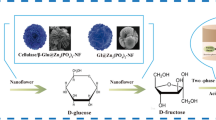
Abstract
One-pot conversion of cellulose to valued-added platform chemicals such as alkyl levulinate is a promising and challenging process of biomass utilization due to the insolubility of cellulose in alcohol. In this work, commercial heteropoly acid combined with postsynthesized Sn-Beta zeolite was employed to transform cellulose to methyl levulinate (MLE) in one pot. The synergistic catalysis of the homogeneous strong Brønsted (B) acid and the solid strong Lewis (L) acid can effectively realize the depolymerization of solid cellulose and the subsequent isomerization, dehydration and hydration steps to MLE. 55% and 62% of MLE yields were obtained from α-cellulose and microcrystalline cellulose, respectively, at 160 °C for 10 h. Various carbohydrates besides cellulose can also be efficiently converted to MLE in high yields over the bifunctional catalyst system. The effects of the amount of B acid and L acid, the reaction temperature and time and the amount of cellulose on the production of MLE were investigated in detail. Finally, the reaction pathway of cellulose to MLE was revealed. Recyclability tests indicated that the binary catalyst can be reused without decrease of catalytic activity.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of chemicals and fuels from renewable biomass resources attracts more and more attention with the decrease of fossil resources and increasing concerns on environmental problems. Nonedible lignocellulose is the main component of plant biomass, in which cellulose accounts for about 40–50% (Chen et al. 2017). Cellulose is a polysaccharide made from glucose units linked by β-1,4-glycosidic bonds, which contains abundant intra- and intermolecular hydrogen bonds. Therefore, cellulose is insoluble in most conventional solvents including water and alcohol, which renders it difficult to be transformed to small molecules (Zhou et al. 2015a). In recent years, many researchers devote a lot of efforts to transforming cellulose to value-added platform chemicals such as glucose, polyols, alkyl glucosides, 5-hydroxymethylfurfural (HMF), levulinic/lactic acid and their esters. Among these platform chemicals, alkyl levulinate is one of important versatile chemical feedstocks with wide potential industrial applications as solvents and additives in the flavoring and fragrance industries or in (bio)diesel and gasoline (Démolis et al. 2014; Deng et al. 2017). One-pot production of alkyl levulinates from cellulose in alcohol is more difficult than from the conversion of monosaccharides, the alcoholysis of furfuryl alcohol, or the esterification of levulinic acid due to the insolubility of cellulose (Scheme S1); but this pathway is more simple and economic than others because it does not need to separate these intermediate products. One-pot conversion of cellulose to alkyl levulinate is a complex process containing several steps including depolymerization of β-1,4-glycosidic bonds, dehydration of monosaccharide and rehydration of furfural derivative, etc. (Tominaga et al. 2016; Zhu et al. 2017). Various acids including homogeneous acids and solid acids are employed to accomplish this process. Démolis et al. (2014) reviewed the related works in literature before 2014, and many new developments emerged continuously in recent 4–5 years. H2SO4 is the most widely used mineral acid catalyst for the conversion of cellulose to alkyl levulinates (Hishikawa et al. 2013; Kang and Yu 2015; Xu et al. 2015a). The easily separated solid acids such as modified heteropoly acids (HPAs), sulfated metal oxides, zeolites, niobium-based phosphate and sulfonated organic polymers were also employed to catalyze cellulose conversion to alkyl levulinates (Ding et al. 2015; Morales et al. 2014; Peng et al. 2011; Saravanamurugan and Riisager 2013; Song et al. 2016; Xu et al. 2015b; Yu et al. 2017; Zhang et al. 2018). However, solid catalysts are generally less active than homogeneous catalysts due to the difficult accessibility of the active sites to the glycosidic bonds of solid cellulose. Additionally, bifunctional catalyst systems with Brønsted (B) and Lewis (L) acidities have been designed for efficient conversion of glucose based substrates, where L acid sites promote the isomerization of glucose to fructose that is more facile to be converted to alkyl levulinate. For example, sole B acid of p-toluenesulfonic acid gave 20% of methyl levulinate (MLE) yield from cellulose conversion at 180 °C for 5 h; the MLE yield was increased to 70% by addition of an L acid of In(OTf)3 as co-catalyst (Tominaga et al. 2011). H-USY with extraframework Al as L acid sites and framework Al as B acid sites showed 49% of MLE yield from glucose conversion at 160 °C for 20 h; however, the solid H-USY is insufficient for cellulose conversion and only 13% of MLE yield was obtained (Saravanamurugan and Riisager 2013). Conformal sulphated zirconia monolayers supported on SBA-15 with L and B acidity gave 25% of ethyl levulinate from glucose at around 140–150 °C (Morales et al. 2014). Over zirconia-zeolite hybrid ZrY6(0.5) with L and B acidity, 67% of MLE yield was obtained from microwave-assisted conversion of glucose at 180 °C for 3 h; whereas MLE yield (27%) was still low for cellulose conversion (Li et al. 2017). Our previous work also reported B and L acid sites synergistically catalyzed glucose conversion to MLE. With Al2(SO4)3 as catalyst, 64% of MLE yield was obtained from glucose at 160 °C for 2.5 h; but at the same reaction conditions, only 19% of MLE was obtained from α-cellulose conversion due to ineffective depolymerization and decrystallization of α-cellulose (Zhou et al. 2014). Solid catalyst of mesoporous H-USY showed lower activity than homogeneous Al2(SO4)3 for glucose conversion, which gave 32% of MLE yield at 160 °C for 5 h; moreover, the catalyst was ineffective for depolymerization of β-glycosidic bonds, and even for cellobiose conversion only 20% of MLE yield was obtained (Zhou et al. 2015b). Very recently, our group employed dual solid strong acids (SO42−/ZrO2 and Sn-Beta) to catalyze glucose conversion, 49% of MLE yield was obtained at 160 °C for 5 h. The activity of the dual solid acid catalyst for depolymerization of β-glycosidic bonds was higher than mesoporous H-USY due to the strong B acidity of SO42−/ZrO2; 37% of MLE yield was achieved from cellobiose conversion (Jiang et al. 2018).
Therefore, in order to efficient conversion of recalcitrant cellulose and improvement of the yield of alkyl levulinate, it is necessary to ensure good accessibility of the active sites to β-glycosidic bonds of cellulose, strong B acidity for depolymerization of β-glycosidic bonds and subsequent dehydration and hydration steps, and enough L acidity for glucose isomerization to fructose. It is known that HPAs have strong B acidity, high proton mobility, and high solubility in various solvents (Deng et al. 2012; Hu et al. 2015; Okuhara 2002; Wang and Yang 2015), which is beneficial for depolymerization of solid cellulose. In addition, HPAs are easy to be separated by extraction with diethyl ether for reuse compared to mineral acids. These fascinating architectures of HPAs attract much interest of researchers, which are widely used in the field of biomass conversion in recent years. For example, Deng et al. (2010, 2011) used HPAs to convert cellulose to methyl glucosides (MGs) in methanol. Tian et al. (2010) employed H3PW12O40 (HPW) to catalyze the hydrolysis of cellulose to glucose in water. Li et al. (2012) reported low-temperature hydrolysis of cellulose catalyzed by concentrated HPW under microwave irradiation. Ogasawara et al. (2011) utilized concentrated aqueous solution of heteropolyacids to saccharify natural lignocellulose biomass and polysaccharides. Zhang et al. (2012) directly converted cellulose to glycolic acid with phosphomolybdic acid as catalyst in a water medium and oxygen atmosphere. Besides, metal cation and quaternary ammonium cation modified HPAs and supported HPAs are also usually employed to catalyze the conversion of cellulose and other carbohydrates to valuable chemicals (Rataboul and Essayem 2011; Sun et al. 2016; Zhang et al. 2016). HPAs in combination with Ru/C were utilized to realize the depolymerization and hydrogenation of cellulose to sugar alcohols (Geboers et al. 2010).
In the present work, a bifunctional catalyst system composed of commercial HPA (HPW or H4SiW12O40 (HSiW)) as a B acid and Sn-Beta as an L acid is designed to catalyze the direct conversion of cellulose to MLE. The strong B acidity and the solubility of HPA and the superiority of Sn-Beta for glucose isomerization to fructose make the bifunctional catalyst system highly active for cellulose conversion to MLE. The B and L acidity was optimized firstly, and then the effects of the reaction conditions including the reaction temperature and time and the amount of cellulose on the production of MLE were investigated. The bifunctional catalyst system was also tested for conversion of other carbohydrates to MLE. Based on the obtained results, the reaction pathway of cellulose conversion to MLE over the bifunctional catalyst was revealed. Finally, the recycling of the bifunctional catalyst system was investigated.
Experimental section
Materials
H3PW12O40 (HPW), H4SiW12O40 (HSiW), methyl α-glucoside (α-MG), methyl β-glucoside (β-MG), fructose, levulinic acid (LeA) and furfural (FF) were purchased from Aladdin Reagent Co. (China). α-Cellulose with degree of polymerization (DP) of 1177 and microcrystalline cellulose (MCC) with DP of ~ 200 were purchased from Sigma-Aldrich and Alfa Aesar, respectively. Glucose monohydrate was purchased from Tianjin Kermel Chemical Reagent, China. Mannose and sucrose were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Cellobiose was purchased from J & K Scientific Ltd, China. Methyl levulinate (MLE) and methyl lactate (MLA) were purchased from TCI Shanghai, China. Starch, SnCl4·5H2O, nitric acid (65–68%), sulphuric acid (98%), diethyl ether, methanol and naphthalene were purchased from commercial resources (AR grade). All chemicals were used as purchased without further purification.
Sn-Beta was prepared from Al-Beta by the postsynthesis method. Al-Beta zeolite with nSi/nAl ratio of 13.8 was purchased from Nankai University Catalyst Co. (China). The detailed procedure for preparing Sn-Beta was given in supporting information according to literature (Yang et al. 2017, 2018). The nominal content of Sn is 2 wt%.
Reaction test and product analysis
Transformation of cellulose was performed in an autoclave reactor (80 mL) with a Teflon liner. Methanol, cellulose, and catalyst (Sn-Beta and/or B acid) were added into the autoclave successively under stirring, and then the autoclave was sealed. The atmosphere in the autoclave was replaced four times with N2 and then 0.4 MPa N2 was charged. Afterwards, the reactor was heated to the desired temperature with a rate of 4 °C min−1. After the reaction was over, the reactor was quenched in ice water to room temperature. The reaction mixture was diluted by methanol and centrifugated to obtain solid and liquid solution. The solid is composed of unreacted cellulose, Sn-Beta and probably a small amount of solid humins. It was washed three times with methanol and then dried at 100 °C overnight. The conversion of cellulose was estimated assuming the weight of Sn-Beta was constant before and after reaction and the weight of solid humins was negligible. Therefore, the weight of the unreacted cellulose equal to the weight of the dried solid subtracting the weight of Sn-Beta. In this way, the conversion of cellulose was calculated. The products in liquid solution were analyzed by GC and HPLC, respectively. Yields of MLE and MLA were quantified using naphthalene as the internal standard on a GC equipped with an FID detector. Other products including α-MG, β-MG, LeV and FF were detected and quantified with the external standard method on a Shimadzu LC-20AT HPLC analysis system equipped with an Aminex HPX-87H column (300 mm × 7.8 mm) and a refractive index detector (RID-10A). H2SO4 (0.005 M, 0.5 mL min−1) was used as the mobile phase under the column temperature of 60 °C. Yields for all products are mole yield except for MLA. The yield of MLA is carbon yield because one C6 unit can produce two MLA molecules. The calculating formulas are as follows.
Results and discussion
Promotion effect of Sn-Beta on the conversion of α-cellulose to MLE catalyzed by various B acids
Conversion of α-cellulose to MLE over different catalysts is listed in Table 1. In the blank experiment (entry 1), only a very small amount of α-cellulose was converted and no product was detected by GC and HPLC. With the addition of only L acid of Sn-Beta, the conversion of α-cellulose is still very low and no product was observed (entry 2). This is different from glucose as substrate, where retro-aldol products such as MLA were formed (Jiang et al. 2018; Yang et al. 2017, 2018). The result indicates L acid of Sn-Beta is almost incapable to depolymerize β-glycosidic bonds of cellulose. Contrarily, the sole B acid such as H2SO4, HPW or HSiW can effectively depolymerize cellulose, and the conversion of α-cellulose was about 70% (entries 3, 5 and 7). The yield of MLE was ~ 20% over sole B acid. A considerable amount of MG was produced over sole B acid catalyst; moreover, the amount of α-MG was always larger than β-MG due to that α-MG is more stable in view of steric hindrance. This is in accordance with that reported in literature (Deng et al. 2010, 2011). The amount of MG over HPAs was higher than over H2SO4 probably due to the stronger acidity of HPAs than H2SO4 (Okuhara 2002). A few of LeA and FF were also observed. The formation of FF from hexoses has been reported in literature, but the detailed mechanism for FF formation is still unclear (Jin and Enomoto 2011). The total yields of all products detected by GC and HPLC are much lower than the corresponding conversion of α-cellulose over sole B acid. It implies most cellulose was depolymerized to soluble oligomers that cannot be detected by GC and HPLC (Jing et al. 2016). The yield of MLE increased more than twice in the co-existance of L acid and B acid (entries 4, 6 and 8), proving the significant promotion effect of L acid of Sn-Beta on the formation of MLE. Meanwhile, the amount of MG decreased remarkably. It is known that Sn-Beta is an excellent catalyst for aldose-ketose isomerization (Moliner et al. 2010), so the formed MG can be quickly transformed to MLE via fructose. In addition, the amount of MLA increased in the presence of Sn-Beta, which is a retro-aldol product of hexose (Yang et al. 2017, 2018). The retro-aldol and the dehydration of hexose are two competing reaction pathways in the co-presence of B acid and L acid; but, the retro-aldol reaction will be restrained in the presence of strong B acid (Jiang et al. 2018). So, the yield of MLA is low in the present work. In addition, the activity of dual solid acids for the conversion of cellulose (entries 9 and 10) is much lower than homogeneous B acid contained catalyst due to the difficult accessibility of B acid sites of solid B acid to the β-glycosidic bonds of solid cellulose. The higher activity of Amberlyst-15 contained system than SO42−/ZrO2 contained system can be ascribed to the large porosity and the organic properties of the former.
Acidity and amount of HSiW and Sn-Beta
XRD pattern, N2 physisorption and structure parameters of Sn-Beta are shown in supporting information (Figs. S1, S2 and Table S1). It can be seen that Sn-Beta has pure *BEA structure. N2 physisorption isotherm implies that Sn-Beta is a microporous material. The acidity of HSiW and Sn-Beta was studied by FT-IR of pyridine adsorption (Fig. S3). Sn-Beta mainly has L acid sites (0.14 mmol g−1, calculated by the peak at 1450 cm−1 at 150 °C) with trace amount of B acid sites derived from the residue Al (Fig. S3a). After loading with HSiW, the strength of peak at 1450 cm−1 does not change obviously, implying that the L acidity of HSiW was much weaker than that of Sn-Beta. However, a large number of B acid sites were observed (Fig. S3b). Moreover, the strength of the band (1540 cm−1) ascribed to B acid sites almost does not decrease with elevation of temperature, implying that HSiW is a strong B acid. Given complete dissociation of HSiW, the density of B acid sites is 1.39 mmol g−1.
Both B acid and L acid are important for efficient conversion of cellulose to MLE, therefore the amounts of HSiW and Sn-Beta were optimized. First, fixing the amount of Sn-Beta, the effect of HSiW amount was investigated (Fig. 1a). At the initial stage, the conversion of α-cellulose and the yield of MLE increased quickly, proving again the significance of strong B acid for cleavage of β-glycosidic bonds of cellulose and subsequent dehydration and hydration reactions. Once the weight of HSiW exceeded 56 mg (the molar ratio of B/L acid sites = 5.6), the conversion of α-cellulose and the yield of MLE increased slowly. The amount of MG increased first and then decreased to zero with the increase in the concentration of HSiW, showing that B acid catalyzed the depolymerization of cellulose to the intermediate MG and MG can be further converted to MLE. The yield of MLA was low and reduced with the increase in the concentration of HSiW due to that the formation of MLA was restrained in the presence of strong B acid (Jiang et al. 2018).
The amount of Sn-Beta also affects the yield of MLE remarkably (Fig. 1b). The yield of MLE increased with the amount of Sn-Beta and reached a maximum of 55% at 50 mg of Sn-Beta (the molar ratio of B/L acid sites = 22). Afterwards, the yield of MLE reduced slightly probably because excess L acid sites led to more side-reactions such as formation of more MLA and humins. Meanwhile, the yield of MG decreased from 21.1% (Table 1, entry 7) to ~ 0. The yield of MLA increased slightly with the amount of Sn-Beta, but its amount always maintained at a low level (< 5%). These results confirm again the ability of Sn-Beta zeolite for isomerization and retro-aldol reactions of sugars, and the cooperative catalysis of B acid and L acid in the conversion of carbohydrates containing glucose units to alkyl levulinate, as reported in literature (Jiang et al. 2018; Liu et al. 2017, 2018; Tominaga et al. 2011).
Effects of reaction temperature and time
The effects of the reaction temperature and time on the conversion of α-cellulose and the yields of two main products of MLE and MG are depicted in Fig. 2. Higher temperature are favorable for the depolymerization of β-1,4-glycosidic bonds of cellulose and the formation of MLE. Shorter time was needed to obtain identical conversion of α-cellulose and yield of MLE at higher temperature. The yield of MLE decreased slightly at 170 °C when the reaction time exceeded 5 h. This implies MLE is unstable and can be further converted to other byproducts at such reaction conditions. Similar phenomenon was also reported in literature (Dai et al. 2018; Song et al. 2016; Yang et al. 2019). The maximum yield of MLE (54–55%) was obtained at 160 °C for 10 h or 170 °C for 5 h, which is higher than most results reported in literature (Table S2); moreover, higher MLE yield (62%) can be obtained over the present catalyst system if MCC is used as substrate as discussed below. The yield of MG reduced as the reaction time increased, which is consistent with the literature (Deng et al. 2010). It confirms again MG is an intermediate for MLE production.
Effects of α-cellulose loading
It is highly desirable that one pot can convert a large amount of substrate and obtain a lot of target product. Thus, the effect of α-cellulose loading on the production of MLE was investigated. The results in Fig. 3 show that the conversion of α-cellulose decreased slowly when the amount of α-cellulose increased. This can be ascribed to the relatively insufficient B acid sites for depolymerization of α-cellulose. The yield of MLE decreased more significantly than the conversion of α-cellulose, whereas the amount of the intermediate MG did not increase remarkedly. This suggests more oligosaccharides were formed, which could not be quickly transformed further due to the insufficient B acidity of the catalyst. When the reaction time is prolonged to 10 h, these oligosaccharides were gradually transformed and thus the yield of MLE was increased from 34 to 45% at the amount of α-cellulose of 1.11 g (corresponding to the content of 9.3 wt%).
Conversion of various carbohydrates to MLE
The catalytic performance of HSiW combined with Sn-Beta was also tested for conversion of other carbohydrates to MLE. First, cellobiose, the basic repeating structural unit of cellulose and a d-glucose dimer linked by β-1,4-glycosidic bond (Garves 1988), was used as a substrate to producing MLE (Fig. 4a). Cellobiose is much more facile to be converted than α-cellulose, and 87% of cellobiose was converted just as the reaction temperature reached 150 °C. The intermediate MG was generated as a main product with a yield of 42% and only 2% of MLE yield was produced at this moment. At the reaction time of 0.5 h, cellobiose was completely converted; meanwhile the yield of MLE increased to 11%, but the yield of MG was unchanged. It indicates that part MG was transformed to MLE, meanwhile some MG was formed again from cellobiose. Afterwards, the amount of MG decreased accompanying with the increase of MLE yield when the reaction time was extended further. The yield of MLE reached a maximum of 69% at the reaction time of 7 h. Glucose is the monomer constituting cellulose. Its conversion to MLE is illustrated in Fig. 4b. Glucose is more easily converted than cellobiose. Moreover, higher MLE yield was obtained from glucose than from cellobiose at the same reaction time. It shows that production of MLE from glucose is easier than from cellobiose. The yield of MLE increased quickly accompanying with the fast decrease in the yield of MG at the initial reaction time of 3 h. Further increasing the reaction time to 10 h, MG disappeared and the yield of MLE reached 72%.
The conversion of other carbohydrates is listed in Table 2. The yield of MLE from glucose, mannose and fructose was 62%, 65% and 69% (entries 3 and 4), respectively, at 150 °C for 3 h. This indicates Sn-Beta can effectively catalyze the isomerization of glucose and mannose to fructose in methanol, and fructose is more facile to be converted to MLE (entry 2). It is worth mentioning that HSiW itself can catalyze efficient conversion of fructose to MLE (entry 1); this is consistent with the results that fructose can be converted smoothly in the presence of only strong B acid (Liu et al. 2013a, b). A slight higher MLE yield (78%) is obtained over solo HSiW than HSiW combined with Sn-Beta; it implies that the presence of L acid leads to more side reactions such as the formation of MLA via retro-aldol reaction. When α-MG was used as a substrate (entry 6), the yield of MLE is lower than from monosaccharides. This is consistent with our previous results that formation of MLE via MG pathway is slower than via fructose pathway (Jiang et al. 2018). Sucrose, a disaccharide, made of a glucose unit and a fructose unit is also easily transformed (entries 7 and 8) just like monosaccharide. The α,β-1,2-glycosidic bond in sucrose is more facile to be depolymerized than β-1,4-glycosidic bond of cellobiose, thus higher MLE yield is achieved from the former at the same reaction conditions. Starch, a macromolecular polysaccharide containing α-glycosidic bonds, is much more difficult to be converted (entry 9) than monosaccharide and disaccharide, but its conversion is much more easy than cellulose consisted of β-1,4-glycosidic bonds. MCC is considered as the most recalcitrant cellulose with high crystallinity, which can also be depolymerized effectively over HSiW and then be converted to MLE over HSiW combined with Sn-Beta catalyst (entries 10 and 11). In comparison with α-cellulose, slight higher MLE yield was obtained from MCC due to its lower DP.
Reaction pathway of cellulose conversion to MLE catalyzed by HPA and Sn-Beta
Depolymerization of cellulose is the initial key step for the conversion of cellulose to small molecule compound, which is mainly catalyzed by B acid. The acidity of H2SO4, HPW and HSiW in methanol (theoretical [H+] = 0.01 mol L−1) was detected. The pH value decreased in the order of H2SO4 (1.31) < HPW (0.42) < HSiW (0.30) (Table S3). The reaction rate of glycosidic bond cleavage for H2SO4, HPW and HSiW (Table S4) is accordant with the order of pH value of these acids in methanol. Additionally, no MG was given over Sn-Beta, implying L acid cannot effectively catalyze depolymerization of cellulose. These results proved that dissociated protons are active sites for the depolymerization of cellulose in methanol to MG.
The conversion of α-MG and glucose over sole Sn-Beta and HSiW was studied. No fructose and its derivates were given over Sn-Beta with MG as a substrate (Table S5), indicating that the catalyst cannot directly catalyze the isomerization of MG. But, fructose was quickly formed from glucose over Sn-Beta (Fig. S5a). MG is in a ring structure which cannot be converted to a linear structure due to that the α/β hydroxyl was etherified with methanol (Guo et al. 2018; Josephson et al. 2018). The isomerization of glucose to fructose involved 1,2-intramolecular hydride shift over L acid catalyst (Li et al. 2014), however, the path is impossible for MG. The hydrolysis of MG to glucose was studied over HSiW. It indicated that MG was hydrolyzed to glucose quickly using the strong B acid catalyst (Table S5). The conversion of glucose over HSiW was also studied (Fig. S5b); MG was formed quickly and the amount of MLE increased slowly accompanying the decrease of MG amount. In comparison with the result over HSiW combined with Sn-Beta catalyst (Fig. 4b), the amount of MLE is much lower over HSiW alone. Contrarily, fructose can be quickly transformed to MLE over HSiW alone (Table 2, entry 1). These results proved Sn-Beta mainly plays an important role in the isomerization of glucose to fructose.
For the conversion of cellulose to MLE, water was mainly from the dehydration of fructose intermediate. L acid of Sn-Beta provided the active sites for the isomerization of glucose to fructose, whereas HSiW showed high activity and selectivity for the dehydration of fructose to MLE. Additionally, MLA was mainly produced over Sn-Beta. The initial reaction rate on each step for the formation of MLE and the by-product of MLA over Sn-Beta and HSiW is given in Table S5.
According to the above results, the possible reaction pathway of cellulose conversion in methanol to MLE catalyzed by HPA and Sn-Beta is illustrated in Scheme 1. Cellulose was firstly depolymerized to MG and then MG was hydrolyzed to glucose, both of which were catalyzed by HSiW. Glucose was isomerized to fructose over the L acid sites of Sn-Beta. Finally, fructose was converted to MLE over the B acid sites of HSiW. If there is no L acid in the reaction system, MG can also be converted to MLE. This can be corroborated by the formation of MLE over only B acid catalyst (Table 2, entry 5 and Fig. S4); but this pathway is much slower than L acid catalyzed one. Therefore, Sn-Beta zeolite showed notable promotion effect on the conversion of cellulose to MLE catalyzed by heteropoly acid.
Reusability of the catalyst
The reusability of the catalyst was investigated in the conversion of α-cellulose to MLE. After each reaction, Sn-Beta along with the unconverted cellulose was separated by centrifugation, and washed with DMSO and methanol for three times, separately. Finally, it was dried at 120 °C overnight for the next reaction run. The amount of the unconverted cellulose equaled to the weight of the dried solid minus the weight of Sn-Beta. In the next run the total weight of cellulose included the weight of the unconverted cellulose. HSiW in the solution was separated as following procedure. The solvent was removed by rotary evaporation. Then MLE and by-products were separated by extraction with cyclohexane for five times. The remaining solid was recycled for the next reaction run. In order to prove the stability of the catalyst system, the yield of MLE in recycling reaction was controlled below 20% (Fig. 5). It can be seen that the yield of MLE has no decrease after 5 runs. XRD pattern of Sn-Beta after the 1st run is shown in Fig. S1. The structure is kept well except for slight decrease in the crystallinity. XRD pattern of Sn-Beta after five runs was also measured (Fig. S6). The structure is still preserved well. The surface area of the spent Sn-Beta after five runs lost slightly (about 7%), while the micropore volume is close to that of the fresh Sn-Beta (Table S1). Analyzing the reaction mixture of the 1st run by ICP, only 0.43% Sn leached from Sn-Beta. These results suggest that Sn-Beta is stable enough to tolerate the strong acid environment and the bifunctional catalyst system is reusable for the transformation of cellulose to MLE in methanol.
Conclusion
In conclusion, one-pot conversion of solid cellulose to MLE can be fulfilled efficiently in methanol over heteropoly acid combined with Sn-Beta zeolite. Homogeneous heteropoly acid provides B acid sites for primary depolymerization of β-1,4-glycosidic bonds and subsequent hydrolysis and dehydration/hydration reactions, while solid Sn-Beta zeolite offers L acid sites for efficient isomerization of glucose to fructose, which promotes effectively the formation of MLE.
References
Chen SS, Maneerung T, Tsang DCW, Ok YS, Wang CH (2017) Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem Eng J 328:246–273. https://doi.org/10.1016/j.cej.2017.07.020
Dai J, Peng LC, Li H (2018) Intensified ethyl levulinate production from cellulose using a combination of low loading H2SO4 and Al(OTf)3. Catal Commun 103:116–119. https://doi.org/10.1016/j.catcom.2017.10.007
Démolis A, Essayem N, Rataboul F (2014) Synthesis and applications of alkyl levulinates. ACS Sustain Chem Eng 2:1338–1352. https://doi.org/10.1021/sc500082n
Deng WP, Liu M, Zhang QH, Tan XS, Wang Y (2010) Acid-catalysed direct transformation of cellulose into methyl glucosides in methanol at moderate temperatures. Chem Commun 46:2668–2670. https://doi.org/10.1039/b925723c
Deng WP, Liu M, Zhang QH, Wang Y (2011) Direct transformation of cellulose into methyl and ethyl glucosides in methanol and ethanol media catalyzed by heteropolyacids. Catal Today 164:461–466. https://doi.org/10.1016/j.cattod.2010.10.055
Deng WP, Zhang QH, Wang Y (2012) Polyoxometalates as efficient catalysts for transformations of cellulose into platform chemicals. Dalton Trans 41:9817–9831. https://doi.org/10.1039/C2DT30637A
Deng L, Chang C, An R, Qi XG, Xu GZ (2017) Metal sulfates-catalyzed butanolysis of cellulose: butyl levulinate production and optimization. Cellulose 24:5403–5415. https://doi.org/10.1007/s10570-017-1530-4
Ding DQ, Xi JX, Wang JJ, Liu XH, Lu GZ, Wang YQ (2015) Production of methyl levulinate from cellulose: selectivity and mechanism study. Green Chem 17:4037–4044. https://doi.org/10.1039/c5gc00440c
Garves K (1988) Acid catalyzed degradation of cellulose in alcohols. J Wood Chem Technol 8:121–134. https://doi.org/10.1080/02773818808070674
Geboers J, de Vyver SV, Carpentier K, de Blochouse K, Jacobs P, Sels B (2010) Efficient catalytic conversion of concentrated cellulose feeds to hexitols with heteropoly acids and Ru on carbon. Chem Commun 46:3577–3579. https://doi.org/10.1039/c001096k
Guo Q, Ren LM, Kumar P, Cybulskis VJ, Mkhoyan KA, Davis ME, Tsapatsis M (2018) A chromium hydroxide/MIL-101(Cr) MOF composite catalyst and its use for the selective isomerization of glucose to fructose. Angew Chem Int Ed 57:4926–4930. https://doi.org/10.1002/anie.201712818
Hishikawa Y, Yamaguchi M, Kubo S, Yamada T (2013) Direct preparation of butyl levulinate by a single solvolysis process of cellulose. J Wood Sci 59:179–182. https://doi.org/10.1007/s10086-013-1324-8
Hu L, Lin L, Wu Z, Zhou SY, Liu SJ (2015) Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl Catal B Environ 174–175:225–243. https://doi.org/10.1016/j.apcatb.2015.03.003
Jiang LY, Zhou LP, Chao JY, Zhao HT, Lu TL, Su YL, Yang XM, Xu J (2018) Direct catalytic conversion of carbohydrates to methyl levulinate: synergy of solid Brønsted acid and Lewis acid. Appl Catal B Environ 220:589–596. https://doi.org/10.1016/j.apcatb.2017.08.072
Jin F, Enomoto H (2011) Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions. Energy Environ Sci 4:382–397. https://doi.org/10.1039/c004268d
Jing SS, Cao XF, Zhong LX, Peng XW, Zhang XT, Wang S, Sun RC (2016) In situ carbonic acid from CO2. ACS Sustain Chem Eng 4:4146–4155. https://doi.org/10.1021/acssuschemeng.6b00623
Josephson TR, DeJaco RF, Pahari S, Ren LM, Guo Q, Tsapatsis M, Siepmann JI, Vlachos DG, Caratzoulas S (2018) Cooperative catalysis by surface Lewis acid/silanol for selective fructose etherification on Sn-SPP zeolite. ACS Catal 8:9056–9065. https://doi.org/10.1021/acscatal.8b01615
Kang SM, Yu J (2015) Effect of methanol on formation of levulinates from cellulosic biomass. Ind Eng Chem Res 54:11552–11559. https://doi.org/10.1021/acs.iecr.5b03512
Li XT, Jiang YJ, Wang LL, Meng LQ, Wang W, Mu XD (2012) Effective low-temperature hydrolysis of cellulose catalyzed by concentrated H3PW12O40 under microwave irradiation. RSC Adv 2:6921–6925. https://doi.org/10.1039/c2ra21022c
Li YP, Head-Gordon M, Bell AT (2014) Analysis of the reaction mechanism and catalytic activity of metal-substituted Beta zeolite for the isomerization of glucose to fructose. ACS Catal 4:1537–1545. https://doi.org/10.1021/cs401054f
Li H, Fang Z, Luo J, Yang S (2017) Direct conversion of biomass components to the biofuel methyl levulinate catalyzed by acid-base bifunctional zirconia-zeolites. Appl Catal B Environ 200:182–191. https://doi.org/10.1016/j.apcatb.2016.07.007
Liu RL, Chen JZ, Huang X, Chen LM, Ma LL, Li XJ (2013a) Conversion of fructose into 5-hydroxymethylfurfural and alkyl levulinates catalyzed by sulfonic acid-functionalized carbon materials. Green Chem 15:2895–2903. https://doi.org/10.1039/c3gc41139g
Liu Y, Liu CL, Wu HZ, Dong WS (2013b) An efficient catalyst for the conversion of fructose into methyl levulinate. Catal Lett 143:1346–1353. https://doi.org/10.1007/s10562-013-1094-3
Liu J, Yang BB, Wang XQ, Liu C, Yang RZ, Dong WS (2017) Glucose conversion to methyl levulinate catalyzed by metal ion-exchanged montmorillonites. Appl Clay Sci 141:118–124. https://doi.org/10.1016/j.clay.2017.02.017
Liu J, Wang XQ, Yang BB, Liu CL, Xu CL, Dong WS (2018) Highly efficient conversion of glucose into methyl levulinate catalyzed by tin-exchanged montmorillonite. Renew Energy 120:231–240. https://doi.org/10.1016/j.renene.2017.12.104
Moliner M, Román-Leshkov Y, Davis ME (2010) Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc Natl Acad Sci USA 107:6164–6168. https://doi.org/10.1073/pnas.1002358107
Morales G, Osatiashtiani A, Hernández B, Iglesias J, Melero JA, Paniagua M, Brown DR, Granollers M, Lee AF, Wilson K (2014) Conformal sulfated zirconia monolayer catalysts for the one-pot synthesis of ethyl levulinate from glucose. Chem Commun 50:11742–11745. https://doi.org/10.1039/c4cc04594g
Ogasawara Y, Itagaki S, Yamaguchi K, Mizuno N (2011) Saccharification of natural lignocellulose biomass and polysaccharides by highly negatively charged heteropolyacids in concentrated aqueous solution. Chemsuschem 4:519–525. https://doi.org/10.1002/cssc.201100025
Okuhara T (2002) Water-tolerant solid acid catalysts. Chem Rev 102:3641–3666. https://doi.org/10.1021/cr0103569
Peng LC, Lin L, Li H, Yang QL (2011) Conversion of carbohydrates biomass into levulinate esters using heterogeneous catalysts. Appl Energy 88:4590–4596. https://doi.org/10.1016/j.apenergy.2011.05.049
Rataboul F, Essayem N (2011) Cellulose reactivity in supercritical methanol in the presence of solid acid catalysts: direct synthesis of methyl-levulinate. Ind Eng Chem Res 50:799–805. https://doi.org/10.1021/ie101616e
Saravanamurugan S, Riisager A (2013) Zeolite catalyzed transformation of carbohydrates to alkyl levulinates. ChemCatChem 5:1754–1757. https://doi.org/10.1002/cctc.201300006
Song CH, Liu SJ, Peng XW, Long JX, Lou WY, Li XH (2016) Catalytic conversion of carbohydrates to levulinate ester over heteropolyanion-based ionic liquids. Chemsuschem 9:1–11. https://doi.org/10.1002/cssc.201601080
Sun Z, Xue LF, Wang ST, Wang XH, Shi JY (2016) Single step conversion of cellulose to levulinic acid using temperature-responsive dodeca-aluminotungstic acid catalysts. Green Chem 18:742–752. https://doi.org/10.1039/c5gc01730k
Tian J, Wang JH, Zhao S, Jiang CY, Zhang X, Wang XH (2010) Hydrolysis of cellulose by the heteropoly acid H3PW12O40. Cellulose 17:587–594. https://doi.org/10.1007/s10570-009-9391-0
Tominaga K, Mori A, Fukushima Y, Shimada S, Sato K (2011) Mixed-acid systems for the catalytic synthesis of methyl levulinate from cellulose. Green Chem 13:810–812. https://doi.org/10.1039/c0gc00715c
Tominaga K, Nemoto K, Kamimura Y, Yamada A, Yamamoto Y, Sato K (2016) A practical and efficient synthesis of methyl levulinate from cellulosic biomass catalyzed by an aluminum-based mixed acid catalyst system. RSC Adv 6:65119–65124. https://doi.org/10.1039/c6ra15638j
Wang SS, Yang GY (2015) Recent advances in polyoxometalate-catalyzed reactions. Chem Rev 115:4893–4962. https://doi.org/10.1021/cr500390v
Xu GZ, Chang C, Fang SQ, Ma XJ (2015a) Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose. Renew Energy 78:583–589. https://doi.org/10.1016/j.renene.2015.01.054
Xu XL, Zhang XL, Zou WJ, Yue HJ, Tian G, Feng SH (2015b) Conversion of carbohydrates to methyl levulinate catalyzed by sulfated montmorillonite. Catal Commun 62:67–70. https://doi.org/10.1016/j.catcom.2015.01.011
Yang XM, Bian JJ, Huang JH, Xin WW, Lu TL, Chen C, Su YL, Zhou LP, Wang F, Xu J (2017) Fluoride-free and low concentration template synthesis of hierarchical Sn-Beta zeolites. Green Chem 19:692–701. https://doi.org/10.1039/c6gc02437h
Yang XM, Liu Y, Li XX, Ren JX, Zhou LP, Lu TL, Su YL (2018) Synthesis of Sn-containing nano-sized Beta zeolite as efficient catalyst for transformation of glucose to methyl lactate. ACS Sustain Chem Eng 6:8256–8265. https://doi.org/10.1021/acssuschemeng.8b00177
Yang XM, Yang JR, Gao BB, Lu TL, Zhou LP (2019) Conversion of glucose to methyl levulinate over Sn–Al–β zeolite: role of Sn and mesoporosity. Catal Commun 130:105783. https://doi.org/10.1016/j.catcom.2019.105783
Yu F, Zhong RY, Chong H, Smet M, Dehaen W, Sels BF (2017) Fast catalytic conversion of recalcitrant cellulose into alkyl levulinates and levulinic acid in presence of soluble and recoverable sulfonated hyperbranched poly(arylene oxindole)s. Green Chem 19:153–163. https://doi.org/10.1039/C6GC02130A
Zhang JZ, Liu X, Sun M, Ma XH, Han Y (2012) Direct conversion of cellulose to glycolic acid with a phosphomolybdic acid catalyst in a water medium. ACS Catal 2:1698–1702. https://doi.org/10.1021/cs300342k
Zhang XY, Zhang D, Sun Z, Xue LF, Wang XH, Jiang ZJ (2016) Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl Catal B Environ 196:50–56. https://doi.org/10.1016/j.apcatb.2016.05.019
Zhang XY, Li Y, Xue LF, Wang ST, Wang XH, Jiang ZJ (2018) Catalyzing cascade production of methyl levulinate from polysaccharides using heteropolyacids HnPW11MO39 with Brønsted/Lewis acidic sites. ACS Sustain Chem Eng 6:165–176. https://doi.org/10.1021/acssuschemeng.7b02042
Zhou LP, Zou HJ, Nan JX, Wu L, Yang XM, Su YL, Lu TL, Xu J (2014) Conversion of carbohydrate biomass to methyl levulinate with Al2(SO4)3 as a simple, cheap and efficient catalyst. Catal Commun 50:13–16. https://doi.org/10.1016/j.catcom.2014.02.021
Zhou LP, Yang XM, Xu JL, Shi MT, Wang F, Chen C, Xu J (2015a) Depolymerization of cellulose to glucose by oxidation–hydrolysis. Green Chem 17:1519–1524. https://doi.org/10.1039/c4gc02151g
Zhou LP, Zhao HT, Cui LL, Bai YQ, Bian JJ, Lu TL, Su YL, Yang XM (2015b) Promotion effect of mesopore on the conversion of carbohydrates to methyl levulinate over H-USY zeolite. Catal Commun 71:74–78. https://doi.org/10.1016/j.catcom.2015.08.017
Zhu SH, Guo J, Wang X, Wang JG, Fan WB (2017) Alcoholysis: a promising technology for conversion of lignocellulose and platform chemicals. Chemsuschem 10:2547–2559. https://doi.org/10.1002/cssc.201700597
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (Grant: 21503192), the State Key Laboratory of Catalysis in DICP (N-16-02). The program of Young Key Teacher of Zhengzhou University is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, S., Yang, X., Zhang, Y. et al. Efficient conversion of cellulose to methyl levulinate over heteropoly acid promoted by Sn-Beta zeolite. Cellulose 26, 9135–9147 (2019). https://doi.org/10.1007/s10570-019-02743-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02743-z