (CO; 2184 cm−1), carbon dioxide (CO2; 2364 cm−1), alkane groups (–CH3, –CH2–; 2800–2980 cm−1), and O–H groups (water vapor, 3500–3700 cm−1) are detected for pure cotton (Fig. 7a). Carbon dioxide and water vapor are derived from the dehydration process, while carbonyl, ether, and alkanes are released by the depolymerization of cellulose during pyrolysis (Brancatelli et al. 2011). After coating with PEI-HCCP, peaks belonging to N–H (~ 3200–3300 cm−1) can be clearly observed (Fig. 7b). The intensity of the signals for most of the volatiles is lowered for cotton after coating. To further analyze the changes in thermal decomposition products, the intensity of total and selected typical products (carbonyl, carbon monoxide, and ether) against time/temperature was evaluated, as shown in Fig. 7c. After coating with poly(PEI-co-HCCP), the main decomposition time/temperature is lowered from 19.2 min/424 °C to 16.5 min/370 °C. This temperature difference can be attributed to the early decomposition of Cotton-P10H5, which agrees with TGA observations (Fig. 3). The peak intensities of three specific decomposed products for Cotton-P10H5 are significantly reduced after coating, according to the Beer–Lambert law (Chan et al. 2018; Liu et al. 2018b).
Conclusion
A crosslinked coating of poly(PEI-co-HCCP) was applied to cotton fabric using a simple two-step procedure under ambient conditions. TGA revealed that the coating changed the thermal decomposition process and increased the char yield of cotton. Using PEI and HCCP solution concentrations of 10 wt% and 5 wt%, respectively, the cotton exhibited an LOI value of 33.8%, self-extinguishing behavior in vertical flame testing, and an 85% reduction in peak HRR. Furthermore, the poly(PEI-co-HCCP) coating dramatically reduced the concentration of evolved toxic gases. This coating also maintained effectiveness after a single laundering cycle, providing a useful platform by which durable flame retardant finishing could be applied to improve textile fire safety.
Abbreviations
- PEI:
-
Branched polyethyleneimine
- HCCP:
-
Hexachlorocyclotriphosphazene
- Poly(PEI-co-HCCP):
-
Crosslinked polymers produced by reaction between PEI and HCCP
- LOI:
-
Limiting oxygen index
- MCC:
-
Microscale combustion calorimeter
- TGA:
-
Thermogravimetric analysis
- SEM:
-
Scanning electron microscopy
- TG–FTIR:
-
Thermogravimetry–Fourier transform infrared spectroscopy
- HRR:
-
Heat release rate
- Tp :
-
Temperature at peak HRR
- THR:
-
Total heat release
References
Alongi J, Malucelli G (2015) Cotton flame retardancy: state of the art and future perspectives. RSC Adv 5:24239–24263. https://doi.org/10.1039/C5RA01176K
Brancatelli G, Colleoni C, Massafra MR, Rosace G (2011) Effect of hybrid phosphorus-doped silica thin films produced by sol–gel method on the thermal behavior of cotton fabrics. Polym Degrad Stabil 96:483–490. https://doi.org/10.1016/j.polymdegradstab.2011.01.013
Castellano A, Colleoni C, Iacono G, Mezzi A, Plutino MR, Malucelli G, Rosace G (2019) Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym Degrad Stabil 162:148–159. https://doi.org/10.1016/j.polymdegradstab.2019.02.006
Chan SY, Si L, Lee KI, Ng PF, Chen L, Yu B, Hu Y, Yuen RKK, Xin JH, Fei B (2018) A novel boron–nitrogen intumescent flame retardant coating on cotton with improved washing durability. Cellulose 25:843–857. https://doi.org/10.1007/s10570-017-1577-2
Chen S, Li X, Li Y, Sun J (2015) Intumescent flame-retardant and self-healing superhydrophobic coatings on cotton Fabric. ACS Nano 9:4070–4076. https://doi.org/10.1021/acsnano.5b00121
Dutkiewicz M, Przybylak M, Januszewski R, Maciejewski H (2018) Synthesis and flame retardant efficacy of hexakis (3-(triethoxysilyl) propyloxy) cyclotriphosphazene/silica coatings for cotton fabrics. Polym Degrad Stabil 148:10–18. https://doi.org/10.1016/j.polymdegradstab.2017.11.018
Edwards B, Hauser P, El-Shafei A (2015) Nonflammable cellulosic substrates by application of novel radiation-curable flame retardant monomers derived from cyclotriphosphazene. Cellulose 22:275–287. https://doi.org/10.1007/s10570-014-0497-7
Fontenot KR, Nguyen MM, Al-Abdul-Wahid MS, Easson MW, Chang S, Lorigan GA, Condon BD (2015) The thermal degradation pathway studies of a phosphazene derivative on cotton fabric. Polym Degrad Stabil 120:32–41. https://doi.org/10.1016/j.polymdegradstab.2015.04.032
Gordon S (2006) Cotton fibre quality. In: Gordon S, Hsieh Y-L (eds) Cotton: science and technology. Woodhead Publishing, Cambridge, pp 68–95
Haile M, Fincher C, Fomete S, Grunlan JC (2015) Water-soluble polyelectrolyte complexes that extinguish fire on cotton fabric when deposited as pH-cured nanocoating. Polym Degrad Stabil 114:60–64. https://doi.org/10.1016/j.polymdegradstab.2015.01.022
Horrocks A (1986) Flame-retardant finishing of textiles. Rev Prog Color Relat Top 16:62–101. https://doi.org/10.1111/j.1478-4408.1986.tb03745.x
Jiang Z, Xu D, Ma X, Liu J, Zhu P (2019) Facile synthesis of novel reactive phosphoramidate siloxane and application to flame retardant cellulose fabrics. Cellulose 26:5783–5796. https://doi.org/10.1007/s10570-019-02465-2
Kasprzak A, Popławska M, Bystrzejewski M, Łabędź O, Grudziński IP (2015) Conjugation of polyethylenimine and its derivatives to carbon-encapsulated iron nanoparticles. RSC Adv 5:85556–85567. https://doi.org/10.1039/C5RA17912B
Köhler J, Kühl S, Keul H, Möller M, Pich A (2014) Synthesis and characterization of polyamine-based cyclophosphazene hybrid microspheres. J Polym Sci Part A Polym Chem 52:527–536. https://doi.org/10.1002/pola.27028
Laufer G, Kirkland C, Morgan AB, Grunlan JC (2012) Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromol 13:2843–2848. https://doi.org/10.1021/bm300873b
Li X, Chen H, Wang W, Liu Y, Zhao P (2015) Synthesis of a formaldehyde-free phosphorus–nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym Degrad Stabil 120:193–202. https://doi.org/10.1016/j.polymdegradstab.2015.07.003
Li S, Lin X, Liu Y, Li R, Ren X, Huang TS (2019) Phosphorus–nitrogen–silicon-based assembly multilayer coating for the preparation of flame retardant and antimicrobial cotton fabric. Cellulose 26:4213–4223. https://doi.org/10.1007/s10570-019-02373-5
Liu W, Chen L, Wang Y-Z (2012) A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym Degrad Stabil 97:2487–2491. https://doi.org/10.1016/j.polymdegradstab.2012.07.016
Liu L, Huang Z, Pan Y, Wang X, Song L, Hu Y (2018a) Finishing of cotton fabrics by multi-layered coatings to improve their flame retardancy and water repellency. Cellulose 25:4791–4803. https://doi.org/10.1007/s10570-018-1866-4
Liu Y, Wang Q-Q, Jiang Z-M, Zhang C-J, Li Z-F, Chen H-Q, Zhu P (2018b) Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J Anal Appl Pyrol 135:289–298. https://doi.org/10.1016/j.jaap.2018.08.024
Lyon RE, Walters R (2002) A microscale combustion calorimeter. Federal Aviation Administration, Washington, DC
Mayer-Gall T, Knittel D, Gutmann JS, Opwis K (2015) Permanent flame retardant finishing of textiles by allyl-functionalized polyphosphazenes. ACS Appl Mater Interfaces 7:9349–9363. https://doi.org/10.1021/acsami.5b02141
Morgan AB (2019) The future of flame retardant polymers-unmet needs and likely new approaches. Polym Rev 59:25–54. https://doi.org/10.1080/15583724.2018.1454948
Nguyen T-M, Chang S, Condon B, Slopek R, Graves E, Yoshioka-Tarver M (2013) Structural effect of phosphoramidate derivatives on the thermal and flame retardant behaviors of treated cotton cellulose. Ind Eng Chem Res 52:4715–4724. https://doi.org/10.1021/ie400180f
Nie S, Jin D, Yang J-N, Dai G, Luo Y (2019) Fabrication of environmentally-benign flame retardant cotton fabrics with hydrophobicity by a facile chemical modification. Cellulose 26:5147–5158. https://doi.org/10.1007/s10570-019-02431-y
Pan H, Song L, Ma L, Pan Y, Liew KM, Hu Y (2014) Layer-by-layer assembled thin films based on fully biobased polysaccharides: chitosan and phosphorylated cellulose for flame-retardant cotton fabric. Cellulose 21:2995–3006. https://doi.org/10.1007/s10570-014-0276-5
Qiu S, Xin W, Yu B, Feng X, Mu X, Yuen RKK, Yuan H (2017) Flame-retardant-wrapped polyphosphazene nanotubes: a novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J Hazard Mater 325:327–339. https://doi.org/10.1016/j.jhazmat.2016.11.057
Shafizadeh F, Bradbury AG, DeGroot WF, Aanerud TW (1982) Role of inorganic additives in the smoldering combustion of cotton cellulose. Ind Eng Chem Prod Res Dev 21:97–101. https://doi.org/10.1021/i300005a021
Tawiah B, Yu B, Yang W, Yuen RKK, Fei B (2019) Facile flame retardant finishing of cotton fabric with hydrated sodium metaborate. Cellulose 26:4629–4640. https://doi.org/10.1007/s10570-019-02371-7
Wang S, Sui X, Li Y, Li J, Xu H, Zhong Y, Zhang L, Mao Z (2016) Durable flame retardant finishing of cotton fabrics with organosilicon functionalized cyclotriphosphazene. Polym Degrad Stabil 128:22–28. https://doi.org/10.1016/j.polymdegradstab.2016.02.009
Wang D, Zhong L, Zhang C, Zhang F, Zhang G (2018) A novel reactive phosphorous flame retardant for cotton fabrics with durable flame retardancy and high whiteness due to self-buffering. Cellulose 25:5479–5497. https://doi.org/10.1007/s10570-018-1964-3
Weil ED, Levchik SV (2008) Flame retardants in commercial use or development for textiles. J Fire Sci 26:243–281. https://doi.org/10.1177/0734904108089485
Wen P, Tai Q, Hu Y, Yuen RKK (2016) Novel cyclotriphosphazene-based intumescent flame retardant (IFR) against the combustible polypropylene. Ind Eng Chem Res 55:298018–298024. https://doi.org/10.1021/acs.iecr.6b01527
Xu F, Zhong L, Xu Y, Zhang C, Zhang F, Zhang G (2019) Highly efficient flame-retardant and soft cotton fabric prepared by a novel reactive flame retardant. Cellulose 26:4225–4240. https://doi.org/10.1007/s10570-019-02374-4
Yang G, Wu W-H, Wang Y-H, Jiao Y-H, Lu L-Y, Qu H-Q, Qin X-Y (2018) Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J Hazard Mater 366:78–87. https://doi.org/10.1016/j.jhazmat.2018.11.093
Zhao B, Liu Y-T, Zhang C-Y, Liu D-Y, Li F, Liu Y-Q (2017) A novel phosphoramidate and its application on cotton fabrics: synthesis, flammability and thermal degradation. J Anal Appl Pyrol 125:109–116. https://doi.org/10.1016/j.jaap.2017.04.011
Zope IS, Foo S, Seah DGJ, Akunuri AT, Dasari A (2017) Development and evaluation of a water-based flame retardant spray coating for cotton fabrics. ACS Appl Mater Interfaces 9:40782–40791. https://doi.org/10.1021/acsami.7b09863
Acknowledgments
The authors want to acknowledge the National Natural Science Foundation of China (Grant 21975226) and China Scholarship Council (CSC: 201808140038). The authors wish to express thanks to Miss Kai-Li Song for her assistance with limiting oxygen index (LOI) tests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, B., Kolibaba, T.J., Lazar, S. et al. Facile two-step phosphazine-based network coating for flame retardant cotton. Cellulose 27, 4123–4132 (2020). https://doi.org/10.1007/s10570-020-03047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03047-3