Abstract
In this study, cotton fabrics were reported to be typically functionalized by loading silver nanowires (AgNW) on the surface of the polydopamine modified cotton fabric. Firstly, AgNW were prepared by a polyol method and then a polydopamine-modified cotton fabric was prepared by being immersed in AgNW dispersion by the dip-coating method. The resulting silver nanowire/polydopamine/cotton-based nanocomposites (APCN) has a surface specific resistance as low as 2.4 Ω and has good durability and flexibility. In addition, the electrothermal properties of APCN were investigated by applied voltage. The result showed that the composite material can reach 80 °C in a short time under the voltage of 1.8 V, and conform to the power balance model. The steady-state temperature of the composite material is closely related to the voltage, and has a quadratic relationship with the voltage and expresses linear relation with the electric power.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, electrothermal heating elements have attracted growing attention due to their applications in the fields of thermal therapy, medical electrothermal and defrosting (Sui et al. 2011; Zhang et al. 2017a). Joule heating is the process of current through a conductor generating heat. With the continuous expansion of human work and life, people necessitates excellent thermal-protective method to keeping warm in the low-temperature environment and harsh weather conditions (Wang et al. 2010). Electrothermal products are a kind of active heating products that convert electric energy into heat energy. Currently, wearable protective clothes still have shortcomings such as insufficient flexibility, slow response, and high-voltage requirement. The fabrication of the electrothermal products with light-weight, low-voltage, flexible, environmentally friendly, fast response and recovery is the current challenges (Ilanchezhiyan et al. 2015; Luo et al. 2016; Wang et al. 2018).
Personal heating protective clothing needs to consider many performances. First generation of electrothermal material generally combined metal or inorganic materials such as copper, silver and nickel with textiles (Jin and Hai 2011; Li et al. 2013). However, these materials have weakness likes heavy mass, stiffness and inflexible. Subsequently, second generation of electrothermal materials used conductive polymers like polypyrrole or polyaniline as conductive fillers, but still exist problem of uneven heating and low electrothermal conversion (Hakansson et al. 2004; Lee et al. 2003). With the development of nanotechnology, carbon nanotubes (Jang et al. 2011; Yoon et al. 2010), graphene (Bae et al. 2012) and metal nanowires (Caroline et al. 2012) are expected to become candidates for a new generation of electrothermal composite materials. However, carbon-based materials require a vacuum environment or a toxic solvent to exhibit excellent conductivity (Chen et al. 2015; Kang et al. 2011). The low aspect ratio of most metallic nanowire and complex preparation process limits their uses (Azulai et al. 2009; Hong et al. 2012). Among them, AgNW is currently the hottest conductive fillers due to its excellent electrical conductivity, relatively cheaper, formation controllably and not intertangle easily (Coskun et al. 2011; Gelves et al. 2010; Jason et al. 2015). Simultaneously, AgNW combining with other polymers has become a promising trend for further strengthening AgNW adhesion with matrix (Choi et al. 2015; Ji et al. 2015; Kim et al. 2013).
Cotton fiber is the most common type of cellulose, and widely used in clothing due to its high wettability, permeability and comfort (Zhang et al. 2018). Cotton also need to be compatible with certain excellent electrical properties with the development of electronic textiles (Giesz et al. 2017). Therefore, the functionalization of cotton fabrics is necessary for the development of protective clothing. Cotton fabrics can combine with nanoparticles in various ways to exhibit superior electrical conductivity (Ashraf et al. 2014; Cai et al. 2018; Tang et al. 2013). Some researches showed that microfiber can combine nanowire through entanglement and electrostatic attraction (Cao et al. 2018a; Cui et al. 2015). But the materials still exists certain durability problems because of fiber’s three-dimensional structure (Nateghi and Shateri-Khalilabad 2015). As a derivative of dopa, polydopamine (PDA) can form a functionalized coating on the surface of the material to achieve surface functional modification of the material (Waite 1983). The researches have shown that PDA can functionalize lots of substances, including graphene (Mi et al. 2012), polymers (Wu et al. 2011) and metals nanomaterials (Gaminian and Montazer 2017), etc. On the other hand, cotton modified by PDA can generate excellent physicochemical properties and better adhesion to the metal nanoparticle (Cheng et al. 2018; Gaminian and Montazer 2017; Yang et al. 2017). However, the researches about the PDA modified nanowires were few and remain to be studied.
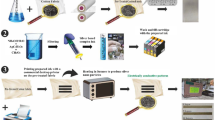
In this paper, silver nanowire/polydopamine/cotton-based nanocomposites (APCN) were prepared by the dip-coating method. This method is popular because of simple ingredients, controllable process, easy implementation and even distribution of filler. AgNW prepared by the polyol method can reach nanometer scale in diameter. The presence of PDA can generate faster and stronger surface bonding between AgNW and cotton fabric. The APCN has a low surface specific resistance while ensuring certain durability and flexibility. At 1.8 V, the composite can reach 80 °C in a short period of time and show excellent electrothermal stability. Based on these properties, APCN can have the potential to be a personal heating protective clothing.
Experimental
Materials
Silver nitrate (AgNO3), sodium chloride (NaCl), polyvinylpyrrolidone (PVP, K = 30) and hydrochloric acid (HCl) was purchased from Sinopharm Chemical Reagent Co., Ltd (China). Ethylene glycol (EG) was supplied by Shanghai Lingfeng Chemical Reagent Co., Ltd (China). Tris(hydroxymethyl) aminomethane and dopamine hydrochloride were purchased from Shanghai Aladdin Chemical Reagent Co., Ltd. (China). Anhydrous alcohol was obtained from Changshu Hongsheng Fine Chemical Co., Ltd (China). The cotton fabric (plain weave, 50 weft cm −1, 50 warp cm−1, 100 g cm−2) were supplied by Shaoxing Shamanlou Textile Co., Ltd. (China), which was ultrasonically cleaned in acetone, absolute ethanol and deionized water for 10 min to remove surface grease and other impurities.
Preparation of AgNW
AgNW were prepared according to the polyol method as shown in Fig. 1 (Korte et al. 2008). 0.2 g of AgNO3, 0.4 g of PVP and 20 ml of EG were mixed in a beaker and stirred for 30 min until the solids were completely dissolved. A small amount of a prepared 0.1 mol/L NaCl-EG solution was added, and the reaction was carried out by heating at 170 °C for about 2 h. After the reaction was finished, naturally cooled to room temperature to obtain a gray solution. The liquid was taken out, and the acetone was uniformly mixed as appropriate and centrifuged at a rotational speed of 5000 rpm for 30 min. After centrifugation, the supernatant was removed and the precipitate was left for use. Then, an appropriate amount of ethanol was added to the centrifuge tube and uniformly mixed, and centrifuged at 5000 rpm for 30 min. After the end of the centrifugation, 20 ml of ethanol was added to the washed precipitate to mix and prepare an ethanol dispersion of AgNW.
Preparation of APCN
The preparation of APCN was schematically illustrated in Fig. 2. 0.12 g of Tris was dissolved in 100 ml of deionized water and titrated with HCl to prepare a Tris–HCl buffer solution (pH = 8.5). Then, dopamine hydrochloride was added to 10 ml buffer solution according to a standard of 2 g/L. The cotton fabric (2.5 cm × 2.5 cm) was dipped in the dopamine solution for 24 h, then washed and dried. The PDA-modified cotton fabric was immersed 10 times in AgNW ethanol dispersion for 1 min each time. At the same time, preparation of simply impregnating cotton fabric with AgNW solution as a control group.
Characterization
Scanning electron microscopy (SEM, FlexSEM 1000, Japan) was used to investigate the surface morphology of the AgNW and fabrics. Fourier transform infrared spectrum (FTIR) were collected by an FTIR spectrometer (Nicolet 5700, USA). The surface specific resistivity of the fabric was measured using a digital universal analyzer (VICTOR VC890D, China) according to the AATCC 76-2005 test standard. The contact angle test of the cotton fabric and the PDA-modified cotton fabric was carried out using an optical contact angle measuring instrument (OCA15EC, Germany). Electrical current as a function of applied voltage for the samples was measured by a regulated DC power supply (APS3005S, China). An infrared thermal imaging camera (FLTR T420, USA) was used to record the temperature distributions of the fabric at the applied voltages.
Results and discussion
SEM morphology of AgNW by polyol method
In recent years, AgNW has attracted extensive attention due to their small size, large aspect ratio, large surface energy composition, and unique physical and chemical properties. AgNW can be prepared through various methods (Xin and Zhao 2009). Among them, the polyol method was the most popular AgNW synthesis method (Yao et al. 2014). The preparation of nanowires by polyol method has the advantages of high yield, relatively short time, low cost and controllable reaction conditions (Pal et al. 2009). In this paper, the AgNW were successfully prepared by the most popular polyol method. As shown in Fig. 3, the SEM photographs of the AgNW networks and AgNW can be clearly seen. It has good dispersibility and good linear structure. Through measuring the single AgNW, the average diameter of the prepared AgNW is 91.83 nm and reaches the nanometer scale.
Characterization of PDA-modified cotton fabric
The mechanism of self-polymerization of PDA is still unclear, but this don’t affect its application in many fields. Many scholars have studied its molecular structure (Dreyer et al. 2012). PDA can functionally modify the surface of the material due to its adhesion and has abundant functional groups. The PDA-modified surface is mainly reacted by Schiff-base and Michael addition reaction. The infrared spectrum of the fabric is shown in Fig. 4. According to the measured structure, the main characteristic peaks of the infrared spectrum of cotton are: 3340.1 cm−1 OH stretching vibration, 2904 cm−1 CH stretching vibration, 1650 cm−1 H–O–H adsorption water absorption peak, 1427.1 cm−1 CH2 bending vibration, 1369.2 cm−1 CH bending vibration, 1334.5 cm−1 OH in-plane deformation vibration, 1276.6 cm−1 CH bending vibration, 1160–1100 cm−1 ring C–O–C asymmetric in-plane stretching vibration, 1060–1000 cm−1 C–O stretching vibration of ring C–O–C (Abidi et al. 2010; Park et al. 2013). The PDA-modified cotton fabric showed a new absorption peaks at 1608.3 cm−1 and 1508.1 cm−1. It’s caused by the bending vibration and shearing vibration of NH in PDA, and represent the characteristic absorption peak of PDA (Jiang et al. 2011; Shi et al. 2015). The characteristic peak of the C=C skeleton in the benzene ring at 1600 cm−1 overlaps with the peak of NH at 1608.3 cm−1 (Centeno and Shamir 2008; Ju et al. 2011). The above analysis shows that PDA-modified cotton fabric was successfully prepared by self-polymerization of dopamine.
PDA-modified cotton fabric can still maintain good hydrophilicity. The results of the contact angle measurement are shown in Fig. 5. The cotton fabric itself is white, and the contact angle of the fabric is 43.9°. Modified by PDA, the color of cotton fabric turns brown and its contact angle decrease by about 7°, which maintains better hydrophilicity.
Electrical property analysis of APCN
The changing trend of the surface specific resistance value during the dip-coating process is shown in Fig. 6. The resistance value of the surface of the cotton fabric decreases with the increase of the dipping times. It is mainly due to the deposition of AgNW on the surface of the cotton fabric to form a conductive loop that makes the overall resistance decrease. In the first dipping, the amount of AgNW deposited on the surface of the cotton fabric has not yet reached the condition of forming a conductive loop, so the resistance value material reaches above 10 MΩ and hardly exhibits conductivity. During the following process, the electrical resistance of PDA-modified cotton fabric decreases quickly due to the surface having much adsorption of AgNW. Finally, the resistance value of the cotton fabric reaches about 8 Ω, while the PDA-modified cotton fabric gradually becomes saturated and the curve begins to stabilize. The resistance value of APCN eventually reaches 2.4 Ω. It demonstrates that the PDA-modified cotton fabric can adsorb more AgNW and reaches a lower stable resistance in the same condition.
SEM morphology of APCN
As shown in Fig. 7a–c, the cotton fabric has a smooth fiber surface and a characteristic stripe structure. After dipping-coating process, the PDA-modified cotton fabric changed from brown to gray because of the existence of AgNW. From the SEM photographs of APCN (Fig. 7d–f), the AgNW covers the surface of the cotton fiber, and masks the original surface stripe morphology. AgNW can distribute on the fibers uniformly, which shows that AgNW can have enough interfacial interaction with the cotton fabric with the help of PDA. In addition, it can be visualized from the image that AgNW is not only deposited on the surface of a single fiber, but also cross-linked among fibers to form a conductive network.
Electrothermal behavior analysis of APCN
The electrothermal behavior of the APCN was investigated by applying different voltages vary from 1 to 1.6 V, and its infrared images at different applied voltages are shown in Fig. 8. The temperature/time curve of the APCN at different voltages is shown in Fig. 9a. Due to the Joule effect, the temperature of the material is constantly increasing. By monitoring the temperature change of the composite material by infrared imager, the steady-state temperature (Tm) of the APCN can reach 41 °C higher than the average body temperature (~ 37 °C) at the voltage U = 1 V, which is suitable for personal heating protective devices. At voltage U = 1.6 V, the APCN can reach a higher steady-state temperature of 63 °C in a short time (~ 75 s), which performs superior electrothermal performance. These mainly depend on the excellent conductivity of the AgNW. It can be found that the electrothermal behavior of the composite depends on the magnitude of the applied voltage. The steady state temperature increases with the increase of the applied voltage.
To further analyze the electrothermal performance of the composite, the time–temperature curve is divided into three parts (El-Tantawy 2001): the temperature growth (heating) zone (0–75 s), the equilibrium (steady-state temperature) zone (75–180 s), and the temperature decay (cooling) zone (180–360 s).
In the first region, the temperature with time can be expressed in an empirical formula
where T0 and Tm are the initial ambient temperature and the steady-state temperature respectively; Tt is the arbitrary temperature at time t; \( \tau_{g} \) is the characteristic growth time constant, indicating the degree of temperature rise. When \( {\text{t}} = \tau_{g} \), \( \frac{{T_{t} - T_{0} }}{{T_{m} - T_{0} }} = 1 - {\text{e}}^{ - 1} \approx 0.632 \), that is, \( \tau_{g} \) is the time when temperature difference ΔT reaches 0.632ΔTm.
In the second region in equilibrium, composite reaches the highest temperature and remains unchanged. It is known from the law of conservation of energy that the heat obtained from the voltage during this time is equal to the loss of radiation and convection. Thus, the heat transferred by radiation and convection, hr+c, is expressed as:
where I is the current, U is the applied voltage and A is sample area (m2).
In the third zone, after the operating power is turned off, according to Newton’s law of cooling, the sample is gradually cooled and the temperature is lowered with time. This can be described by the following empirical formula:
where \( \tau_{d} \) is the characteristic growth time constant, indicating the degree of temperature rise. When \( {\text{t}} = \tau_{d} \), \( \frac{{T_{t} - T_{0} }}{{T_{m} - T_{0} }} = {\text{e}}^{ - 1} \approx 0.368 \), wherein, \( \tau_{d} \) is the time when temperature difference ΔT falls to 0.368ΔTm.
There are different electrothermal behaviors for different applied voltages or materials. The electrothermal effect of a material can be evaluated by the electrothermal coefficient, which is defined as follows:
According to Eqs. (1)–(4), the characteristic parameters: \( h_{r + C} \), \( \tau_{g} \), \( \tau_{d} \), \( \alpha \) of APCN under applied voltage is listed in Table 1.
Through parameters obtained by the above analysis, APCN can compare with the parameters of other electrothermal materials such as carbon nanotubes, graphene and other AgNW (Cao et al. 2018b; Gueye et al. 2017; Sun et al. 2018; Tian et al. 2017; Yao et al. 2018; Zhang et al. 2017b). Among them, the steady-state temperature and electrothermal coefficient are important parameters for evaluating electrothermal materials. As shown in Fig. 10, it shows that APCN has superior electrothermal coefficient, and it can respond quickly to achieve higher steady-state temperatures at lower voltages.
The steady-state temperature of the APCN increases with the increase of the applied voltage as shown in Fig. 9b. On the one hand, according to the formula P = IU = U2/R, the electric power is converted into heat by Joule heat. The higher the voltage, the higher the power, and the higher the temperature of the electrothermal material. Figure 9c shows that there is a high linear correlation between steady-state temperature and power, and it can be more intuitively represented from the second formula of the power balance model. According to this result, the desired steady-state temperature can be obtained by controlling the electric power or voltage within a certain range.
According to the existing data analysis, the power conservation model is used to derive the electrothermal performance under the voltage of 1.8 V (as shown in Fig. 9d). It can be found that the theoretical and experimental electric heating can be well matched. The error value ε of the steady-state temperature (Tm) is only 3 °C. This indicates that the composite can well match the power balance model at voltages below 1.8 V.
Durable electrothermal properties of APCN
It is well known that cotton fabrics are non-conductive and have a surface specific resistance of about 1015 Ω. However, cotton fabrics can have electrical conductivity after adsorption of AgNW through the dip-coating process. The surface specific resistance of the APCN is as low as 2.4 Ω, and its current–voltage (I–U) curve is shown in Fig. 11a. It can be seen from the fitting analysis that the electrical conductivity of the APCN can better match Ohm’s law, that is, U = IR. At the same time, the durability of the APCN was further studied. After a certain bending angle (Fig. 11b) and 2000 bending cycles at a bending radius of ~ 0.5 cm (Fig. 11c), the resistance change of the APCN is little and still maintains good electrical conductivity under at certain bending stress.
The operational stability of the APCN was tested by a cyclic heating/cooling test. Figure 11d shows the temperature response of the APCN at a 1.8 V cycle voltage. By performing 10 cycles of the cyclic heating/cooling test at 1.8 V, the steady-state temperature of the composite material only has a difference of 2–3 °C. This indicates that the structure of the APCN is not damaged due to the thermal stability of the cotton fabric. The above analysis suggests that APCN has good thermal stability and usability.
Conclusion
In summary, personal heating protective materials based on APCN were successfully prepared. The prepared AgNW has a diameter of less than 100 nm and reaches a nanometer scale. The addition of PDA not only maintains the hydrophilicity of the cotton fabric, but also makes electrothermal material reach a lower resistance value of 2.4 Ω. The resistance of the APCN can well match Ohm’s law and has good flexibility and durability, and it hardly changes under 2000 bending cycles and under different bending angles. APCN has excellent electrothermal properties and the heat energy is associated with the applied voltage. The steady-state temperature increases with the increase of the applied voltage. The material can reach a steady-state temperature of ~ 80 °C in a short time (~ 75 s) at a voltage of 1.8 V, which can conform to the power balance model. At the same time, the APCN has good electrical and thermal stability. In the cyclic heating/cooling test tests, the steady-state temperature changes little, and it can meet the practicality of personal heating protective clothing. In general, APCN as flexible electrical heating elements can be used in the field of physiotherapy device, functional textiles and wearable smart clothing.
References
Abidi N, Cabrales L, Hequet E (2010) Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fiber. Cellulose 17:309–320
Ashraf S, Saifur R, Sher F, Khalid ZM, Mehmood M, Hussain I (2014) Synthesis of cellulose–metal nanoparticle composites: development and comparison of different protocols. Cellulose 21:395–405
Azulai D, Belenkova T, Gilon H, Barkay Z, Markovich G (2009) Transparent metal nanowire thin films prepared in mesostructured templates. Nano Lett 9:4246
Bae JJ et al (2012) Heat dissipation of transparent graphene defoggers. Adv Funct Mater 22:4819–4826
Cai D, Zhou J, Duan P, Luo G, Zhang Y, Fu F, Liu X (2018) A hierarchical structure of l-cysteine/Ag NPs/hydrogel for conductive cotton fabrics with high stability against mechanical deformation. Cellulose 25:7355–7367
Cao M, Wang M, Li L, Qiu H, Padhiar MA, Yang Z (2018a) Wearable rGO-Ag NW@cotton fiber piezoresistive sensor based on the fast charge transport channel provided by Ag nanowire. Nano Energy 50:528–535
Cao M, Wang M, Li L, Qiu H, Yang Z (2018b) Effect of graphene-EC on Ag NW-based transparent film heaters: optimizing the stability and heat dispersion of films. ACS Appl Mater Interfaces 10:1077–1083
Caroline C, Céline M, Eléonore M, Henda B, Alexandre C, Jean-Pierre S (2012) Highly flexible transparent film heaters based on random networks of silver nanowires. Nano Research 5:427–433
Centeno SA, Shamir J (2008) Surface enhanced Raman scattering (SERS) and FTIR characterization of the sepia melanin pigment used in works of art. J Mol Struct 873:149–159
Chen TL, Ghosh DS, Marchena M, Osmond J, Pruneri V (2015) Nanopatterned graphene on a polymer substrate by a direct peel-off technique. ACS Appl Mater Interfaces 7:5938–5943
Cheng D, He M, Ran J, Cai G, Wu J, Wang X (2018) In situ reduction of TiO2 nanoparticles on cotton fabrics through polydopamine templates for photocatalysis and UV protection. Cellulose 25:1413–1424
Choi S et al (2015) Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 9:6626–6633
Coskun S, Aksoy B, Unalan HE (2011) Polyol synthesis of silver nanowires: an extensive parametric study. Cryst Growth Des 11:4963–4969
Cui H-W, Suganuma K, Uchida H (2015) Highly stretchable, electrically conductive textiles fabricated from silver nanowires and cupro fabrics using a simple dipping-drying method. Nano Research 8:1604–1614
Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW (2012) Elucidating the structure of poly (dopamine). Langmuir ACS J Surf Coll 28:6428
El-Tantawy F (2001) Joule heating treatments of conductive butyl rubber/ceramic superconductor composites: a new way for improving the stability and reproducibility? Eur Polym J 37:565–574
Gaminian H, Montazer M (2017) Decorating silver nanoparticles on electrospun cellulose nanofibers through a facile method by dopamine and ultraviolet irradiation. Cellulose 24:3179–3190
Gelves GA, Sundararaj U, Haber JA (2010) Electrostatically dissipative polystyrene nanocomposites containing copper nanowires. Macromol Rapid Commun 26:1677–1681
Giesz P, Mackiewicz E, Nejman A, Celichowski G, Cieślak M (2017) Investigation on functionalization of cotton and viscose fabrics with AgNWs. Cellulose 24:409–422
Gueye MN, Carella A, Demadrille R, Simonato J-P (2017) All-polymeric flexible transparent heaters. ACS Appl Mater Interfaces 9:27250–27256
Hakansson E, Kaynak A, Lin T, Nahavandi S, Jones T, Hu E (2004) Characterization of conducting polymer coated synthetic fabrics for heat generation. Synth Met 144:21–28
Hong S, Yun SN, Choi S, Song IT, Lee H (2012) Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv Funct Mater 22:4711–4717
Ilanchezhiyan P, Zakirov AS, Kumar GM, Yuldashev SU, Cho HD, Kang TW, Mamadalimov AT (2015) Highly efficient CNT functionalized cotton fabrics for flexible/wearable heating applications. RSC Adv 5:10697–10702
Jang HS, Sang KJ, Nahm SH (2011) The manufacture of a transparent film heater by spinning multi-walled carbon nanotubes. Carbon 49:111–116
Jason NN, Shen W, Cheng W (2015) Copper nanowires as conductive ink for low-cost draw-on electronics. ACS Appl Mater Interfaces 7:16760–16766
Ji S, He W, Wang K, Ran Y, Ye C (2015) Thermal response of transparent silver nanowire/PEDOT:PSS film heaters. Small 10:4951–4960
Jiang J, Zhu L, Zhu L, Zhu B, Xu Y (2011) Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 27:14180–14187
Jin FW, Hai RL (2011) Research on wearable sensors based on knitted fabrics with silver plating fiber. Adv Mater Res 331:36–39
Ju K-Y, Lee Y, Lee S, Park SB, Lee J-K (2011) Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromol 12:625–632
Kang J et al (2011) High-performance graphene-based transparent flexible heaters. Nano Lett 11:5154–5158
Kim TY, Kim YW, Lee HS, Kim H, Yang WS, Suh KS (2013) Uniformly Interconnected silver-nanowire networks for transparent film heaters. Adv Funct Mater 23:1250–1255
Korte KE, Skrabalak SE, Xia Y (2008) Rapid synthesis of silver nanowires through a CuCl-or CuCl. J Mater Chem 18:437–441
Lee JY, Dong WP, Lim JO (2003) Polypyrrole-coated woven fabric as a flexible surface-heating element. Macromol Res 11:481–487
Li C, Liu H, Li Z (2013) Structure and electric conductive heating performance of silver-plated filament knitted fabrics. J Text Res 34:52–56
Luo J, Lu H, Zhang Q, Yao Y, Chen M, Li Q (2016) Flexible carbon nanotube/polyurethane electrothermal films. Carbon 110:343–349
Mi Y et al (2012) A simple and feasible in situ reduction route for preparation of graphene lubricant films applied to a variety of substrates. J Mater Chem 22:8036–8042
Nateghi MR, Shateri-Khalilabad M (2015) Silver nanowire-functionalized cotton fabric. Carbohydr Polym 117:160–168
Pal A, Shah S, Devi S (2009) Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent. Mater Chem Phys 114:530–532
Park JH, Oh KW, Choi H-M (2013) Preparation and characterization of cotton fabrics with antibacterial properties treated by crosslinkable benzophenone derivative in choline chloride-based deep eutectic solvents. Cellulose 20:2101–2114
Shi Z et al (2015) Enhanced colloidal stability and antibacterial performance of silver nanoparticles/cellulose nanocrystal hybrids. J Mater Chem B 3:603–611
Sui D, Huang Y, Huang L, Liang J, Ma Y, Chen Y (2011) Flexible and transparent electrothermal film heaters based on graphene materials. Small 7:3186–3192
Sun H et al (2018) Large-area self-assembled reduced graphene oxide/electrochemically exfoliated graphene hybrid films for transparent electrothermal heaters. Appl Surf Sci 435:809–814
Tang B, Kaur J, Sun L, Wang X (2013) Multifunctionalization of cotton through in situ green synthesis of silver nanoparticles. Cellulose 20:3053–3065
Tian S, He P, Chen L, Wang H, Ding G, Xie X (2017) Electrochemical fabrication of high quality graphene in mixed electrolyte for ultrafast electrothermal heater. Chem Mater 29:6214–6219
Waite JH (1983) Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis L. J Biol Chem 258:2911–2915
Wang F, Gao C, Holmér I (2010) Effects of air velocity and clothing combination on heating efficiency of an electrically heated vest (EHV): a pilot study. J Occup Environ Hyg 7:501–505
Wang D, Li D, Zhao M, Xu Y, Wei Q (2018) Multifunctional wearable smart device based on conductive reduced graphene oxide/polyester fabric. Appl Surf Sci 454:218–226
Wu J et al (2011) Mussel-inspired chemistry for robust and surface-modifiable multilayer films. Langmuir 27:13684–13691
Xin H, Zhao X (2009) Solvothermal synthesis and formation mechanism of chain-like triangular silver nanoplate assemblies: application to metal-enhanced fluorescence (MEF). Appl Surf Sci 255:7361–7368
Yang J, Xu H, Zhang L, Zhong Y, Sui X, Mao Z (2017) Lasting superhydrophobicity and antibacterial activity of Cu nanoparticles immobilized on the surface of dopamine modified cotton fabrics. Surf Coat Technol 309:149–154
Yao T, Wei H, Wang S, Tao Z, Cheng L (2014) One step synthesis of silver nanowires used in preparation of conductive silver paste. J Mater Sci Mater Electron 25:2929–2933
Yao X, Hawkins SC, Falzon BG (2018) An advanced anti-icing/de-icing system utilizing highly aligned carbon nanotube webs. Carbon 136:130–138
Yoon YH, Song JW, Kim D, Kim J, Park JK, Oh SK, Han CS (2010) Transparent film heater using single-walled carbon nanotubes. Adv Mater 19:4284–4287
Zhang L, Baima M, Andrew TL (2017a) Transforming commercial textiles and threads into sewable and weavable electric heaters. ACS Appl Mater Interfaces 9:32299–32307
Zhang T-Y et al (2017b) A large-strain, fast-response, and easy-to-manufacture electrothermal actuator based on laser-reduced graphene oxide. Appl Phys Lett 111:121901
Zhang C, Zhou G, Rao W, Fan L, Xu W, Xu J (2018) A simple method of fabricating nickel-coated cotton fabrics for wearable strain sensor. Cellulose 25:4859–4870
Funding
This work is jointly supported by “the Fundamental Research Funds for the Central Universities (2232018G-01)”, by the National Key Research and Development Program of China (Grant No. 2016YFC0802802) and by Fok Ying Tung (huoyingdong) Education Foundation (151071).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Z., Yu, W. & Du, Z. Study of electrothermal properties of silver nanowire/polydopamine/cotton-based nanocomposites. Cellulose 26, 5995–6007 (2019). https://doi.org/10.1007/s10570-019-02506-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02506-w