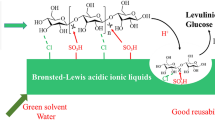
Abstract
Conversion of cellulose to chemicals is an economic and environmental route for biomass utilization. In this work, efficient conversion of cellulose to alkyl levulinates and levulinic acid was realized by oxidation pretreatment combined with alcoholysis over Al2(SO4)3 catalyst. Proper pre-oxidation conditions including oxidation temperature and time are important. By pre-oxidation, part of hydroxymethyl groups on cellulose was converted to carboxyl groups which provide the Brønsted acid sites near the glycosidic bonds to improve the depolymerization of cellulose to monosaccharide. Al2(SO4)3·18H2O can play both Brønsted and Lewis acid roles in methanol and catalyze the conversion of monosaccharide to alkyl levulinates and levulinic acid. After pre-oxidation at optimized conditions, cellulose can be converted into methyl levulinate and levulinic acid over Al2(SO4)3 in methanol efficiently, and total yield of methyl levulinate and levulinic acid can reach 66.8% at 180 °C for 3 h. Furthermore, the simple and cheap Al2(SO4)3 catalyst is recyclable which is important for the practical application.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is a sustainable feedstock for production of renewable biofuels and chemicals. Cellulose, the main component of lignocellulose, is a polysaccharide of anhydroglucose units which are linked by β-1,4-glycosidic bonds. Because of the highly repetitive structural character, cellulose is a promising candidate for synthesis of the simple value-added chemical products (Akin and Yuksel 2019; Podrojková et al. 2018; Zhou et al. 2019a; Zhang et al. 2015). Levulinic acid (LA) and alkyl levulinate are important chemicals which can be widely applied in the fields of fuel additives, agricultural chemicals, flavor and fragrance additives, polyacrylates and so on (Ahmad et al. 2016; Chatterjee et al. 2015; Li et al. 2018; Zhang et al. 2018a). Biomass can be used as renewable and sustainable source for synthesis of LA and alkyl levulinate (Chen et al. 2018; Li et al. 2017; Tiong et al. 2017). Compared to monosaccharides (e.g. glucose and fructose) (Babaei et al. 2018; Jiang et al. 2018; Zhang et al. 2019a) and furfuryl alcohol (the intermediates of cellulose conversion) (Zhang et al. 2019b), cellulose is the more desirable substrate. One pot synthesis of LA and alkyl levulinate from cellulose eliminates the separation and purification processes for the intermediates including glucose and furfuryl alcohol. Thus, considerable effort has been devoted to this attractive strategy.
Various catalytic systems have been developed for the direct synthesis of alkyl levulinate and LA from cellulose (Deng et al. 2017; Huang et al. 2018; Liu et al. 2018; Wang et al. 2017; Tominaga et al. 2016; Yu et al. 2017). By using liquid mineral acid as catalyst is a conventional method (Dai et al. 2018; Kang and Yu 2015; Wu and Fu 2012). Meanwhile, acidic ionic liquids have also been reported for conversion of cellulose to LA (Khan et al. 2018a, b). Considering the simple separation and recyclable characters of heterogeneous catalysts, many solid acids including heteropoly acids (HPAs) (Démolis et al. 2016; Song et al. 2016; Zhang et al. 2018a, b), sulfated solid materials (Morales et al. 2014; Peng et al. 2011; Shi et al. 2018; Xu et al. 2015), zeolites (Li et al. 2017; Saravanamurugan and Riisager 2013; Zhou et al. 2015b, 2019b) and niobium-based phosphate (Ding et al. 2015) were used to catalyze the production of alkyl levulinate from cellulose. However, the catalytic activities of these solid acids were generally low. Generally, for the solid acids, alkyl levulinate yield with cellulose as substrate was much lower than that with glucose as substrate. By using H-USY as catalyst, 49% of methyl levulinate (MLE) yield was obtained after 20 h under 160 °C when glucose was the substrate. However, when cellulose was used as substrate, only 13% of MLE yield was obtained under the same reaction conditions (Saravanamurugan and Riisager 2013). For the heterogeneous solid acid systems, both the reactant of cellulose and the catalyst are in solid phase, and insoluble in the solvent. Acid sites cannot attack the glycosidic bonds in cellulose efficiently because solid–solid mass transfer is very difficult. Besides catalyst systems mentioned above, homogenous catalyst including Brønsted (B) and Lewis (L) acid sites were also designed for production of alkyl levulinate from cellulose. Tominaga and coworkers applied 2-naphthalenesulfonic acid and In(OTf)3 to catalyze the conversion of cellulose and 75% MLE was obtained (Tominaga et al. 2011). As a simple and cheap catalyst, Al2(SO4)3·18H2O which can play both B and L acid roles in methanol was also used for the production of MLE from glucose and cellulose (Zhou et al. 2014). Similar with the solid acids mentioned above, much lower MLE yield was obtained with cellulose as substrate than that with glucose as substrate.
Contact of β-1,4-glycosidic bonds with the active sites is the primary step for the depolymerization of cellulose. However, abundant intra- and intermolecular hydrogen bonds and van der Waals forces make the cellulose chains tightly packed and prevent the diffusion of acid sites (H+/H3O+) to β-1,4-glycosidic bonds. This is why most of the acids are low efficient for the hydrolysis of cellulose. Considering the diffusion problem of liquid and solid catalyst to internal crystalline structure of cellulose, our group has reported an alternative strategy for depolymerization of cellulose to monosaccharide (Zhou et al. 2015a). Pre-oxidation of cellulose can selectively convert the hydroxymethyl groups on cellulose to carboxyl groups. The generated carboxyl groups can catalyze the depolymerization of cellulose to glucose in water. Additional acid is not needed for depolymerization of cellulose to monosaccharide in this method (Zhou et al. 2015a). However, to realize the synthesis of alkyl levulinate and LA, the catalyst for conversion of monosaccharide to alkyl levulinate and LA is necessary. As reported in our previous literature, Al2(SO4)3 is a cheap, simple, efficient and recyclable catalyst for production of methyl levulinate from glucose in methanol (Zhou et al. 2014). Here, pretreatment cellulose by oxidation combined with alcoholysis over Al2(SO4)3 was applied for the efficient conversion of recalcitrant cellulose to alkyl levulinates and LA. Oxidation pretreatment decreased the crystallinity index and degree of polymerization resulting in the increase of reactivity of cellulose. As a simple and recyclable catalyst, Al2(SO4)3 can efficiently catalyze the conversion of oxidized cellulose to alkyl levulinates and LA. This work provides an attractive strategy for synthesis of value-added chemicals from cellulose.
Materials and methods
Material
Microcrystalline cellulose was purchased from Alfa Aesar (China). Al2(SO4)3·18H2O (≥ 99.0%), Al(NO3)3·9H2O (≥ 99.0%) and AlCl3·6H2O (≥ 97.0%) were obtained from Tianjin Fengchuan Chemical Reagent (China). Glucose monohydrate (Glu, analytical reagent) was obtained from Tianjin Kermel Chemical Reagent (China). Fructose (≥ 99.0%), methyl α-d-glucopyranoside (α-MG, ≥ 98.0%), methyl β-d-glucopyranoside (β-MG, ≥ 98.0%), and LA (≥ 99.0%) were purchased from Aladdin Reagent (China). Methyl levulinate (MLE, ≥ 99.0%) was purchased from TCI Shanghai, China. Other reagents used in this work were obtained from commercial sources.
Pretreatment cellulose by oxidation
Microcrystalline cellulose was oxidized in a tube furnace with O2 as the oxidant. Firstly, O2 was pumped in a quartz tube (30 cm × 2 cm) containing 2.0 g of cellulose at a flowing rate of 50 mL min−1. Then, the temperature of the furnace was elevated to set value with a rate of 5 °C min−1. After oxidation for designed time, the furnace was cooled down to room temperature to get the oxidized cellulose. The oxidized cellulose was denoted as Cellulose–x–y, where x and y represented the oxidation temperature and time, respectively.
Characterization of the oxidized cellulose
Panalytical X’pert PRO diffractometer was applied at 40 kV to get the X-ray diffraction (XRD) patterns of the cellulose. Radiation of the diffractometer was Cu Kα (λ = 0.15418). The FT-IR spectra of the cellulose were obtained by a Bruker Tensor II instrument. Mixture of cellulose (~ 2 mg) and KBr (~ 200 mg) was used to prepare the pellet. Acid density of the samples was determined by a titration method according to the literature (Hu et al. 2001). Firstly, 1.0 g of cellulose was added to 40 mL of NaOH aqueous solution (0.05 mol L−1). After stirring for 24 h, solid–liquid separation was carried out by centrifugation. Then, 10 mL of the obtained liquid was titrated by HCl aqueous solution (0.05 mol L−1). Finally, the acid density of cellulose was calculated based on the titration result. Intrinsic viscosity of the cupriethylenediamine solution containing cellulose (0.5 M), measured by the ASTM standard D1795-96, was used to calculate the average degree of polymerization of cellulose.
Catalytic conversion of oxidized cellulose
Catalytic tests were carried out in a stainless autoclave reactor equipped with heating system and magnetic stirrer. Designed amount of oxidized cellulose and catalyst were added to 15 mL of methanol in reactor under stirring. After air in the reactor was replaced by N2 for 4 times, the pressure of N2 was charged to 0.2 MPa. Then, the autoclave was heated. After reaction at designed temperature for desired time, quench the reactor in ice-water, and analyze the obtained reaction mixture.
Product analysis
Solid–liquid separation of the reaction mixture was realized by centrifugation. After the solid was dried to constant weight, conversion of cellulose was calculated according to the weight loss after reaction. The obtained liquid phase was diluted by methanol to 50 mL for analysis. By using naphthalene as internal standard, an Agilent 7890A GC equipped with a FID detector was used to analyze the product of methyl levulinate. Capillary column of HP-5 was used for the separation of products. Shimadzu LC-20AT HPLC with a mobile phase of H2SO4 (0.005 M) aqueous solution was applied to analyze other products. Column of Aminex HPX-87H and refractive index detector of RID-10A were used for the separation and detection of products, respectively.
Conversion and yield measurement
The conversion of cellulose was calculated according to the Eq. (1) where mB and mA represent the weight of cellulose before and after reaction, respectively.
Yields for the products were calculated according to the Eqs. (2) to (6), where YMLE, YLA, YGlu, Yα-MG and Yβ-MG are the yields of methyl levulinate, laevulinic acid, glucose, methyl α-d-glucopyranoside and methyl β-d-glucopyranoside, respectively; nMLE, nLA, nGlu, nα-MG and nβ-MG are the molar amount of these products as mentioned above, respectively; nC6H11O5 unit is the molar amount of glucopyranosyl unit in cellulose.
Results and discussion
Characterization of the oxidized cellulose
Figure 1 shows the SEM images of the cellulose samples. After pretreatment, no obvious change can be observed on the surface structure of the cellulose, and morphology was kept. Pre-oxidation was applied to convert the hydroxymethyl groups at C6 of the glucose unit of cellulose to carboxyl groups (Zhou et al. 2015a). The impact of this method on the crystalline structure of the cellulose was investigated by XRD. As shown in Fig. 2, all the samples before and after treatment have similar diffraction peaks at 2θ ≈ 15.7°, 22.6° and 34.6° which are the characteristic peaks for the cellulose I structure (French and Cintrón 2013; Shakouri and Nazockdast 2018). The corresponding tabular XRD results are listed in Table S1. Pre-oxidation method has obvious impact on the crystallinity of the samples. Based on the intensity in the region of 2θ ≈ 18.3° and intensity of the peak at 2θ ≈ 22.6° as listed in Table S1, crystallinity index of the samples were calculated (Table 1) (Shakouri and Nazockdast 2018). Compared to untreated cellulose, crystallinity of the oxidized samples is lower. When the pre-oxidation time was 10 h, crystallinity of the cellulose decreases slightly from 87.1 to 81.3% with the elevation of oxidation temperature from 170 to 200 °C. However, further increase the temperature to 210 °C, crystallinity of the sample decreases obviously to 67.6%. With the increase of oxidation time, crystallinity also decreases. After oxidation at 200 °C for 20 h, crystallinity of 68.2% was obtained, which is much lower than that of the sample oxidized at 200 °C for 5 h (84.3%). Average degree of polymerization (DP) of cellulose was also collected (Table 1). However, Cellulose-170-10, Cellulose-180-10 and Cellulose-200-5 have similar DP indicating lower oxidation temperature and/or short reaction time did not change the DP of cellulose. When the temperature is higher than 190 °C, further increase the oxidation temperature would lead to the dramatic decrease of DP implying the split of cellulose chains. For example, DP value of Cellulose-210-10 is only 55. Meanwhile, increase of the oxidation time at proper temperature would also cause the decrease of DP. Based on the results mentioned above, it can be concluded that when the oxidation temperature is low and/or the time is shorter, depolymerization of the cellulose chains is slight, but the cleavage of the hydrogen bonds between the chains can occur which causing the decrease of crystallinity. Only when the oxidation temperature and time are proper (for example, at 200 °C for 10 h), depolymerization of the cellulose can occur.
FT-IR method was applied to detect the carboxyl groups which were derived from the hydroxymethyl groups on cellulose by pre-oxidation. As shown in Fig. 3, the intensity of band at 898 cm−1 reflects the crystalline structure of cellulose (Proniewicz et al. 2001). With the elevation of oxidation temperature (Fig. 3a) or prolongation of time from 5 to 20 h at 200 °C (Fig. 3b), intensity of 898 cm−1 band decreased indicating the formation of more disordered structure (Proniewicz et al. 2001). This is consistent with the XRD results as mentioned above. The band at 1732 cm−1 is assigned to C=O vibration of the carboxyl group in cellulose (Saito et al. 2006; Zhou et al. 2015a). Intensity of band at 1732 cm−1 increased with the elevation of temperature implying the increase of carboxyl group amount (Fig. 3a). Similar trend can be observed when the oxidation time increased at 200 °C. Changes of carboxyl group amount reflected in FT-IR are in accordance with the results of acid amount of the samples listed in Table 1. When the temperature elevated from 170 to 200 °C, acid amount increased from 0.16 to 0.68 mmol g−1; however, further elevated the temperature to 210 °C, acid amount increased dramatically to 1.52 mmol g−1. Besides, acid amount also increased with the prolongation of pre-oxidation time. For example, when the oxidative treatment time was 20 h under 200 °C, 1.72 mmol g−1 of acid amount was obtained which is much higher than that for the sample treated for 5 h (0.2 mmol g−1). Figure 4 shows the plot of the cleavage of glycosidic bonds in pre-oxidation versus the acid density. The cleavage of glycosidic bonds are correlated linearly to the acid amount. This explains the decrease of DP after oxidation treatment mentioned above. We had proved that the DP of cellulose treated in N2 was much higher than that in O2 under the same treatment conditions (Zhou et al. 2015a). This indicates the main reason for the decrease of DP is not the thermal decomposition. Thus, the acid sites on cellulose formed in oxidation catalyze the depolymerization of cellulose and this is the primary reason for the decrease of DP.
Screening catalysts
Conversion of oxidized cellulose to MLE is a complex process including depolymerization, isomerization, dehydration and so on. Both B and L acid sites are needed. Al2(SO4)3·18H2O can play both the L and B acid roles in methanol. It is an efficient catalyst for the conversion of glucose to MLE (Zhou et al. 2014). Thus, Al2(SO4)3·18H2O was used as catalyst in this work. Meanwhile, some mineral acids and other Al3+ salts were also tested as catalysts (Table 2). For comparison, the untreated and pre-oxidized cellulose samples were applied as reactants in absence of catalyst, respectively (Table 2). As shown in Table 2, pre-oxidation cannot improve the conversion of cellulose without catalyst; only 5% of conversion was obtained no matter oxidized or un-oxidized celluloses were used. When B acid (HCl or H2SO4) was used as catalyst, conversion of the oxidized cellulose was improved. Meanwhile, catalytic performance of H2SO4 is better than that of HCl (Dai et al. 2018). However, yields of MLE and LA were only 11.0% and 1.3% when H2SO4 was used, although conversion of oxidized cellulose reached 52%. Besides a few of glucose (Glu), a large amount of MG including α-MG and β-MG were obtained which can be converted to MLE via fructose over L acid sites. When Al2(SO4)3·18H2O was used as catalyst, conversion of oxidized cellulose reached 66% which is higher than that for H2SO4. Meanwhile, higher MLE (38.5%) and LA (4.7%) yields and lower MG yield (11.2%) were obtained compared to that over H2SO4. Thus Al2(SO4)3·18H2O is a better catalyst than H2SO4, because the L acid sites in Al2(SO4)3·18H2O can improve the conversion of cellulose to MLE and LA (Huang et al. 2018). Furthermore, pre-oxidation of cellulose indeed facilitates the production of MLE. When the untreated cellulose was used as substrate, both the cellulose conversion and the MLE yield were lower. Moreover, AlCl3·6H2O and Al(NO3)3·9H2O were also tested as catalysts. Al(NO3)3·9H2O has no catalytic activity on conversion of oxidized cellulose. For AlCl3·6H2O, both cellulose conversion and MLE yield were much lower than that over Al2(SO4)3·18H2O. It had been reported that pH of Al2(SO4)3·18H2O methanol solution is lower than Al(NO3)3·9H2O or AlCl3·6H2O methanol solution (Zhou et al. 2014). This might be the reason for the better catalytic performance of Al2(SO4)3·18H2O than Al(NO3)3·9H2O and AlCl3·6H2O. In this work, formic acid (FA) was also detected which is inevitable in the production of MLE and LA from cellulose (Khan et al. 2018a).
Effects of pre-oxidation conditions
Effects of pre-oxidation temperature and time on production of MLE from cellulose were studied. As shown in Fig. 5, when the oxidation temperature was in the range from 170 to 190 °C, conversions of cellulose were comparable and products distributions were similar. Further elevation of the temperature led to the increase of cellulose conversion. When the oxidation temperature increased from 190 to 210 °C, cellulose conversion increased from 54 to 70% indicating that higher oxidation temperature than 190 °C can improve the depolymerization of cellulose obviously. This is consistent with the DP results listed in Table 1. Otherwise, when the oxidation temperature was 200 °C, total yield of MLE and LA was the highest. Lower oxidation temperature cannot realize the efficient depolymerization of cellulose. For example, when the temperature is 190 °C, the DP value of the treated cellulose (194) is comparable to that for untreated sample (213). This is unfavorable for the following production of MLE and LA from cellulose. When the oxidation temperature is 210 °C, total yield of MLE and LA is slightly lower than that for 200 °C. However, higher conversion for 210 °C means lower selectivity of MLE and LA. Furthermore, total yield of α-MG and β-MG for 210 °C is much lower. As mentioned above, MG including α-MG and β-MG is a potential feedstock for production of MLE and/or LA under proper reaction conditions. Thus, 200 °C was applied for the pre-oxidation of cellulose in the following study.
Figure 6 shows the effect of pre-oxidation time on the production of MLE and LA from cellulose. When the pre-oxidation time increased from 1 to 5 h, conversion of oxidized cellulose and yield of MLE did not changed obviously. This is consistent with the characterization result mentioned above. After oxidation at 200 °C for 5 h, the DP value of the cellulose (209) is similar to that of the untreated cellulose (213). Further prolongation of the treatment time from 5 to 10 h, conversion of cellulose and yield of MLE increased obviously. This is related to the obvious decrease of DP of the treated samples in this time range. When the cellulose oxidized at 200 °C for 10 h was used as substrate, 66% cellulose conversion and 38.5% MLE yield can be obtained; meanwhile, the DP value of the cellulose-200-10 is 82. When the treatment time further increased to 20 h, DP value decreased to 39, and cellulose conversion further increased to 71%. However, slight decrease of the MLE yield was observed. This phenomenon also existed in the sample of cellulose-210-10 as mentioned in Fig. 5. As listed in Table 1, acid amount of cellulose-210-10 and cellulose-200-20 were 1.52 and 1.72 mmol g−1, respectively. The singularly high acid amount was caused by the over-oxidation of the cellulose; besides oxidation of hydroxymethyl group, oxidative cleavage of C–C bonds might occur. Oxidative cleavage of C–C bonds can also lead to the formation of carboxyl groups; however, the destruction of the glucopyranosyl unit is unfavorable for the production of the MLE. Besides MLE, LA is also our target product. From Fig. 6, it can be seen that yield of LA increased with the prolongation of treatment time; meanwhile, yields of α-MG and β-MG decreased. It seems that the formation of LA is related to the decrease of MG. The formation pathway of LA was investigated by using α-MG and MLE as substrate, respectively. As shown in Table 3, α-MG is unstable under the reaction conditions. The main products of the α-MG conversion over Al2(SO4)3·18H2O were MLE and LA; meanwhile some unknown by-products formed. Furthermore, when α-MG was used as substrate, MLE yield decreased and LA yield increased with the prolongation of time. Is the LA formed by hydrolysis of MLE in the presence of water provided by Al2(SO4)3·18H2O? To answer this question, conversion of MLE over Al2(SO4)3·18H2O was also carried out. Conversion of MLE indeed occurred under the same reaction conditions (Table 3). However, hydrolysis of MLE is just one part of the MLE conversion. For example, when the reaction was 3 h, only 6.9% of LA yield was obtained while 17% MLE conversion was observed. This explains the decrease of MLE yield with prolongation of time when α-MG was used as substrate. Meanwhile, this also explains the decrease of MLE yield with the prolongation of time from 3 to 10 h when oxidized cellulose was used as substrate under 180 °C as shown in Fig. 5. Based on the results listed in Table 3, it can be concluded that formation of LA was realized by direct conversion of MG and hydrolysis of MLE. Meanwhile, direct conversion of the MG to LA is the main route.
Effects of reaction conditions
Figure 7 shows the time courses of the cellulose conversion and products distributions under different reaction temperatures. Higher reaction temperature means higher conversion rate of cellulose and higher formation rates of MLE and LA. When the reaction temperature were 160 and 180 °C, 34% and 81% cellulose conversion can be obtained after reaction for 1 h, respectively. Meanwhile, total yield of MLE and LA were 12% and 53.4%, respectively. Otherwise, trends of time courses of MLE formation under 160, 170 and 180 °C are different. Under 160 °C, MLE yield increased with the prolongation of time within 10 h. When the temperature was 170 °C, MLE yield increased within the first 5 h and kept comparable in the following 5 h. Under 180 °C, MLE yield increased within the first 3 h and decreased in the following 7 h. This indicates that MLE is unstable under the temperature higher than 180 °C. For the tested reaction temperatures, LA yields increased as the prolongation of time. Meanwhile, α-MG and β-MG yields increased at 160 °C and decreased at 170 and 180 °C with the increase of time. As reported in previous reports, MG is an intermediate compound in the conversion of cellulose to MLE (Ahmad et al. 2016; Zhang et al. 2019a). In this work, low temperature (160 °C) is unfavorable for the conversion of MG to MLE and/or LA. Glucose are also detected and listed in this work; however, the amount is very small. Considering the effects of temperature and time on the cellulose conversion and products distributions, higher temperature and shorter time were needed to realize the efficient production of MLE and LA. The total yield of MLE and LA can reach 66.8% at 180 °C for 3 h.
Figure 8 shows the effect of catalyst amount on the conversion of cellulose to MLE and LA. Compared to blank experiment, addition of slight Al2(SO4)3·18H2O (0.05 mmol) improved the cellulose conversion and products yields dramatically indicating the critical role of Al2(SO4)3·18H2O in this reaction. Yield of MLE increased when the amount of Al2(SO4)3·18H2O increased from 0.05 to 0.3 mmol, and then decreased when the Al2(SO4)3·18H2O amount further increased to 0.4 mmol. Differently, yield of LA increased while yields of glucose, α-MG and β-MG decreased with the increase of Al2(SO4)3·18H2O amount from 0.05 to 0.4 mmol. Considering the dosage of catalyst, 0.3 mmol Al2(SO4)3·18H2O is proper for this reaction. Changes of the products distributions are related to the reaction pathway which had been discussed above.
One-pot conversion of large amount of cellulose to a lot of MLE and LA is also one respect of the efficiency of the catalytic system. Thus, the effect of cellulose amount on the production of MLE and LA was studied. As shown in Fig. 9, when the cellulose amount increased from 0.34 to 0.972 g, cellulose conversion changed from 83 to 82% and the total yield of MLE and LA changed from 66.8 to 62.7%. These changes are slight indicating the amount of active sites provided by the Al2(SO4)3·18H2O is very large. This is desirable for the industrialization of the conversion of cellulose to MLE and LA. Besides methanol, ethanol and n-butanol were also applied as solvents to produce the corresponding alkyl levulinate and LA from cellulose over Al2(SO4)3·18H2O (Fig. 10). With the increase of the carbon chain, the corresponding cellulose conversion, alkyl levulinate yield and LA yield decreased. This might be caused by that the longer carbon chain leads to the greater steric hindrance effect.
Reuse of the catalyst
Catalyst of Al2(SO4)3 can be reused easily in this work. Firstly, unconverted cellulose was separated from the reaction mixture. After removing of methanol from the solution by evaporation, CH2Cl2 was used as solvent, in which Al2(SO4)3 was un-dissolvable, to re-dissolve the products. The solid Al2(SO4)3 was separated from the CH2Cl2 solution. After drying under room temperature, the obtained Al2(SO4)3 was used for the next run. Figure 11 shows the MLE yields of the reuse tests. Yield of MLE after five runs did not decrease compared to that for the first run indicating the recyclability of the Al2(SO4)3 catalyst. This is important for the practical application of the catalyst.
Conclusion
Pre-oxidation strategy was applied for the conversion of cellulose. By pre-oxidation, hydroxymethyl groups on cellulose can be converted to carboxyl groups which can improve the following conversion of cellulose to MLE and LA over catalyst. Proper pre-oxidation conditions are important for the following conversion of cellulose. A simple salt of Al2(SO4)3·18H2O, which can play both B and L acid roles in methanol, was applied as an efficient catalyst for this conversion. After optimization of reaction conditions, total yield of MLE and LA can reach 66.8%. Furthermore, Al2(SO4)3 is stable and recyclable in this work. This work provides an attractive strategy for synthesis of value-added chemicals from cellulose.
References
Ahmad E, Alam MI, Pant KK, Haider MA (2016) Catalytic and mechanistic insights into the production of ethyl levulinate from biorenewable feedstocks. Green Chem 18:4804–4823
Akin O, Yuksel A (2019) Novel hybrid process for the conversion of microcrystalline cellulose to value-added chemicals: part 3: detailed reaction pathway. Cellulose 26:2999–3008
Babaei Z, Chermahini AN, Dinari M (2018) Alumina-coated mesoporous silica SBA-15 as a solid catalyst for catalytic conversion of fructose into liquid biofuel candidate ethyl levulinate. Chem Eng J 352:45–52
Chatterjee C, Pong F, Sen A (2015) Chemical conversion pathways for carbohydrates. Green Chem 17:40–71
Chen Z, Ma X, Xu L, Wang Y, Long J (2018) Catalytic conversion of duckweed to methyl levulinate in the presence of acidic ionic liquids. Bioresour Technol 268:488–495
Dai J, Peng L, Li H (2018) Intensified ethyl levulinate production from cellulose using a combination of low loading H2SO4 and Al(OTf)3. Catal Commun 103:116–119
Démolis A, Eternot M, Essayem N, Rataboul F (2016) Influence of butanol isomers on the reactivity of cellulose towards the synthesis of butyl levulinates catalyzed by liquid and solid acid catalysts. New J Chem 40:3747–3754
Deng L, Chang C, An R, Qi X, Xu G (2017) Metal sulfates-catalyzed butanolysis of cellulose: butyl levulinate production and optimization. Cellulose 24:5403–5415
Ding D, Xi J, Wang J, Liu X, Lu G, Wang Y (2015) Production of methyl levulinate from cellulose: selectivity and mechanism study. Green Chem 17:4037–4044
French AD, Cintrón MS (2013) Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 20:583–588
Hu H, Bhowmik P, Zhao B, Hamon MA, Itkis ME, Haddon RC (2001) Determination of the acidic sites of purified single-walled carbon nanotubes by acid–base titration. Chem Phys Lett 345:25–28
Huang YB, Yang T, Lin YT, Zhu YZ, Li LC, Pan H (2018) Facile and high-yield synthesis of methyl levulinate from cellulose. Green Chem 20:1323–1334
Jiang L, Zhou L, Chao J, Zhao H, Lu T, Su Y, Yang X, Xu J (2018) Direct catalytic conversion of carbohydrates to methyl levulinate: synergy of solid Brønsted acid and Lewis acid. Appl Catal B Environ 220:589–596
Kang S, Yu J (2015) Effect of methanol on formation of levulinates from cellulosic biomass. Ind Eng Chem Res 54:11552–11559
Khan AS, Man Z, Bustam MA, Kait CF, Nasrullah A, Ullah Z, Sarwono A, Ahamd P, Muhammad N (2018a) Dicationic ionic liquids as sustainable approach for direct conversion of cellulose to levulinic acid. J Clean Prod 170:591–600
Khan AS, Man Z, Bustam MA, Nasrullah A, Ullah Z, Sarwono A, Shah FU, Muhammad N (2018b) Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr Polym 181:208–214
Li H, Fang Z, Luo J, Yang S (2017) Direct conversion of biomass components to the biofuel methyllevulinate catalyzed by acid-base bifunctional zirconia-zeolites. Appl Catal B Environ 200:182–191
Li S, Deng W, Wang S, Wang P, An D, Li Y, Zhang Q, Wang Y (2018) Catalytic transformation of cellulose and its derivatives into functionalized organic acids. Chemsuschem 11:1995–2028
Liu L, Li Z, Hou W, Shen H (2018) Direct conversion of lignocellulose to levulinic acid catalyzed by ionic liquid. Carbohydr Polym 181:778–784
Morales G, Osatiashtiani A, Hernández B, Iglesias J, Melero JA, Paniagua M, Brown DR, Granollers M, Lee AF, Wilson K (2014) Conformal sulfated zirconia monolayer catalysts for the one-pot synthesis of ethyl levulinate from glucose. Chem Commun 50:11742–11745
Peng L, Lin L, Li H, Yang Q (2011) Conversion of carbohydrates biomass into levulinate esters using heterogeneous catalysts. Appl Energy 88:4590–4596
Podrojková N, Oriňak A, Oriňaková R, Procházková L, Čuba V, Patera J, Smith RM (2018) Effect of different crystalline phase of ZnO/Cu nanocatalysts on cellulose pyrolysis conversion to specific chemical compounds. Cellulose 25:5623–5642
Proniewicz LM, Paluszkiewicz C, Weselucha-Birczyńska A, Majcherczyk H, Barański A, Konieczna A (2001) FT-IR and FT-Raman study of hydrothermally degradated cellulose. J Mol Struct 596:163–169
Saito T, Nishiyama Y, Putaux J-L, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Saravanamurugan S, Riisager A (2013) Zeolite catalyzed transformation of carbohydrates to alkyl levulinates. ChemCatChem 5:1754–1757
Shakouri Z, Nazockdast H (2018) Microstructural development and mechanical performance of PLA/TPU blends containing geometrically different cellulose nanocrystals. Cellulose 25:7167–7188
Shi X-L, Hu Q, Chen Y, Wang F, Duan P (2018) Conversion of biomass components to methyl levulinate over an ultra-high performance fiber catalyst in impellers of the agitation system. J Ind Eng Chem 65:264–271
Song C, Liu S, Peng X, Long J, Lou W, Li X (2016) Catalytic conversion of carbohydrates to levulinate ester over heteropolyanion-based ionic liquids. Chemsuschem 9:1–11
Tiong YW, Yap CL, Gan S, Yap WSP (2017) One-pot conversion of oil palm empty fruit bunch and mesocarp fiber biomass to levulinic acid and upgrading to ethyl levulinate via indium trichloride-ionic liquids. J Cleaner Prod 168:1251–1261
Tominaga K, Mori A, Fukushima Y, Shimada S, Sato K (2011) Mixed-acid systems for the catalytic synthesis of methyl levulinate from cellulose. Green Chem 13:810–812
Tominaga K, Nemoto K, Kamimura Y, Yamada A, Yamamoto Y, Sato K (2016) A practical and efficient synthesis of methyl levulinate from cellulosic biomass catalyzed by an aluminum-based mixed acid catalyst system. RSC Adv 6:65119–65124
Wang K, Ye J, Zhou M, Liu P, Liang X, Xu J, Jiang J (2017) Selective conversion of cellulose to levulinic acid and furfural in sulfolane/water solvent. Cellulose 24:1383–1394
Wu X, Fu J, Lu X (2012) One-pot preparation of methyl levulinate from catalytic alcoholysis of cellulose in near-critical methanol. Carbohydr Res 358:37–39
Xu X, Zhang X, Zou W, Yue H, Tian G, Feng S (2015) Conversion of carbohydrates to methyl levulinate catalyzed by sulfated montmorillonite. Catal Commun 62:67–70
Yu F, Zhong R, Chong H, Smet M, Dehaen W, Sels BF (2017) Fast catalytic conversion of recalcitrant cellulose into alkyl levulinates and levulinic acid in the presence of soluble and recoverable sulfonated hyperbranched poly(arylene oxindole)s. Green Chem 19:153–163
Zhang J, Li J, Tang Y, Lin L, Long M (2015) Advances in catalytic production of bio-based polyester monomer 2,5-furandicarboxylic acid derived from lignocellulosic biomass. Carbohydr Polym 130:420–428
Zhang X, Li Y, Xue L, Wang S, Wang X, Jiang Z (2018a) Catalyzing cascade production of methyl levulinate from polysaccharides using heteropolyacids HnPW11MO39 with Brønsted/Lewis acidic sites. ACS Sustain Chem Eng 6:165–176
Zhang X, Zhang H, Li Y, Bawa M, Wang S, Wang X, Jiang Z (2018b) First triple-functional polyoxometalate Cs10.6[H2.4GeNb13O41] for highly selective production of methyl levulinate directly from cellulose. Cellulose 25:6405–6419
Zhang Y, Chen X, Lyu X, Zhao G, Zhao T, Han L, Xiao W (2019a) Aluminum phosphotungstate as a promising bifunctional catalyst for biomass carbohydrate transformation to methyl levulinate under mild conditions. J Clean Prod 215:712–720
Zhang Z, Hu X, Zhang S, Liu Q, Hu S, Xiang J, Wang Y, Lu Y (2019b) Direct conversion of furan into levulinate esters via acid catalysis. Fuel 237:263–275
Zhou L, Zou H, Nan J, Wu L, Yang X, Su Y, Lu T, Xu J (2014) Conversion of carbohydrate biomass to methyl levulinate with Al2(SO4)3 as a simple, cheap and efficient catalyst. Catal Commun 50:13–16
Zhou L, Yang X, Xu J, Shi M, Wang F, Chen C, Xu J (2015a) Depolymerization of cellulose to glucose by oxidation-hydrolysis. Green Chem 17:1519–1524
Zhou L, Zhao H, Cui L, Bai Y, Bian J, Lu T, Su Y, Yang X (2015b) Promotion effect of mesopore on the conversion of carbohydrates to methyl levulinate over H-USY zeolite. Catal Commun 71:74–78
Zhou L, Zhang S, Li Z, Zhang Z, Liu R, Yun J (2019a) WCl6 catalyzed cellulose degradation at 80 °C and lower in [BMIM]Cl. Carbohydr Polym 212:289–296
Zhou S, Yang X, Zhang Y, Jiang L, Zhou L, Lu T, Su Y (2019b) Efficient conversion of cellulose to methyl levulinate over heteropoly acid promoted by Sn-Beta zeolite. Cellulose 26:9135–9147
Acknowledgments
We acknowledge the National Natural Science Foundation of China (21875222, 21503192, 21802125) and the Natural Science Foundation of Henan Province (182300410122).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflcit of interest
All authors declare that they have no conflcit of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, L., Gao, D., Yang, J. et al. Conversion of recalcitrant cellulose to alkyl levulinates and levulinic acid via oxidation pretreatment combined with alcoholysis over Al2(SO4)3. Cellulose 27, 1451–1463 (2020). https://doi.org/10.1007/s10570-019-02903-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02903-1