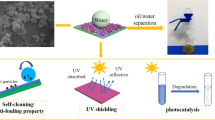
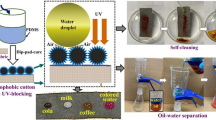
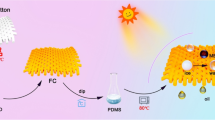
Abstract
Superhydrophobic cotton fabrics with improved ultraviolet resistance were prepared via in situ growing NH2-MIL-125(Ti)(Ti-MOF) nanoparticles on cotton fibers and subsequently coating with polydimethylsiloxane (PDMS) under room temperature. The synergetic effect of Ti-MOFs nanoparticles and PDMS was critical and essential for obtaining a superhydrophobic coating with anti-UV property. The surface microstructure, chemical composition, and superhydrophobic property of the as-prepared fabrics were characterized by K/S value, scanning electron microscopy, X-ray photoelectron spectroscopy, energy dispersive spectroscopy, and water contact angle (WCA) measurements. The as-prepared cotton fabrics not merely exhibited desirable superhydrophobic property with a water contact angle (WCA) of 154.7 ± 0.7° and a sliding angle of 3.6°, but also displayed the considerable UV resistance. The oil–water separation, stability of UV protection and superhydrophobicity were also investigated. The superhydrophobicity, anti-ultraviolet property, and K/S value of as-prepared PDMS/Ti-MOFs@cotton fabrics were stable after 300 cycles of abrasion and 5 cycles of washing. The facile synthesis technique provided a simple method for scalable construction of multifunctional fabrics.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past few years, superhydrophobic surfaces have received considerable attention due to the increased demands of water-repellent textiles (Zhou et al. 2019), self-cleaning coatings (Cheng et al. 2019; Yang et al. 2019a; Zhou et al. 2018), oil-separation (Cheng et al. 2017, 2018; Dong et al. 2019) etc. Cotton fabric has been widely used in daily life due to its renewability, biodegradability, low cost, and good mechanical properties. Despite the excellent properties of cellulose material, some inherent features such as being hydrophilic and poor resistance to ultraviolet (UV) radiation inhibited its extensive application in advanced fields. Consequently, the practical construction of robust fluorine-free superhydrophobic cotton fabrics with improved ultraviolet resistance was highly desirable (Agrawal et al. 2019; Jiang et al. 2019).
Inspired by lotus leaves in nature, rough surface structure and low surface energy were considered as the two main characteristics to construct a superhydrophobic surface (Liu et al. 2017). In order to produce superhydrophobic surface, some nano-materials such as SiO2 (Lahiri et al. 2019), MnO2 (Li and Guo 2017), CuO (Li et al. 2012), Ag, TiO2 (Chen et al. 2016, 2018; Yang et al. 2018), ZnO (Yang et al. 2019b), carbon nanotubes (Emam 2019), fly ash (Khan et al. 2018), ormosil (Cao et al. 2016), and graphene oxide (Gu et al. 2019) have been used to increase surface roughness. As novel multifunctional crystalline nano-materials, metal–organic frameworks (MOFs) have been widely used in many areas, such as sensor (Li et al. 2019), high-capacity adsorbents (Cui et al. 2019), gas separation (Brandt et al. 2019), catalysis (Xu et al. 2019), etc. Recently, MOFs have been used for the oil–water separation (Kim et al. 2019; Lei et al. 2018; Mukherjee et al. 2016; Zhu et al. 2017). For example, Lei et al. (2018) constructed a hydrophobic ZIF-8/melamine sponge with excellent oil absorption ability. Miao et al. (2018) developed a self-cleaning and antibacterial coating by mixed ZIF-8 nanoparticles with polyvinylidene fluoride (PVDF) colloidal particle suspension and then modified with 1H, 1H, 2H, 2H-perfluorooctyltriethoxysilane. Mao et al. (2017) have prepared a ZIF-8/reduced graphene-oxide hydrogel for wastewater remediation via one step self-assembly strategy. Jayaramulu et al. (2016) have developed superhydrophobic highly fluorinated graphene oxide/ZIF-8 composites and used for efficient oil absorption. Li and Guo (2018) fabricated a stable superhydrophobic F-ZIF-8@Kevlar fabric by a facile layer-by-layer hot-pressing (HoP) technology. Lu et al. (2018) have prepared a multi-functional cotton fabric with super-hydrophobic, antibacterial, and UV resistance properties via benign layer-by-layer (LbL) deposition ZnBDC on the fabric surface. Although great progress has been made, most of superhydrophobic materials mentioned above mainly focused on ZIF-8 MOF materials. However, the research of multifunctional textiles based on titanium-based MOFs was still rare (Abdelhameed et al. 2017).
Very recently, Emam and Abdelhameed (2017) revealed anti-UVR textiles with good laundering durability can be constructed via incorporation of nano-NH2-MIL-125(Ti) into natural textiles by one-pot process. So, it is very feasible to construct multifunctional cotton textiles with superhydrophobicity and UV resistance through the combination nano Ti-MOFs with low surface energy materials. However, to the best of our knowledge, the immobilization of Ti-MOFs on fabrics to obtain both stable superhydrophobicity and anti-UVR properties and maintain its nature properties such as durability, flexibility, and breathability of cotton textile has not been thoroughly studied.
Here, as a part of our ongoing studies on the development of multifunctional textiles (Bu et al. 2018, 2019; Gu et al. 2017; Huang et al. 2019; Mai et al. 2018), we now present a facile and energy-saving method to fabricate superhydrophobic cotton textiles with improved ultraviolet resistance via in situ growing nano-Ti-MOFs on cotton fibers and subsequently coating with polydimethylsiloxane (PDMS) under room temperature. These functional superhydrophobic fabrics were promising for improved ultraviolet resistance, efficient oil–water emulsions separation, and effective self-cleaning. The surface microstructure of the obtained cotton textiles were investigated by K/S value, scanning electron microscopy (SEM) coupled to energy dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS) analysis. The wettability and anti-UVR effect were studied respectively. The washing fastness, stability, and self-cleaning application of the modified cotton textiles were also discussed. This facile fluorine-free and inexpensive method was easy to be applied in large scale.
Experimental
Materials
2-aminoterephthalic acid (99%), and Titanium isopropoxide (Ti(OiPr)4) were obtained from Shanghai Aladdin Reagent Co., Ltd (China). N,N-dimethylformamide (DMF), methanol (AR), and ethanol (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd (China) and used with received. Polydimethylsiloxane (PDMS) prepolymer (Sylgard 184A) and the curing agent (Sylgard 184B) were bought from Dow Corning Corporation. Cotton fabric (plain weave fabric, 107 g/m2) was bought from local supermarkets and was cleaned with ethanol and deionized water before for further processing.
Synthesis of Ti-MOFs@fabric
A piece of cotton fabric (5 cm × 5 cm) was soaked in 30 mL of methanol solution containing 1.5 mmol of titanium isopropoxide. Then the mixture solution was stirred for 30 min at room temperature. A 12 mL of DMF solution containing 2-aminoterephthalic acid (1.2 mmol) was drop-wise added for above mixture solution. The obtained mixtures were then incubated at room temperature for 24 h. Afterward, the modified cotton (Ti-MOFs@cotton) fabric was taken out from mixtures solution and washed three times with deionized water and dried at 80 °C for 2 h. A various numbers of complete cycles were performed using the same procedure to prepare textiles with highly MOFs content and labelled as (Ti-MOFs)n@cotton fabric, where n is the deposition numbers.
Preparation of superhydrophobic PDMS/Ti-MOFs@cotton fabric
The superhydrophobic cotton fabrics were obtained by soaking the resulted (Ti-MOFs)n@cotton fabric in THF solution containing 3% PDMS (polydimethylsiloxane) pre-polymer and curing agent with a volume ratio of 10:1 for 5 min and subsequently baking at 80 °C for 3 h. The resulted cotton fabric treated with different deposition numbers was named as PDMS/(Ti-MOFs)n@cotton fabric (n = 1, 2, 3), respectively.
Analyses and measurements
The surface morphologies of pristine and obtained superhydrophobic fabrics were characterized with scanning electron microscopy (SEM, JEOL JSM IT300 A, Japan) after sputtering gold. Fourier transform infrared (FTIR) spectra were collected on a FTIR spectrometer (Ten-sor 27, Bruker, Germany) at attenuated total reflectance (ATR) mode was used to determine the functional groups of before and after modification samples. X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi) with a monochromatic Al Ka was used to determine the chemical composition of before and after modification samples. The K/S value of different specimens was analyzed using an X-Rite Color i7 bench top spectrophotometer (X-Rite, USA) based on D65 illuminant and 10° observer. The surface wetting characteristic of each step of modification samples were estimated by measuring the water static contact (WCA) angles using a Dataphysics OCA 30 contact angle system (Dataphysics, Germany) at room temperature with a 3 μL water droplet in all measurements. The average values were obtained by measuring the same sample at least five different positions. The water sliding angles (WSA) of different samples were obtained with 10 μL drops of water according to the published procedure (Zimmermann et al. 2009).
Ultraviolet resistance of as-obtained PDMS/(Ti-MOFs)n@cotton fabric was determined by measuring percentage transmission of UV radiations on UV–vis-NIR Spectrophotometer (UV-HD 902C) as per AATCC 18830 standard. The samples were cut into a circle having a diameter of 5 cm and tested at room temperature.
Mechanical stability: The mechanical stability of as-fabricated superhydrophobic cotton sample was subjected to the scratch test using sandpaper as an abrasion source. The superhydrophobic fabric with a length of 10 cm was kept in directly contact with sandpaper and moved repeatedly with load at 50 g weight and abrasive speed of approximately 2–8 cm/s. The WCA and WSA after abrading with sandpaper were used to determine the mechanical stability.
Laundering durability of superhydrophobic fabrics: The superhydrophobic fabrics (5 cm × 5 cm) were washed using 50 mL of sodium dodecanesulfonate (2.0%, w/w) aqueous solution in a 100 mL of beaker with stirring (300 rpm, magnetic stirrer) at 30 °C for 10 min. After then, the fabric was rinsed with deionized water and dried at 60 °C. The wettability of the laundered fabrics was examined through the WCA test.
For the chemical stability test: a piece of modified cotton fabric was immersed in hydrochloric acid (pH = 2), saturated NaCl solution (pH = 7), NaOH solution (pH = 12), and acetone for a few hours, respectively. After then, the superhydrophobic fabrics were washed with water and dried in a vacuum oven at room temperature. For the environmental stability evaluation, the superhydrophobic fabrics were heated up to 150 °C for 1 h or cooled at − 196 °C for 2 h.
Oil/water separation test of PDMS/(Ti-MOFs)3@cotton fabric: A simple device was used for the continuous oil–water separation, which consisted of the superhydrophobic fabric bag filled with melamine sponges, and a peristaltic pump. The whole oil–water separation process was carried out using n-hexane as model oil according to our previous work (Bu et al. 2018). The oil–water separation efficiency was calculated according to the following Eq. (1).
where mo and m1 represented the added oil mass initially and the collected oil mass, respectively.
Air flows of pristine and modified fabrics were measured according to ISO 9237:1995 standard with the air permeability tester (Wenzhou Darong Textile Instrument Co., Ltd, China). The handling properties were measured with the Electronic fabric stiffness tester (NingboTextile Instrument Co., Ltd, China) according to GB/T 18318.1-2009.
Results and discussions
Synthesis process of superhydrophobic PDMS/Ti-MOFs@cotton fabric with improved ultraviolet resistance
In this work, the colorful superhydrophobic PDMS/Ti-MOFs@cotton fabric with improved ultraviolet resistance was prepared via two-step simple process. The whole process was carried out without high reaction temperature and pressure. The preparation procedure was explained in Scheme 1. After cotton fabrics were immersed into a mixture solution of titanium isopropoxide and 2-aminoterephthalic acid for 24 h, the cotton fabric was stained with Ti-MOFs particles. As seen in Scheme 1, the dyed cotton fabrics exhibited brilliant yellow colors because of the underlying color of the deposited Ti-MOFs particles. At the same time, Ti-MOFs particles coated on the surface of fabric make the surface rougher by conversion of micro-structured fiber into micro-/nano-structured fiber surface. Further, PDMS was used to produce low surface energy and the superhydrophobic fabric was produce. The synergetic effect of Ti-MOFs nanoparticles and PDMS was critical to obtain this superhydrophobic cotton fabric.
Effect of deposition cycles on the K/S value of Ti-MOFs@cotton fabric
K/S value was often used to compare the color strength of different samples. The fabric was stained after Ti-MOFs nanoparticles depositing on the fiber surface, which ascribed to the underlying color of the deposited Ti-MOFs nanoparticles. As displayed in Fig. 1, the different color fabric can be observed indicated the deposit with different Ti-MOFs nanoparticles on the fabric surface. Upon increasing the deposition time from one to third cycle, the K/S value increased from 3.76 to 7.39, which implied the higher content of Ti-MOFs goes into the fabric surface. After PDMS coating, the K/S values of different samples have a little change, implying that there is no dissolution of the deposited Ti-MOFs during PDMS coating.
Correspondingly, the water contact angle (WCA) of the different modified fabric was measured. The water contact angle treated with different deposition cycles was 149.7 ± 1.7°, 151.6 ± 1.8°, and 154.7 ± 0.7°, respectively. Based on the above results, the PDMS/(Ti-MOFs)3@cotton fabric was chosen for further study because it exhibited a higher WCA (154.7 ± 0.7°) and higher K/S value.
Surface microstructures, morphology and chemical composition
The surface morphology structure of the pristine and modified cotton fabrics was characterized by scanning electron microscopy (SEM). As illustrated in Fig. 2a, d, the surface of pristine cotton fabric was smooth without micro/nano-structures. After in situ direct growth of Ti-MOFs, a lot of particles uniform cover on the fiber surfaces, as shown in Fig. 2e. After further treatment the above surface with PDMS, a uniform film with lots of nanoscale protuberances was observed on the fiber surface (Fig. 2f). This hierarchical micro/nano-structures increase the roughness of modified fiber surface, which is a crucial factor to achieve superhydrophobicity. The WCA of the PDMS/(Ti-MOFs)3@cotton fabric is 154.7 ± 0.7°, displaying good superhydrophobicity. The pristine and Ti-MOFs coated cotton fabrics show hydrophilic. As control, the water contact angle of cotton fabric only covering of PDMS film was about 135.47°, suggesting that the synergistic effect of Ti-MOFs and PDMS was crucial for the construction of robust superhydrophobicity.
Then, atomic force microscopy (AFM) was used to further characterize the surface roughness of Ti-MOFs and PDMS coated fiber surface. The arithmetic average roughness (Ra) of the pristine cotton fabric was 11.5 nm according to the AFM image (Fig. 3a). The Ra value increased to 38.6 nm after the deposition of Ti-MOFs nano-particles. Further, after PDMS modification, the Ra value increased to 51.5 nm (Fig. 3c), which may be ascribed to the special cross-linking structure of PDMS (Cao et al. 2016), suggesting the PDMS/Ti-MOFs coating increased roughness of the fiber surface. Furthermore, SEM–EDS analysis was carried out to characterize the chemical composition of the modified cotton fabric. Figure 3e demonstrated the fabric surface contains C, O, Si and Ti elements. The appearance of the Ti and Si peaks was attributed to Ti-MOFs and PDMS, respectively. The EDS mapping of C, Ti, and Si was displayed in Fig. 3d. The EDS mapping clearly demonstrated that these elements well distributed on the scanning white square region of PDMS/(Ti-MOFs)3@cotton fabric surface.
The surface chemical composition of cotton fabrics before and after Ti-MOFs deposition and PDMS coating were further confirmed by Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS). As shown in Fig. 4a, for the pristine cotton fabric, only many strong absorption peaks appear corresponding to the stretching vibrations of –OH (3326 cm−1), and C–H (2904 cm−1). After coating with Ti-MOFs, the appearance of a new strong peak at 773 cm−1 attributed to the vibration of O–Ti–O (Dan-Hardi et al. 2009; Emam and Abdelhameed 2017). Additionally, two new peaks appeared at 1543 cm−1 and 1430 cm−1 after deposited Ti-MOFs ascribed to the COO− vibrational modes from the MOF linker (Dwyer et al. 2018), demonstrating successful synthesis of Ti-MOFs@cotton fabric.
Electronic structure and chemical composition of before and after modified fabrics were confirmed by XPS. As seen in the wide-scan XPS spectrum (Fig. 5a), two new peaks appear at 399.6 eV, and 458.7 eV corresponding to N 1s, and Ti 2p after depositing of Ti-MOFs, respectively, indicating the existence of N and Ti elements. In the wide-scan XPS spectrum of pristine cotton fabric surface, there are no N 1s and Ti 2p peak. This proved that the Ti-MOFs has been deposited on fabric surface. After PDMS coating, two new peaks were observed at 101.0 (Si 2p) and 153.0 eV (Si 2s) (Fig. 5a), indicating PDMS film has fully coated on the surface of Ti-MOFs@cotton fabric. In the Ti 2p core level spectrum (Fig. 5b), symmetric peaks for Ti 2p 3/2 and Ti 2p 1/2 appeared at 458.9 eV and 464.9 eV, respectively, which suggests the existence of a normal state of titanium IV bounded to oxygen for the titanium-oxo cluster, similar to the reported data for Ti-MOFs (Wang et al. 2015). The above results all showed the PDMS/Ti-MOFs has successful deposited on the fabric surface.
Self-cleaning properties of the superhydrophobic fabrics
The self-cleaning properties of the as-prepared PDMS/(Ti-MOFs)3@cotton fabrics were examined with red dyes powder as model contaminant (Fig. S1). In the process of self-cleaning, we can see that the red dye powder can be immediately dissolved by water drop and rolled away from the superhydrophobic fabric surface without no residue. This observation confirmed that PDMS/(Ti-MOFs)3@cotton fabrics shows good self-cleaning performance.
Continuous oil–water separation process of superhydrophobic fabrics
We built up a simple continuous oil–water separation apparatus via combining the robust superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric with three-dimensional porous sponges, as illustrated in Fig. 6. N-Hexane (dyed with oil red O) was selected as the oil target to study continuous separation of oil–water mixture. Figure 6a–c shows the continuous separation process under the assistance of peristaltic pump. When the superhydrophobic fabric bag filled with melamine sponge came into contact with the oil slick, the oil was absorbed into the superhydrophobic fabric bag and rapidly removed from the water surface. No visible water droplets were observed in the recovered hexane (Fig. 6c). After 10 cycles, the separation efficiency was still as high as more than 98.5%, demonstrating an excellent separation performance of this superhydrophobic fabric bag (Fig. 6d). To further evaluate the separation efficiency of the superhydrophobic oil absorption bag, different oils such as methyl silicone oil, vegetable oil, and gasoline were used to carry out this process. As shown in Fig. 6e, the oil absorption bag exhibited excellent adsorption capacity for various oils.
Photographs of the continuous absorption (a–c) process of oil–water mixture (n-hexane dyed with oil red O) from water by using the superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric. d The separation efficiencies for selective absorption of floating oil with repeated cycle numbers, and separation efficiency for different oils e
In additional, the superhydrophobic fabric could also separate surfactant-free oil/water emulsions (Fig. 7). The emulsion was prepared by mixing n-hexane (dyed with oil red) and water in a volume ratio of 1:9 under intensively ultrasonic dispersed for 2 h. The PDMS/(Ti-MOFs)3@cotton fabric with wrapped sponges was put into the above emulsion. During the separation process, the red color of the oil–water emulsions gradually disappeared, and the solution finally became transparent. These results indicated that the n-hexane dispersed in the water had been selectively absorbed by the superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric. In addition, as shown in Fig. 7a, c, light microscopy image showed that there were almost no oil droplets left after separation, indicating that the surfactant-free oil/water emulsions were successfully separated by the PDMS/(Ti-MOFs)3@cotton fabric. This result demonstrated the promising industrial prospects of PDMS/(Ti-MOFs)3@cotton fabric for marine oil spills separations.
Durable anti-ultraviolet properties of superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric
The improvement of anti-UV performance of cotton fabrics has been a subject of concern in textile industry. UV protection factor (UPF) was a significant parameter to measure the UV resistance for the functional textiles. The UV transmittance and UPF values spectra before and after modified are displayed in Fig. 8a, b. The UPF value of pristine cotton fabric was 7.2, while the UPF value of Ti-MOFs coated cotton fabric increased to 32.8, suggesting that the Ti-MOFs improved the UV-blocking ability of the cotton fabric.
The durability of UV resistance against washing and abrasion was studied. The UVA, UVB, and UPF were all measured for the as-prepared superhydrophobic cotton fabric after five washing cycles and 300 abrasion cycles. All the treated samples after washing and abrasion kept good UV-blocking properties (higher UPF value and lower UVA/UVB transmittance) (Fig. 8). From the Fig. 8, we can see that the as-prepared fabric displayed very good blocking effect with UPF value of 26.7 even after 300 cycles of abrasion. In addition, the as-prepared samples exhibited slightly reduction in UVR blocking property after five washing cycles (Fig. S2). These findings suggest that such a UV-resistant superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric had good abrasion resistant and laundering durability.
In addition, the K/S values of different samples after washing and abrasion were further used to evaluate the durability of colorful superhydrophobic PDMS/Ti-MOFs@cotton fabrics. As can be seen from Fig. 9a, the K/S value was still keep 4.51 after 300 cycles of abrasion. At the same time, the K/S value was still keep 3.39 after 5 cycles of washing (Fig. S3). This demonstrated that binding of Ti-MOFs to fabric was stable. At the same time, the K/S value was also tested after the modified fabric treated in different harsh conditions. As seen in Fig. 9b, except for the sample treated with pH = 12, the K/S value of all the tests keep good color depth.
Superhydrophobic stability of PDMS/(Ti-MOFs)3@cotton fabric
The stability of the colorful superhydrophobic cotton fabric by examining the changes of water contact angle against washing and abrasion. As shown in Table 1, after immersing the superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric into HCl (pH = 2), NaCl solution (saturated), and NaOH solution (pH = 12), as well as immersing the fabric in liquid nitrogen, and heated it up to 150 °C for 1 h or frozen at − 196 °C for 2 h, respectively, except for NaOH, the WCAs of all the tests were still over 150°, demonstrating good stability in harsh conditions. In addition, the mechanical stability of superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric for abrasion was tested. After the 300 cycles of abrasion (Fig. 10), the fabric can still keep a WCA of 148.27 ± 0.64°, showing good wear resistance. After laundering for 6 cycles, the WCA shows a slight decrease and the sliding angle is 5.53 ± 0.25°. Furthermore, it was found that rough surface topology structure of the PDMS/(Ti-MOFs)3@cotton fabric was retained after being repeatedly washing (inset of Fig. 10a) or scratched (inset of Fig. 10b), which was essential for the durability of the superhydrophobic fabric. All these results indicated that the as-prepared fabrics show excellent durability of superhydrophobicity and UV resistance. These may be attributed to that the strong covalent bond between the polymer PDMS and the fabric (Deng et al. 2014; Zhou et al. 2012).
On the other hand, the physical properties of pristine cotton fabric and the PDMS/(Ti-MOFs)3@cotton fabric were also investigated. As seen in Table 2, the air permeability for the modified superhydrophobic textiles was slightly lower (30.49 cm/s) than the pristine fabric (about 36.67 cm/s), suggesting the coating treatment has a slight influence for the transparency of fabrics. It is evident that the value of bending length and rigidity slightly increased after treatment, displaying the treatment had little influence on the handling properties of the fabric.
Conclusion
In conclusion, the cotton fabrics with durable superhydrophobicity and ultraviolet resistance properties have been successfully prepared by one-pot directly depositing TiMOF nanoparticles on cotton fabric surfaces and by further hydrophobic treatment with PDMS coating under room temperature without high reaction temperature and pressure. The superhydrophobic PDMS/(Ti-MOFs)3@cotton fabric showed excellent mechanical and environmental stability and exhibited self-cleaning and selectively oil–water separation. Moreover, the superhydrophobic cotton fabric exhibited very good UVR protection and had good durability against washing and abrasion with UPF = 26.7–32.8. In addition, the versatility of as-prepared cotton fabrics was stable in harsh conditions. Furthermore, the superhydrophobic coating has no or negligible adverse effect on the important textile physical properties of the cotton fabric, such as the air permeability, and flexibility. The PDMS/(Ti-MOFs)3@cotton fabrics were highly efficient in oil–water separation for various oil–water mixtures. The method was simple, and low chemical consumption and can also be in a large scale. We believed that these durable multifunctional PDMS/(Ti-MOFs)3@cotton fabric could be utilized in outdoor protect field from solar radiation and rains.
References
Abdelhameed RM, Kamel OMHM, Amr A, Rocha J, Silva AMS (2017) Antimosquito activity of a titanium–organic framework supported on fabrics. Acs Appl Mater Interface 9:22112–22120. https://doi.org/10.1021/acsami.7b03164
Agrawal N, Tan JSJ, Low PS, Fong EWM, Lai Y, Chen Z (2019) Green synthesis of robust superhydrophobic antibacterial and UV-blocking cotton fabrics by a dual-stage silanization approach. Adv Mater Interfaces 6:1900032. https://doi.org/10.1002/admi.201900032
Brandt P, Nuhnen A, Lange M, Mollmer J, Weingart O, Janiak C (2019) MOFs with potential application for SO2-separation and flue gas desulfurization. ACS Appl Mater Interface. https://doi.org/10.1021/acsami.9b00029
Bu Y, Huang J, Zhang S et al (2018) Robust superhydrophobic surface by nature-inspired polyphenol chemistry for effective oil–water separation. Appl Surf Sci 440:535–546. https://doi.org/10.1016/j.apsusc.2018.01.177
Bu Y, Zhang S, Cai Y et al (2019) Fabrication of durable antibacterial and superhydrophobic textiles via in situ synthesis of silver nanoparticle on tannic acid-coated viscose textiles. Cellulose 26:2109–2122. https://doi.org/10.1007/s10570-018-2183-7
Cao C, Ge M, Huang J et al (2016) Robust fluorine-free superhydrophobic PDMS-ormosil@fabrics for highly effective self-cleaning and efficient oil–water separation. J Mater Chem A 4:12179–12187. https://doi.org/10.1039/c6ta04420d
Chen F, Yang H, Liu X et al (2016) Facile fabrication of multifunctional hybrid silk fabrics with controllable surface wettability and laundering durability. ACS Appl Mater Interface 8:5653–5660. https://doi.org/10.1021/acsami.5b11420
Chen D, Mai Z, Liu X et al (2018) UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 25:3635–3647. https://doi.org/10.1007/s10570-018-1790-7
Cheng Q-Y, An X-P, Li Y-D, Huang C-L, Zeng J-B (2017) Sustainable and biodegradable superhydrophobic coating from epoxidized soybean oil and ZnO nanoparticles on cellulosic substrates for efficient oil/water separation. ACS Sustain Chem Eng 5:11440–11450. https://doi.org/10.1021/acssuschemeng.7b02549
Cheng Q-Y, Guan C-S, Wang M, Li Y-D, Zeng J-B (2018) Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr Polym 199:390–396. https://doi.org/10.1016/j.carbpol.2018.07.046
Cheng Q-Y, Guan C-S, Li Y-D, Zhu J, Zeng J-B (2019) Robust and durable superhydrophobic cotton fabrics via a one-step solvothermal method for efficient oil/water separation. Cellulose 26:2861–2872. https://doi.org/10.1007/s10570-019-02267-6
Cui X, Sun X, Liu L et al (2019) In-situ fabrication of cellulose foam HKUST-1 and surface modification with polysaccharides for enhanced selective adsorption of toluene and acidic dipeptides. Chem Eng J 369:898–907. https://doi.org/10.1016/j.cej.2019.03.129
Dan-Hardi M, Serre C, Frot T, Rozes L, Maurin G, Sanchez C, Férey G (2009) a new photoactive crystalline highly porous titanium(IV) dicarboxylate. J Am Chem Soc 131:10857–10859. https://doi.org/10.1021/ja903726m
Deng Z-Y, Wang W, Mao L-H, Wang C-F, Chen S (2014) Versatile superhydrophobic and photocatalytic films generated from TiO2–SiO2@PDMS and their applications on fabrics. J Mater Chem A 2:4178–4184. https://doi.org/10.1039/C3TA14942K
Dong XL, Gao SW, Huang JY et al (2019) A self-roughened and biodegradable superhydrophobic coating with UV shielding, solar-induced self-healing and versatile oil–water separation ability. J Mater Chem A 7:2122–2128. https://doi.org/10.1039/c8ta10869b
Dwyer DB, Lee DT, Boyer S, Bernier WE, Parsons GN, Jones WE (2018) Toxic organophosphate hydrolysis using nanofiber-templated UiO-66-NH2 metal–organic framework polycrystalline cylinders. ACS Appl Mater Interface 10:25794–25803. https://doi.org/10.1021/acsami.8b08167
Emam HE (2019) Generic strategies for functionalization of cellulosic textiles with metal salts. Cellulose 26:1431–1447. https://doi.org/10.1007/s10570-018-2185-5
Emam HE, Abdelhameed RM (2017) Anti-UV radiation textiles designed by embracing with nano-MIL (Ti, In)–metal organic framework. ACS Appl Mater Interface 9:28034–28045. https://doi.org/10.1021/acsami.7b07357
Gu S, Yang L, Huang W et al (2017) Fabrication of hydrophobic cotton fabrics inspired by polyphenol chemistry. Cellulose 24:2635–2646. https://doi.org/10.1007/s10570-017-1274-1
Gu JH, Fan HW, Li CX, Caro J, Meng H (2019) Robust superhydrophobic/superoleophilic wrinkled microspherical MOF@rGO composites for efficient oil–water separation. Angew Chem Int Ed 58:5297–5301. https://doi.org/10.1002/anie.201814487
Huang J, Yang Y, Yang L et al (2019) Fabrication of multifunctional silk fabrics via one step in situ synthesis of ZnO. Mater Lett 237:149–151. https://doi.org/10.1016/j.matlet.2018.11.035
Jayaramulu K, Datta KK, Rösler C, Petr M, Otyepka M, Zboril R, Fischer RA (2016) Biomimetic superhydrophobic/superoleophilic highly fluorinated graphene oxide and ZIF-8 composites for oil–water separation. Angew Chem Int Ed 55:1178–1182
Jiang C, Liu W, Yang M, Liu C, He S, Xie Y, Wang Z (2019) Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil–water separation via thiol-ene click chemistry. Appl Surf Sci 463:34–44. https://doi.org/10.1016/j.apsusc.2018.08.197
Khan MZ, Baheti V, Militky J, Ali A, Vikova M (2018) Superhydrophobicity, UV protection and oil/water separation properties of fly ash/Trimethoxy(octadecyl)silane coated cotton fabrics. Carbohydr Polym 202:571–580. https://doi.org/10.1016/j.carbpol.2018.08.145
Kim ML, Otal EH, Hinestroza JP (2019) Cellulose meets reticular chemistry: interactions between cellulosic substrates and metal–organic frameworks. Cellulose 26:123–137. https://doi.org/10.1007/s10570-018-2203-7
Lahiri SK, Zhang P, Zhang C, Liu L (2019) Robust fluorine-free and self-healing superhydrophobic coatings by H3BO3 incorporation with SiO2-Alkyl-Silane@PDMS on cotton fabric. ACS Appl Mater Interface 11:10262–10275. https://doi.org/10.1021/acsami.8b20651
Lei Z, Deng Y, Wang C (2018) Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J Mater Chem A 6:3258–3263. https://doi.org/10.1039/C7TA10566E
Li D, Guo Z (2017) Stable and self-healing superhydrophobic MnO2@fabrics: applications in self-cleaning, oil/water separation and wear resistance. J Colloid Interface Sci 503:124–130. https://doi.org/10.1016/j.jcis.2017.05.015
Li D, Guo Z (2018) Metal–organic framework superhydrophobic coating on Kevlar fabric with efficient drag reduction and wear resistance. Appl Surf Sci 443:548–557
Li J, Shi L, Chen Y, Zhang Y, Guo Z, Su B-l, Liu W (2012) Stable superhydrophobic coatings from thiol-ligand nanocrystals and their application in oil/water separation. J Mater Chem 22:9774–9781. https://doi.org/10.1039/c2jm30931a
Li Y-z, Fu Z-h, Xu G (2019) Metal–organic framework nanosheets: preparation and applications. Coord Chem Rev 388:79–106. https://doi.org/10.1016/j.ccr.2019.02.033
Liu M, Wang S, Jiang L (2017) Nature-inspired superwettability systems. Nat Rev Mater 2:17036. https://doi.org/10.1038/natrevmats.2017.36
Lu L, Hu C, Zhu Y, Zhang H, Li R, Xing Y (2018) Multi-functional finishing of cotton fabrics by water-based layer-by-layer assembly of metal–organic framework. Cellulose 25:4223–4238. https://doi.org/10.1007/s10570-018-1838-8
Mai Z, Xiong Z, Shu X et al (2018) Multifunctionalization of cotton fabrics with polyvinylsilsesquioxane/ZnO composite coatings. Carbohydr Polym 199:516–525. https://doi.org/10.1016/j.carbpol.2018.07.052
Mao J, Ge M, Huang J, Lai Y, Tang Y (2017) Constructing multifunctional MOF@rGO hydro-/aerogels by the self-assembly process for customized water remediation. J Mater Chem A 5:11873–11881
Miao W, Wang J, Liu J, Zhang Y (2018) Zeolitic imidazolate framework: self-leaning and antibacterial zeolitic imidazolate framework coatings. Adv Mater Interfaces 5:1870068
Mukherjee S, Kansara AM, Saha D et al (2016) An ultrahydrophobic fluorous metal–organic framework derived recyclable composite as a promising platform to tackle marine oil spills. Chem—A Eur J 22:10937–10943. https://doi.org/10.1002/chem.201601724
Wang H, Yuan X, Wu Y et al (2015) Facile synthesis of amino-functionalized titanium metal–organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J Hazard Mater 286:187–194. https://doi.org/10.1016/j.jhazmat.2014.11.039
Xu C, Fang R, Luque R, Chen L, Li Y (2019) Functional metal–organic frameworks for catalytic applications. Coord Chem Rev 388:268–292. https://doi.org/10.1016/j.ccr.2019.03.005
Yang M, Liu W, Jiang C, He S, Xie Y, Wang Z (2018) Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol–gel process. Carbohydr Polym 197:75–82. https://doi.org/10.1016/j.carbpol.2018.05.075
Yang M, Liu W, Jiang C, Liu C, He S, Xie Y, Wang Z (2019a) Facile preparation of robust superhydrophobic cotton textile for self-cleaning and oil–water separation. Ind Eng Chem Res 58:187–194. https://doi.org/10.1021/acs.iecr.8b04433
Yang M, Liu W, Jiang C, Xie Y, Shi H, Zhang F, Wang Z (2019b) Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil–water separation. Colloid Surf A 570:172–181. https://doi.org/10.1016/j.colsurfa.2019.03.024
Zhou H, Wang H, Niu H, Gestos A, Wang X, Lin T (2012) Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating. Adv Mater 24:2409–2412. https://doi.org/10.1002/adma.201200184
Zhou H, Wang H, Niu H, Lin T (2018) Recent progress in durable and self-healing super-nonwettable fabrics. Adv Mater Interfaces 5:1800461. https://doi.org/10.1002/admi.201800461
Zhou Q, Yan B, Xing T, Chen G (2019) Fabrication of superhydrophobic caffeic acid/Fe@cotton fabric and its oil–water separation performance. Carbohydr Polym 203:1–9. https://doi.org/10.1016/j.carbpol.2018.09.025
Zhu H, Zhang Q, Li B-G, Zhu S (2017) Engineering elastic ZIF-8-Sponges for oil–water separation. Adv Mater Interfaces. https://doi.org/10.1002/admi.201700560
Zimmermann J, Seeger S, Reifler FA (2009) Water shedding angle: a new technique to evaluate the water-repellent properties of superhydrophobic surfaces. Text Res J 79:1565–1570
Acknowledgments
The authors thank the National Key Research and Development Program of China (2016YFA0101102), and Educational Commission of Hubei Province (D20181701) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Huang, W., Guo, Z. et al. Robust fluorine-free colorful superhydrophobic PDMS/NH2-MIL-125(Ti)@cotton fabrics for improved ultraviolet resistance and efficient oil–water separation. Cellulose 26, 9335–9348 (2019). https://doi.org/10.1007/s10570-019-02707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02707-3