Abstract
Sophorolipids (SLs) are surface active glycolipids produced by nonpathogenic yeasts, and a combination of SLs with 1,2,3,4-butanetetracarboxyic acid (BTCA) was used as a new durable antimicrobial treatment method for cotton fabrics. The factors influencing the antibacterial property of the fabrics in the finishing process, such as the amount of SLs, treatment temperature, curing time, amount of BTCA, and sodium hypophosphite (SHP) were investigated. An optimized procedure for treating fabrics was two dips and two nips with a wet pickup of 90% in an aqueous solution containing 20 g/L of SHP, 40 g/L of SLs and 90 g/L of BTCA, then drying at 100°C for 2 min and curing at 120°C for 2 min. As a result, 99% of the S. aureus in a concentration of 3.0×104 CFU/mL were killed by the treated fabrics in 1 h contact according to ASTM Standards E2149-10, and the functions are durable against washing. The mechanism of BTCA crosslinking lactonic sophorolipid onto cellulose was investigated by using computational chemistry and experimental methods. As a non-irritant surfactant, sophorolipids proved to be a potential natural antimicrobial agent for textiles or medical products.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, pathogenic microbes can survive on textile materials for days and months, which has become a source of transmission of diseases around the world due to wide applications of textiles in medical and institutional settings (Bockmühl et al. 2019; Metcalf and Lessler 2017; Olival et al. 2017; Angen et al. 2017). Therefore, antibacterial property, as a necessary and imperative function, should be incorporated onto textiles to reduce the transmission of disease effectively. Although various synthetic and natural biocides, such as triclosan, peptides, chitosan, silver nanoparticles, halamine, and quaternary ammonium salts, have been explored for producing antimicrobial textiles, potential toxicity, sensitization, and environmental safety concerns have put them under severe regulatory control (Ma et al. 2019; Si et al. 2018; Cheng et al. 2014; Kang et al. 2016; Smiechowicz et al. 2018). Furthermore, with the concern on environmental safety, natural antibacterial agents have been favored. Sophorolipids (SLs), a group of promising natural surface active glycolipids, also called biosurfactants, can be produced by fermentation of nonpathogenic yeasts and applied as antimicrobial agents in cosmetics, plant protection, bioremediation, medical and food industry (Sekhon et al. 2012; Vaughn et al. 2014; Vrushali et al. 2014; Lourith and Kanlayavattanakul 2009; Borsanyioya et al. 2016; Paulino et al. 2016). However, there is little research on applications of SLs in the textile materials.
SLs are extracellular products produced by several yeast species mainly Candida bombicola (Spencer et al. 1970; Gorin et al. 1961; Gorin and Spencer 1968), consisting of a sophorose sugar head and a long chain hydrophobic fatty acid tail (Konishi et al. 2008; Van Bogaert et al. 2007). When the carboxyl group of the fatty acid moiety is esterified with the hydroxyl group at C4 in one glucose ring, a lactonic sophorose forms. An acidic sophorose structure forms when the carboxyl group is free. Both lactonic and acidic sophorose structures in SLs are shown in Fig. 1, which are biologically produced as a mixture in a ratio. However, the lactonic form possesses better antibacterial property than the acidic form (De Oliveira et al. 2015). Although the antimicrobial property of SLs is prominent, it is difficult to endow SLs with a durable effect on the fabrics.
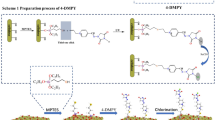
In this article, the biosurfactant SLs, as a new antibacterial agent, were applied to treat the cotton fabrics combined with 1,2,3,4-butanetetracarboxylic acid (BTCA) in a simple finishing process. The effect of treatment conditions on the antibacterial property and anti-crease property of the treated fabrics were investigated. Computational chemistry, attenuated total reflection-Fourier transform infrared (ATR-FTIR), scanning electron microscope (SEM) and liquid chromatography-mass spectrometry (LC–MS) were employed to provide a profound proof on the mechanism of crosslinking SLs onto cellulose. By using this method, an easily biodegradable and ecologically acceptable antibacterial cotton fabric could be provided.
Material and method
Chemicals and materials
Cotton fabric used in this paper was a plain woven 100% cotton (14.8tex, 574 × 480, weighing 120 g/m2). 1,2,3,4-Butanetetracarboxylic acid (BTCA) in industrial grade, and sodium hypophosphite (SHP) monohydrate in the analytical grade were purchased from Sinopharm Chemical Reagent Co., Ltd.. Sophorolipids (SLs) containing 40% of antibacterial ingredients and lactonic sophorolipid (LSL) with a pH value of 3–4 were provided by EVONIK Greater China (EVONIK Industries AG). Nutrient agar powder was purchased from Sinopharm Chemical Reagent Co., Ltd.. Tryptone (OXOIDTM, Unipath Ltd., Basingstoke, England) and yeast extract (OXOIDTM, Unipath Ltd., Basingstoke, England) were biological grade reagents.
Methods
Fabric treatment
The cotton fabrics were dipped and nipped two times with an average wet pickup of 90% in SLs aqueous solution with or without BTCA and SHP. Certain amounts of SLs were mixed in the solutions of BTCA and SHP. Then the wet fabrics were dried at 100 C for 2 min and cured successively at varied temperatures and durations.
Antibacterial activity
A modified ASTM Standards E2149-10 was adopted to examine the antimicrobial performance of the treated samples against S. aureus (ATCC 6538) and E. coli (ATCC 8099). 0.75 g of the fabrics were put into a 250 mL flask containing 75 mL, 0.3 mM PBS buffer with bacteria suspension at a concentration of 3.0 × 104 CFU/mL. Then, these samples were shaken for 1 h at 24°C on a temperature control cyclotron desktop oscillator (ZHWY-200H, Zhicheng Inc., China). The suspension was serially diluted and dropped onto a nutrient agar plate. The plates were then incubated at 37°C for 18 h. Finally, the reduction of bacteria was calculated according to the ASTM method E2149-10.
A = CFU per milliliter for the flask containing the treated substrate after the specified contact time, and B = CFU per milliliter for the flask containing the untreated substrate after the specified contact time.
Attenuated total reflection-Fourier transform infrared (ATR-FTIR)
ATR-FTIR spectra were obtained by using an attenuated total reflectance (ATR) accessory of PerkinElmer spectrometer. The treated fabrics were directly measured by the instrument in the range of 4000–400 cm−1 and at a resolution of 4 cm−1.
LC–MS analysis
One gram of lactonic sophorolipid (LSL) was dispersed in 10 mL distilled water containing 0.05 g sodium silicate, then the liquid was stirred in a water bath at the temperature of 49 °C for 45 min after adjusting the pH value to 10 with hydrochloric acid to stimulate the situation of washing process. The liquid chromatography mass spectrometry (LC–MS) analysis of the solution was performed by a Shimadzu LC–MS-2020 (APCI/MS) instrument.
Scanning electron microscopy (SEM)
The surface morphology of the modified fabrics was observed by using a HITACHI/TM-1000 (Japan). Before testing, the samples were coated with gold.
Laundry test method
Laundry tests of the fabrics were conducted by using a Washtec-P A2 washing machine (Roaches International Ltd.) following the American Association of Textile Chemists and Colorists (AATCC) test method 61-2A, an accelerated procedure. One machine washing is equivalent to 5 household washings.
Wrinkle recovery angle (WRA)
WRAs of the treated fabrics were measured according to AATCC method 66-2010 with a wrinkle recovery tester. Before testing, the fabrics were stored in a conditioning room for 24 h to equilibrate the moisture of fabrics.
Tensile strength testing
The tensile strengths of unmodified and modified cotton fabrics were assessed following a universal material testing machine (H10K-S, Tinius Olsen) according to ASTM D5035 method
Computational modeling
All calculations were performed using a Gaussian 09 package (Frisch et al. 2009). The geometries of sophorolipids and glucopyranose rings were optimized at DFT-B3LYP/6-31G (d,p) level of theory with no solvation model. Based on these optimized results, a Multiwfn program was used to calculate the Hirshfeld charges of sophorolipids and glucopyranose rings (Cao et al. 2015; Hirshfeld 1977; Lu and Chen 2012).
Whiteness measurement
CIE whiteness index (WI) of the fabrics was measured on a Datacolor SF650 spectrophotometer (Datacolor, USA) according to ISO 105-J02: 1997 standard. Each sample was measured four times at various positions to give an average value. The average value of the three pieces of cotton fabrics was recorded.
Add-on of agents
After the cotton fabrics were treated with SLs, BTCA and SHP, the weight changes of fabric could be calculated by the following formula:
W1 (g) is the weight of original fabrics and the W2 (g) is the weight of modified fabrics.
Results and discussion
Optimization of treatment
Amount of antibacterial agent
The antibacterial property of the treated fabric is determined by the amount of SLs incorporated, and the results are shown in Fig. 2. With the increase of the antibacterial agent concentration, the antibacterial property of the fabrics against S. aureus was improved dramatically. And the fabrics treated with 60 g/L of SLs demonstrated a complete kill to the 3.0 × 104 CFU/mL of the bacteria. However, the excessive amount of the antibacterial agent made the treated fabric yellow and sticky, and the CIE whiteness index of them was decreased significantly (~ 30 in CIE white index) compared with the untreated fabrics. Therefore, 40 g/L of SLs is considered as a suitable amount to treat the fabrics, balancing high antibacterial property with proper appearance of the treated fabrics.
Conditions of treatment
Because of the low chemical reactivity between SLs and cotton fabrics, most of SLs could be removed after one-time machine washing from the treated fabrics. To improve the antibacterial effect and durability of the agent, a polycarboxylic acid crosslinker, 1,2,3,4-butanetetracarboxylic acid (BTCA), together with sodium hypophosphite (SHP) as a catalyst, was combined with 40 g/L of SLs to treat the cotton fabrics. BTCA could serve as a crosslinker between cotton cellulose and sophorolipids and provide potential covalent connections. The reaction between BTCA and cellulose requires high temperature. Therefore, the influence of the treatment temperature on the antibacterial property of the modified fabrics was investigated, and the results are shown in Fig. 3a.
The fabrics treated with the mixture of SLs, BTCA, and SHP demonstrated better antimicrobial functions than that of treated with only SLs. When the curing temperature reached 120°C, the antibacterial function of the unwashed fabric showed over 90% kill against S. aureus. However, further raising curing temperature was not beneficial either for keeping the tensile strength of the fabrics or for dramatically improving the durability of antimicrobial functions of the fabrics treated with SLs, BTCA, and SHP. Therefore, the curing temperature of 120°C was selected for the rest treatments.
When investigating the influence of curing time on the antimicrobial performance of the treated fabrics, the curing temperature was fixed at 120°C and other conditions were unchanged. As shown in Fig. 3b, prolonging curing time did not affect the antibacterial property and tensile strength of the treated fabrics significantly. No matter whether the fabrics were washed or not, the antibacterial rates of the fabrics were almost same with the results shown in Fig. 3a. Increasing curing time slightly decreased the tensile strength of the warp direction of the treated fabrics, with the tensile strength retention maintained above 90%. Therefore, 2 min was chosen as the curing time for the rest treatments.
The dosage of SHP and the crosslinker in the finishing process are two important factors that could influence crosslinking of SLs onto the cellulose and consequently affect the antibacterial property and durability of the treated fabrics. Here the treatment conditions were fixed at 120°C for 2 min with varying amounts of crosslinker in the finishing baths.
Comparing the results shown in Fig. 3c with that in Fig. 3a, b, the antimicrobial property of treated fabrics was not enhanced evidently after introducing more BTCA in the finishing bath, while the washed fabrics exhibited over 60% of antimicrobial rates when the concentrations of BTCA was above 90 g/L. However, the tensile strength losses of the treated fabrics continuously increased as BTCA concentration was raised. More SLs could be immobilized on the surface of fabrics by increasing the amount of BTCA, shown in Fig. 3c. However, it seems that BTCA prefers to react with cellulose rather than SLs, indicated by the fact that the increased losses of tensile strengths of the treated fabrics mainly occurred in high concentrations of BTCA in the finishing baths. Thus, 90 g/L of BTCA was determined to serve for the following treatments.
The amount of SHP catalyst could affect the catalytic effect of esterification of BTCA with cellulose and SLs, also the antibacterial properties of the washed fabrics significantly, as shown in Fig. 3d. It is correlated with the amount of BTCA in the system, mostly in a molar ratio of SHP/BTCA = 0.25 − 0.5 (Yang and Bakshi 1996; Yang and Andrews 1991). The antimicrobial performance of the washed fabrics was improved sharply and then declined. According to the known reaction mechanism of the formation of anhydride of BTCA and esterification of the anhydride with cellulose, it seems that metal ions could only be involved in the step of the anhydride formation instead of the esterification (Ji et al. 2016a). The alkali metal ions assist the anhydride formation of BTCA, while the acid anions provided functions on the esterification step of the anhydride by assisting removal of the protons in the intermediate (Ji et al. 2016a; Ji et al. 2015). Then the resulted metal carboxylates could influence the H-bond interactions between carboxylic acid groups within or between BTCA molecules and possibly improve the formation of the anhydride (Ji et al. 2016a, 2015). Therefore, a higher possibility of the reaction between BTCA and SLs was achieved at the ratio of SHP/BTCA = 0.49. Excessive SHP might accelerate the reaction between cellulose and BTCA instead of SLs and BTCA, which will reduce the grafted SLs on the treated fabrics. As a result, the washed fabrics demonstrated a tendency of declining antimicrobial functions.
It is obvious that the acid crosslinker could affect the antibacterial durability of the treated cotton fabrics. And an optimal condition of finishing process was determined as two dips and two nips fabrics with a wet pickup of 90% in an aqueous solution containing 20 g/L of SHP, 40 g/L of SLs and 90 g/L of BTCA. And then the fabric was dried at 100°C for 2 min and cured at 120°C for 2 min. At the same time, the weight gain of modified fabrics finished by this method is about 17%.
Antibacterial property and WRA of treated fabrics
The antibacterial properties of the fabrics treated with BTCA, SHP, and SLs by the optimal processing conditions are shown in Table 1. The fabrics treated with BTCA only also exhibited antimicrobial properties. Because residual carboxylic acid groups of BTCA on the surface of fabrics could disturb reproduction of microbes, resulting very limited antimicrobial effect. The SLs modified fabrics could kill 99% S. aureus by contact. However, the functions of the treated fabrics showed a low kill against E. coli. Similar results were observed on other materials and referred as the difference of the cellular membranes of both bacteria (De Oliveira et al. 2015; Baek et al. 2003). Also, the antibacterial property of SLs sometimes depends on the cell wall structure of microbes, such as gram positive and negative bacteria.
Table 1 also shows the wrinkle recovery angles (WRA) of the modified cotton fabrics, which could be a result of BTCA directly reacting with cellulose only. The WRA of SLs and BTCA treated fabrics is lower than the cotton fabric modified with BTCA only, an indication of BTCA molecules reacting with both cellulose and SLs during the finishing process. In other words, some of the carboxyl groups consumed by SLs could reduce crosslinking reaction of BTCA with cellulose.
However, the antimicrobial property of the finished fabrics against laundering decreased dramatically. One reason might be that the ester bond in lactonic sophorolipids was hydrolyzed in alkaline washing conditions. The pH value of the standard detergent solution is about 10, making the hydrolysis of ester connections favored during the laundering process. Therefore, a liquid chromatography mass spectrometry (LC–MS) analysis was applied to investigate the hydrolysis property of sophorolipids, and the result is shown in Fig. 4
In Fig. 4, a mass to charge ratio (m/z) at 687 (M-1) in the mass spectrum (A) revealed a molecular mass of pure LSL at 688 (Daverey and Pakshirajan 2010) before washing. The mass spectrum (B) revealed that the LSL was hydrolyzed at the pH value of 10. Because the major peak (m/z 621 M-1) fits with the mass of the hydrolyzed LSL molecule shown in Fig. 4b, it indicates that not only the ester bond but also the two acetyl groups in the LSL structure were hydrolyzed. At the same time, the ions m/z 459 and m/z 297 may be assigned to the fragments showed in Fig. 4b (Dengle-Pulate et al. 2014; Shah et al. 2007; Davila et al.1993, 1997; Chen et al. 2014; Wadekar et al. 2012). The ionization method in the mass spectrometer is pressure chemical ionization (APCI), which is considered as a “soft ionization” source inducing preferentially the formation of the de-protonated or protonated molecule without fragmentation (Souverain et al. 2004). Therefore, less fragmentation of the bio-molecules was observed in the MS spectrum of hydrolyzed LSL.
Hydrolysis of the ester bonds connecting between BTCA, cotton fabrics and SLs are also highly vulnerable to be hydrolyzed in alkaline laundering conditions. With increasing washing times, both the antibacterial property and the WRA of the treated fabrics decrease, as shown in Table 1, indicating that hydrolysis of ester bonds from the fabrics is a main cause of low washing durability.
Replacing the alkaline detergent with a neutral detergent in the laundering process could improve the washing durability of the modified fabrics, according to the above analysis (Table 1). The reduction of antibacterial rate and WRAs declined significantly, due to reduced hydrolysis of ester bond connections. However, hydrolysis reaction was only reduced but not completely prevented.
Several structural factors of SLs may also attribute to the low durability of the resulted antimicrobial functions, including steric hindrance and intermolecular interactions. Among the SLs molecules, the configurations of SLs are not regular and stable enough. When one SLs molecule is crosslinked with BTCA, the reactive site of another molecule with BTCA might be blocked. Another reason is that when one or two carboxyl groups of BTCA react with hydroxyl groups of SLs, the chemical activity of rest carboxyl groups in BTCA might be impaired.
Potential mechanism of crosslinking among SLs, BTCA, and cellulose
Gaussian calculations
There are two types of chemical structures in the agent SLs, the lactonic form and the acidic form. Generally, the lactonic form was the main antibacterial ingredient. Then we tried to estimate the chemical reactivity of hydroxyl in LSL by a computational modeling program, and the results are shown in Fig. 5 and Table 2.
Generally speaking, the reaction between BTCA and cellulose should undergo two steps, formation of anhydride and esterification of anhydride with cellulose (Gu and Yang 1998; Yang 1991; Yang and Wang 1998). The glucopyranose rings in the structure of SLs are similar to the structure of cellulose. Therefore, BTCA could react with both SLs and cellulose to form the crosslinked structure. The configurations of LSL in lowest energy are shown in Fig. 5. The LSL could keep its loop structure, and the long alkyl chain is tangled a bit, as shown in Fig. 5a–c from different view angle. All hydroxyl groups on the glucopyranose rings in the LSL (circled in red in Fig. 5a–c) are in the periphery positions of LSL molecules, which improves relatively higher reaction probability because of low steric hindrance. Moreover, the electrostatic potential maps and the numbers of every atoms in the LSL molecule are generated from GaussView in Fig. 5d. The color changes from blue to red mean the electrostatic potential of atoms changes from positive to negative. In LSL molecule, the electrostatic potentials of 8-O (− 0.562) and 9-O (− 0.596) atoms (yellow or red) are more negatively charged than 17-O (− 0.547) and 27-O (− 0.547) in hydroxyl groups, which illustrated the stronger electron-donating ability and higher nucleophilic reaction possibility towards carbonyl groups in BTCA anhydride.
At the same time, the Hirshfeld charges of O atoms in these four hydroxyl groups were calculated by Gaussian 09 W and are showed in Table 2. The 9-O with the Hirshfeld charge of − 0.244554 could be the most likely one to react with BTCA. As we know, the hydroxyl group on C6 in the glucopyranose ring has higher chemical activity compared to other hydroxyls on C2 and C3 positions (Klemm et al. 2005; Luo et al. 2015; Kamide 2005). As well, the Hirshfeld charge of C6 in the glucopyranose rings of cellulose is − 0.246754, also competitive in reacting with BTCA anhydride. A possible chemical reaction is shown in Fig. 6.
ATR-FTIR and morphology analysis
To further determine whether SLs were fixed to the cotton fabrics by BTCA, the fabrics treated with the optimum finishing process were analyzed by ATR-FTIR spectroscopy. As shown in Fig. 7A, the peaks at 2924 cm−1and 2854 cm−1 are due to the asymmetrical stretching and symmetrical stretching of methylene groups (Daverey and Pakshirajan 2009, 2010; Wadekar et al. 2012; Hu and Ju 2001; Ji et al. 2016a). And the peak at 1741 cm−1 and 1712 cm−1 are attributed to the carbonyl group in ester and acid forms, respectively (Ji et al. 2016a, b, 2015). Therefore, the ester bonds generated among BTCA, cellulose, and SLs could be compared by comparing related peaks in FTIR spectra of (a), (b) and (d). These peaks also can be compared with the one of after one-time washing circle (c) and a difference spectrum of subtracting spectrum d from spectrum b (b–d). The results can confirm that the chemical reaction between the BTCA, fabrics, and SLs happened. In addition, the band at 1643 cm−1 was observed due to stretching of the unsaturated C=C bonds in the SLs molecule (e) (Daverey and Pakshirajan 2010). Although the band also can be found in pristine fabric (a), it might be caused by the water (Chung et al. 2004), the subtracted spectrum b–d eliminated the influence of water peak at 1643 cm−1 showed in spectrum (a).
A ATR-FTIR spectra: (a) cotton fabric, (b) antibacterial fabric, (c) one time machine washed fabric, (d) BTCA modified fabric, (b–d) difference spectrum, subtracting (d) from (b), (e) SLs; B SEM images of antibacterial fabrics: (1) control sample, (2) treated with BTCA and SLs, (3) after one-time standard washing using alkali detergent
Surface morphologic changes of the fabrics were observed, shown in Fig. 7B. The surfaces of the antibacterial fabrics seemed to be rougher, indicating that the agent grafted on the surface (B2). In contrast, the untreated cotton fabrics looked smooth and neat (B1), but with some dust particles on it. It appears that SLs were combined with fabrics depending on the crosslinking of BTCA. But after machine washing, parts of SLs were detached from the fabrics because of the hydrolyzation effects.
Conclusion
The durable antimicrobial treating processes and conditions for using sophorolipids and BTCA in treatments of cotton fabrics were investigated, and the influence of curing time, curing temperature, amounts of crosslinker and catalyst on the antibacterial property and tensile strength were discussed. An optimized treatment condition of cotton fabrics was found as two dips and two nips with a wet pickup of 90% in an aqueous solution containing 20 g/L of catalyst, 40 g/L of SLs and 90 g/L of BTCA, and the treated fabrics were dried at 100°C for 2 min, and cured at 120°C for 2 min. The treated cotton fabrics showed better antimicrobial properties against gram-positive bacterium than gram-negative one, and crosslinked SLs on the fabrics with the use of BTCA showed improved washing durability. Hydrolysis of SLs in alkaline washing condition was a main cause of reduced washing durability. Replacing the alkaline detergent with a neutral detergent could improve the durability of antibacterial fabrics. The crosslinking reactions between BTCA with cotton cellulose and SLs were confirmed by using instrumental and supported by computational analyses.
References
Angen Ø, Feld L, Larsen J, Rostgaard K, Skov R, Madsen AM, Larsen AR (2017) Transmission of MRSA to human volunteers visiting a swine farm. Appl Environ Microb 83:e01489-17. https://doi.org/10.1128/AEM.01489-17
Baek SH, Sun XX, Lee YJ, Wang SY, Han KN, Choi JK, Noh JH, Kim EK (2003) Mitigation of harmful algal blooms by sophorolipid. J Microbiol Biotechn 13:651–659
Bockmühl DP, Schages J, Rehberg L (2019) Laundry and textile hygiene in healthcare and beyond. Microb Cell 6:299. https://doi.org/10.15698/mic2019.07.682
Borsanyiova M, Patil A, Mukherji R, Prabhune A, Bopegamage S (2016) Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol 61:85–89. https://doi.org/10.1007/s12223-015-0413-z
Cao J, Ren Q, Chen F, Lu T (2015) Comparative study on the methods for predicting the reactive site of nucleophilic reaction. Sci China Chem 58:1845–1852. https://doi.org/10.1007/s11426-015-5494-7
Chen J, Zhang HR, Liu Y, Fu SM, Liu XL (2014) Metal ions can affect the composition and production of sophorolipids by Wickerhamiella domercqiae Y2A CGMCC 3798. Eur J Lipid Sci Tech 116:1505–1512. https://doi.org/10.1002/ejlt.201300512
Cheng X, Ma K, Li R, Ren X, Huang TS (2014) Antimicrobial coating of modified chitosan onto cotton fabrics. Appl Surf Sci 309:138–143. https://doi.org/10.1016/j.apsusc.2014.04.206
Chung C, Lee M, Choe EK (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Arbohyd Polym 58:17–420. https://doi.org/10.1016/j.carbpol.2004.08.005
Daverey A, Pakshirajan K (2009) Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotech 158:663–674. https://doi.org/10.1007/s12010-008-8449-z
Daverey A, Pakshirajan K (2010) Sophorolipids from Candida bombicola using mixed hydrophilic substrates: production, purification and characterization. Colloid Surf B 79:246–253. https://doi.org/10.1016/j.colsurfb.2010.04.002
Davila AM, Marchal R, Monin N, Vandecasteele JP (1993) Identification and determination of individual sophorolipids in fermentation products by gradient elution high-performance liquid chromatography with evaporative light-scattering detection. J Chromatogr A 648:139–149. https://doi.org/10.1016/0021-9673(93)83295-4
Davila AM, Marchal R, Vandecasteele JP (1997) Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl Microbiol Biot 47:496–501. https://doi.org/10.1007/s002530050962
De Oliveira MR, Magri A, Baldo C, Camilios-Neto D, Minucelli T, Celligoi MAPC (2015) Sophorolipids a promising biosurfactant and it’s applications. Int J Adv Biotechnol Res 6:161–174
Dengle-Pulate V, Chandorkar P, Bhagwat S, Prabhune AA (2014) Antimicrobial and SEM studies of sophorolipids synthesized using lauryl alcohol. J Surfactants Deterg 17:543–552. https://doi.org/10.1007/s11743-013-1495-8
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC et al (2009) Gaussian 09 Revision A.02. Wallingford, CT
Gorin PAJ, Spencer JFT (1968) Galactomannans of Trichosporon fermentans and other yeasts; proton magnetic resonance and chemical studies. Can Can J Chem 46:2299–2304. https://doi.org/10.1139/v68-373
Gorin PAJ, Spencer JFT, Tulloch AP (1961) Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Can J Chem 39:846–855. https://doi.org/10.1139/v61-104
Gu X, Yang CQ (1998) FT-IR and FT-Raman spectroscopy study of the cyclic anhydride intermediates for esterification of cellulose: I. Formation of anhydrides without a catalyst. Res Chem Intermed 24:979–996. https://doi.org/10.1163/156856798X00672
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim 44:129–138. https://doi.org/10.1007/BF00549096
Hu Y, Ju LK (2001) Purification of lactonic sophorolipids by crystallization. J Biotechnol 87:263–272. https://doi.org/10.1016/S0168-1656(01)00248-6
Ji BL, Tang PX, Yan KL, Sun G (2015) Catalytic actions of alkaline salts in reactions between 1, 2, 3, 4-butanetetracarboxylic acid and cellulose: II. Esterification. Carbohyd Polym 132:228–236. https://doi.org/10.1016/j.carbpol.2015.06.070
Ji BL, Qi H, Yan KL, Sun G (2016a) Catalytic actions of alkaline salts in reactions between 1, 2, 3, 4-butanetetracarboxylic acid and cellulose: I. Anhydride formation. Cellulose 23:259–267. https://doi.org/10.1007/s10570-015-0810-0
Ji BL, Zhao CY, Yan KL, Sun G (2016b) Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohyd Polym 144:82–288. https://doi.org/10.1016/j.carbpol.2016.02.036
Kamide K (2005) Cellulose and cellulose derivatives. Elsevier, Amsterdam
Kang CK, Kim SS, Kim S, Lee J, Lee JH, Roh C, Lee J (2016) Antibacterial cotton fibers treated with silver nanoparticles and quaternary ammonium salts. Carbohyd Polym 151:1012–1018. https://doi.org/10.1016/j.carbpol.2016.06.043
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Edit 44:3358–3393. https://doi.org/10.1002/anie.200460587
Konishi M, Fukuoka T, Morita T, Imura T, Kitamoto D (2008) Production of new types of sophorolipids by Candida batistae. J Oleo Sci 57:359–369. https://doi.org/10.5650/jos.57.359
Lourith N, Kanlayavattanakul M (2009) Natural surfactants used in cosmetics: glycolipids. Int J Cosmet Sci 31:255–261. https://doi.org/10.1111/j.1468-2494.2009.00493.x
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Luo CC, Wang H, Chen Y (2015) Progress in modification of cellulose and application. Chem Ind Eng Prog 34:767–773
Ma Y, Li J, Si Y, Huang K, Nitin N, Sun G (2019) Rechargeable Antibacterial N-halamine Films with antifouling function for food packaging applications. ACS Appl Mater Interfaces 11:17814–17822. https://doi.org/10.1021/acsami.9b03464
Metcalf CJE, Lessler J (2017) Opportunities and challenges in modeling emerging infectious diseases. Science 357:149–152. https://doi.org/10.1126/science.aam8335
Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P (2017) Host and viral traits predict zoonotic spillover from mammals. Nature 546:646–650
Paulino BN, Pessôa MG, Mano MCR, Molina G, Neri-Numa IA, Pastore GM (2016) Current status in biotechnological production and applications of glycolipid biosurfactants. Appl Microbiol Biot 100:10265–10293. https://doi.org/10.1007/s00253-016-7980-z
Sekhon KK, Khanna S, Cameotra SS (2012) Biosurfactant production and potential correlation with esterase activity. J Pet Environ Biotechnol 3:133. https://doi.org/10.4172/2157-7463.1000133
Shah V, Badia D, Ratsep P (2007) Sophorolipids having enhanced antibacterial activity. Antimicrob Agents Ch 51:397–400. https://doi.org/10.1128/AAC.01118-06
Si Y, Zhang Z, Wu W, Fu Q, Huang K, Nitin N, Ding B, Sun G (2018) Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci Adv 4:5931. https://doi.org/10.1126/sciadv.aar5931
Smiechowicz E, Niekraszewicz B, Kulpinski P, Dzitko K (2018) Antibacterial composite cellulose fibers modified with silver nanoparticles and nanosilica. Cellulose 25:3499–3517. https://doi.org/10.1007/s10570-018-1796-1
Souverain S, Rudaz S, Veuthey JL (2004) Matrix effect in LC-ESI-MS and LC-APCI-MS with off-line and on-line extraction procedures. J Chromatogr A 1058:61–66. https://doi.org/10.1016/j.chroma.2004.08.118
Spencer JFT, Gorin PAJ, Tulloch AP (1970) Torulopsis bombicola sp. n. Antonie Van Leeuwenhoek 36:129–133. https://doi.org/10.1007/BF02069014
Van Bogaert IN, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biot 76:23–34. https://doi.org/10.1007/s00253-007-0988-7
Vaughn SF, Behle RW, Skory CD, Kurtzman CP, Price NPJ (2014) Utilization of sophorolipids as biosurfactants for postemergence herbicides. Crop Prot 59:29–34. https://doi.org/10.1016/j.cropro.2014.01.014
Vrushali DP, Jnanada J, Sunil B, Asmita P (2014) Application of sophorolipids synthesized using lauryl alcohol as a germicide and fruit-vegetable wash. World J Pharm Pharm Sci 3:1630–1643
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012) Jatropha oil and karanja oil as carbon sources for production of sophorolipids. Eur J Lipid Sci Tech 114:823–832. https://doi.org/10.1002/ejlt.201100282
Yang CQ (1991) FT-IR spectroscopy study of the ester crosslinking mechanism of cotton cellulose. Text Res J 61:433–440. https://doi.org/10.1177/004051759106100801
Yang CQ, Andrews BAK (1991) Infrared spectroscopic studies of the nonformaldehyde durable press finishing of cotton fabrics by use of polycarboxylic acids. J Appl Polym 43:1609–1616. https://doi.org/10.1002/app.1991.070430904
Yang CQ, Bakshi GD (1996) Quantitative analysis of the nonformaldehyde durable press finish on cotton fabric: acid-base titration and infrared spectroscopy. Text Res J 66:377–384. https://doi.org/10.1177/004051759606600604
Yang CQ, Wang X (1998) Formation of five-membered cyclic anhydride intermediates by polycarboxylic acids: thermal analysis and Fourier convert infrared spectroscopy. J Appl Polym Sci 70:2711–2718. https://doi.org/10.1002/(SICI)1097-4628(19981226)70:13%3c2711:AID-APP16%3e3.0.CO;2-Z
Acknowledgments
The authors will thank Dr. Shiyuan Hu and Dr. Tiantian Xie of EVONIK Greater China (EVONIK Industries AG) for providing the antibacterial agents.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, C., Hu, C., Sun, G. et al. Antimicrobial finish of cotton fabrics treated by sophorolipids combined with 1,2,3,4-butanetetracarboxyic acid. Cellulose 27, 2859–2872 (2020). https://doi.org/10.1007/s10570-019-02925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02925-9