Abstract
Deposition of nanoparticles on the surface of a variety of materials is a subject of great interest due to their potential applications in electronic devices, sensing, catalysis and bio-medical sciences. In this context, we have explored and compared various methodologies to generate gold and silver nanoparticles on the surface of cellulose fibers. It was found that boiling of the cellulose fibers in alkaline solution of gold and silver salts led to the formation and immobilization of gold and silver nanoparticles. However, in case of lecithin treated and thiol-modified cellulose fibers, high temperature was not essentially required for the formation and deposition of nanoparticles on cellulose substrate. In both these cases, fairly uniform metal nanoparticles were obtained in good yields (~43 wt% gold loading in case of thiol modified cellulose fibers) at room temperature. Borohydride-reduction method resulted in relatively lower loading (~22 wt%) with a wide size distribution of gold and silver nanoparticles on cellulose fibers. All these nanoparticle–cellulose composites were thoroughly characterized using scanning electron microscopy, energy dispersive X-ray, Fourier transform infrared spectroscopy, UV–visible spectroscopy, and elemental analyzer. Thiol modified cellulose–gold nanoparticle composites served as active catalysts in the reduction of 4-nitrophenol into 4-aminophenol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent times, direct synthesis/growth of metal nanoparticles on substrates is attracting a considerable interest mainly to address various challenges associated with the immobilization of preformed metal nanoparticles on different kinds of substrates. Such nanocomposite materials are important for their potential applications in optics, electronic devices, catalysis, sensors, medical applications, etc. (Baker and Moore 2005; Sergeev 2006; Kwon et al. 2005; Hetrick and Schoenfisch 2006; Drogat et al. 2011; El-Shishtawy et al. 2011; Rotello 2004; Shipway et al. 2000; Serp et al. 2003). For example, antibacterial properties of polymer supported silver nanoparticles are the subject of extensive studies for their applications in wound dressing materials, antimicrobial filters, tissue scaffolds, body wall repairing substances (Son et al. 2004; Wang et al. 2005; Drogat et al. 2011; El-Shishtawy et al. 2011; Lee et al. 2007) and antibacterial packaging materials for preventing bacterial infection in foodstuffs (Tankhiwale and Bajpai 2009; Drogat et al. 2011; El-Shishtawy et al. 2011). A diverse range of substrates such as silica, metal oxides, carbon based materials, and polymers (Rotello 2004; Shipway et al. 2000; Serp et al. 2003) have been used to immobilize metal nanoparticles for various applications. Recently, many investigations have been devoted to developing methodologies for the synthesis of metal nanoparticles using cellulose as a support material (He et al. 2003; Ferraria et al. 2009; Taylor et al. 2005a, b; Barud et al. 2008; de Santa Maria et al. 2009; Drogat et al. 2011; El-Shishtawy et al. 2011) mainly because of its biocompatibility, biodegradability, and specificity towards attracting metal ions. Immobilized metal nanoparticles at cellulosic surfaces are considered harmless to humans and animals due to fairly strong interaction between metal nanoparticles and cellulosic material, and therefore minimizing the risks of contaminating water or organic/biological media (Ferraria et al. 2009).
Ferraria et al. (2009) suggested that if metallic nanostructures are appropriately used in combination with cellulose then it may lead to the miniaturization of electrical circuits and an era of nanoelectronics. These electrical circuits may be printed on paper simply by using inkjet printers. Similarly, “smart paper” or “smart cotton” could potentially be based on nanoparticles structured on cellulosic materials with possibility of a large number of applications in different fields (Ferraria et al. 2009).
Modified cellulosic substrates have been utilized either as supporting materials or as reductants for the synthesis of gold, silver and platinum nanoparticles. These substrates include oxidized cellulose microfibrils (Wu et al. 2008), carboxymethyl cellulose sodium (Chen et al. 2008) and nanoporous cellulose gel (Cai et al. 2008). Main characteristic of cellulose fiber and matrix is its porous texture which acts as a useful substrate for the synthesis of nanoparticles of different metals such as platinum, palladium, silver, copper, gold, cadmium sulphide, iron oxide, etc. (Kotelnikova et al. 1999; He et al. 2003; Wu et al. 2012; Mahmoud et al. 2009; Liu et al. 2011a, b). Cellulose derivative matrix and other polysaccharides having chemical structure similar to cellulose have also been used as dispersant for metal nanoparticles composite materials (Kwon et al. 2005; Huang et al. 2004; Drogat et al. 2011). Nanoporous cellulose gel has also been used as a reducing as well as morphology directing agent to produce selenium nanobelts (Lu et al. 2006). Cellulose acetate nanofibrillar aerogel has been prepared with silver nanoparticles with a maximum silver loading of up to 6 wt% (Luong et al. 2008). Despite all these interesting reports, the loading of metal nanoparticles onto the cellulosic substrate remained low. Therefore, we set out to examine and compare various new routes to produce cellulose–metal nanoparticle composites with a high loading of fairly uniform metal nanoparticles and examined the composite with the highest metal content for its catalytic activity for the reduction of 4-nitrophenol to 4-aminophenol.
Dong and Hinestroza (2009) have recently reported two strategies for the deposition/formation of nanoparticles of gold, platinum and palladium on the surface of modified cellulose fibers (cationic cellulose fibers). In one strategy, citrate-stabilized metal nanoparticles were electrostatically immobilized on cationic cellulosic surfaces. Whereas in their 2nd approach the anionic metal complex ions were adsorbed onto cationic cellulose surfaces which were then reduced with sodium borohydride. Using this approach, high loading density of metal nanoparticles was obtained, but the size distribution of nanoparticles was quite broad. El-Shishtawy et al. (2011) have dipped cellulose fabric in the solution of silver salt and added cetyl trimethyl ammonium bromide and glucose. Subsequently after the addition of sodium hydroxide, antimicrobial fabric was obtained but the loading content/density of silver was low. Ferraria et al. (2009) have developed a method for the synthesis of silver nanoparticles at the surface of ultrathin cellulose films having high loading density of nanoparticles. In their experimental approach, they adopted mild wet chemistry technique by spin coating cellulose ultrathin films on Gallium arsenide substrates and grafted the films with diaminoalkanes (activated by N,N′-carbonyldiimidazole). These amine containing modified cellulosic films acted as anchoring centers for silver ions which helped in the formation/immobilization of silver nanoparticles.
Cellulose is an interesting polymeric material for the immobilization of metal nanoparticles as it contains six hydroxyl groups per cellubiose (repeating) unit of cellulose polymer (Sarrazin et al. 2009). These hydroxyl groups can be functionalized by chemical modification of the cellulose fibers. In this work, we have applied different strategies for treatment of the cellulose fibers in an effort to achieve better size uniformity and loading densities of metal nanoparticles on the surface of modified cellulose fibers. Interestingly thiol modified cellulose fibers–gold nanoparticles composites served as active catalysts for the reduction of 4-nitrophenol into 4-aminophenol. The deposited nanoparticles were investigated by the scanning electron microscopy, energy dispersive X-ray, FTIR spectroscopy, UV–visible spectroscopy and elemental analyzer. Cellulose fibers with such high metal contents may serve as potential substrates for catalysis, antimicrobial scaffolds, and as substrates to enhance the signals in surface enhanced Raman spectroscopy (SERS).
Materials and methods
Materials
South American Pima cotton (Gossypium barbadense) was used as a source of cellulose fiber in this study. Silver nitrate, sodium hydroxide, sodium borohydride, lecithin, n-hexane, mercaptoacetic acid, para-toluene sulphonic acid, and 4-nitrophenol were received from Acros Chemicals and were 99–99.9 % pure. Hydrogen tetrachloroaurate was received from Aldrich (99.8 %). Ultra pure water with specific resistivity of 18 MΩ cm was obtained using Millipore water purification system.
Methodology
In situ reduction of gold and silver nanoparticles on cellulose fibers using sodium borohydride as reducing agent
Two separate loading baths containing 20 mL of 10 mM HAuCl4 and 1 M AgNO3 solutions were taken and pieces of 5 mg cellulose fiber were dipped into each of them for overnight. For control experiment, the same amount of cellulose fiber was dipped into double distilled deionized water for the same duration of time. The soaked fibers were rinsed with absolute ethanol for 1 min to remove excess metal ions. All three pieces of fibers were then dipped into 20 mL of 200 mM NaBH4 solution overnight for reduction of metal ions.
In situ reduction of gold and silver nanoparticles on sodium hydroxide treated cellulose fibers (reduction by refluxing)
5 mg of cellulose fibers, pre-soaked for 12 h in 10 mL of aq. NaOH solutions (0.05 M for gold nanoparticles and 0.05 mM for silver nanoparticles), were refluxed for 10–20 min. 25 mL of 1 mM HAuCl4 and AgNO3 solutions were separately added with constant stirring into two flasks containing cellulose fibers in boiling solutions of sodium hydroxide and refluxed for 1.5 h. The reaction contents were then cooled to room temperature with constant stirring. For control experiment, the same amount of fibers was soaked into double distilled deionized water and the remaining process was completed under exactly the same conditions.
In situ reduction of gold and silver nanoparticles on the surface of cellulose fibers using lecithin as reducing agent
5 mg of cellulose fibers were dipped into two different aliquots of 5 mL of 10 mM lecithin solutions in n-hexane for overnight. Fibers were taken out the next day from the solvent, dried in air and dipped into 10 mL of 1 mM HAuCl4 and silver nitrate solutions in two separate flasks for 24 h at room temperature. For control experiment, the same amount of cellulose fibers was dipped into n-hexane (without lecithin) and treated under the same conditions as described above. FTIR spectrum of lecithin treated cellulose fibers was recorded and compared with untreated cellulose fibers.
In situ reduction of gold and silver nanoparticles on the surface of thiol modified cellulose fibers
100 mg of cellulose fibers were heated with 10 mL of mercaptoacetic acid in the presence of catalytic amount of para-toluene sulphonic acid with constant stirring in an inert atmosphere of argon gas. Heating of the reaction contents was carried out for 30 min at 50 °C, 30 min at 100 °C and finally 30 min at 130 °C. After these heating stages, the reaction contents were cooled to room temperature with constant stirring. The thiol modified cellulose fibers were then filtered using a Buchner funnel attached with a vacuum suction pump, washed with plenty of water to remove any soluble impurities and dried in a vacuum oven at 50 °C for overnight. Thiol modification of cellulose fibers was verified by the comparison of FTIR spectra of untreated cellulose fibers with that of thiol modified cellulose fibers and by means of elemental analyzer. For preparation of metal nanoparticles on thiol modified cellulose fibers, 1 mg of modified cellulose fibers was dipped into 5 mL of 1 mM HAuCl4, and AgNO3 solutions separately and the reaction contents were subjected to gentle shaking for overnight. For control experiment, the same amount of thiol modified cellulose fibers was dipped into 5 mL of double distilled deionized water and treated in the same way as described above.
The fibers containing metal nanopartciles obtained by all above methods were rinsed with double distilled deionized water, dried on filter paper and finally in a vacuum oven for 7 h at 70 °C for further characterization and used in subsequent experiments.
Catalytic activity of thiol modified cellulose–metal nanoparticles composites
The potential of thiol-modified cellulose fibers loaded with gold and silver nanoparticles was examined for the catalytic conversion of 4-nitrophenol into 4-aminophenol. In 5 mL (0.05 mM) 4-nitrophenol, 5 mL (5 mM) sodium borohydride solution was added in the absence and presence of catalysts (15 mg of gold and silver nanoparticles loaded thiol modified cellulose composites). UV–visible absorption spectroscopy was used to monitor the conversion of 4-nitrophenol into 4-aminophenol.
Characterization of cellulose–metal nanoparticle composites
SEM analyses were performed using JSM-7500F field emission scanning electron microscope. JEE-420 vacuum evaporator by JEOL was used for coating some of the samples with carbon before analyses. EDX spectra were recorded with a SEM CamScan IV from Germany. 282A programmable vacuum oven was used for drying the fibers before analyses. FTIR analyses were performed using Bruker Alpha-P FTIR with a diamond ATR attachment. Elemental analysis was performed using elemental analyzer Flash EA 1112 series by Thermo electron corporation. UV–visible spectra were recorded using Agilent 8453 UV–visible spectrophotometer. Digital camera pictures were captured by means of Cyber-shot DSC-H10/B digital camera by SONY.
Results and discussion
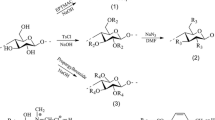
Chemical reduction of metal salts is the most commonly used method for solution phase synthesis of metal nanoparticles. In this method, generally a soluble metal salt is used as a precursor in the presence of a stabilizer and a reducing agent for the synthesis of metal nanoparticles. However, for many applications template assisted synthesis of metal nanoparticles is preferred owing to certain advantages as template not only provides a platform for the synthesis of metal nanoparticles but it also helps the particles anchor on an organic template to adopt shape controlled structures. For the same reasons, we have used cellulose fibers as template to produce metal nanoparticles by adopting 04 different strategies summarized in Fig. 1. The diameter of cellulose fibers was about 10 μm as revealed by field emission scanning electron microscopy. Digital camera pictures of cellulose fibers before and after coating with gold and silver nanoparticles by all recipes are given in Fig. 2.
Digital camera pictures of cellulose fibers before (a) and after the formation of gold (b–e) and silver (f–i) nanoparticles by various treatments. Photographs of cellulose–gold nanoparticle composites formed by borohydride reduction (b), NaOH treatment (c), lecithin treatment (d), and reduction on thiol modified cellulose fibers respectively (e). Photographs of cellulose–silver nanoparticle composites formed by borohydride reduction (f), NaOH treatment (g), lecithin treatment (h), and reduction on thiol modified cellulose fibers (i)
In borohydride reduction experiments, the colour of pre-soaked cellulose fibers (into metal salt solutions) changed immediately after dipping the cellulose fibers into sodium borohydride solution. Purple coloured cellulose fibers were obtained at the end of reduction (~4 h) in case of the gold nanoparticles formation. From field emission scanning electron micrographs (FESEM), it is evident that the particles range in size from 5 to 70 nm and are quite polydisperse in nature (Fig. 3). Some of the particles are in the form of clusters having oval and round shapes with 22 % loading density of gold in composites as shown in the scanning electron micrographs and EDX spectrum in Figure S1.
Scanning electron micrographs of cellulose–gold nanoparticle composites formed by sodium borohydride reduction (a), sodium hydroxide treatment (b), using lecithin as reducing agent (c), and using thiol modified cellulose fibers (d). Scale bars correspond to 100 nm. The size distribution of particles formed by all above mentioned treatments are shown at the bottom of each micrograph (e–h respectively)
The cellulose fibers turned dark brown after the completion of reaction for silver nanoparticles synthesis using borohydride reduction method. From their FESEM micrographs and EDX spectrum shown in Figs. 4 and S2, it is evident that the particles are polydisperse in nature, ranging in size from 15 to 70 nm with spherical and oval morphologies and 19 % loading density of silver in composites was observed.
Scanning electron micrographs of cellulose-silver nanoparticle composites formed by sodium borohydride reduction (a), sodium hydroxide treatment (b), using lecithin as reducing agent (c), and using thiol modified cellulose fibers (d). Scale bars correspond to 100 nm. The size distribution of particles formed by all above mentioned treatments are shown at the bottom of each micrograph (e–h respectively)
Earlier studies on the formation of metal nanoparticles at the surface of cellulose fibers using borohydride reduction method suggest that when the cellulose fibers are immersed in the solution of metal salts (e.g., gold and silver), the metal ions are readily impregnated into the cellulose fibers through the pores. The electron rich oxygen atoms of polar hydroxyl and other electronegative groups of cellulose fibers interact with the electropositive transition metal cations via electrostatic i.e., ion–dipole interactions and as a result of that most of the metal ions get incorporated into the cellulose macromolecules (Kesting 1965; He et al. 2003). The metal ions that are not anchored to cellulose fibers or that are loosely bound are effectively removed by rinsing the treated cellulose fibers with absolute ethanol for approximately 1 min. When these metal cations incorporated porous cellulose fibers are dipped into the solution of sodium borohydride, it immediately reduces the adsorbed metal ions into metal atoms and initiates the nucleation process. The metal nuclei then grow during the course of reaction to form polydisperse metal nanoparticles.
Cellulose is known to be easily hydrolyzed by acid treatment forming water soluble sugars but it is resistant to strong alkali for up to 17.5 % by weight, and also to oxidizing agents (John and Thomas 2008). However, concentrated NaOH (>17.5 % by weight) can hydrolyze cellulose fibers to some extent at room temperature and the degree of hydrolysis strongly depends upon the degree of polymerization of cellulose fibers as well as the concentration of sodium hydroxide used (Egal et al. 2008). In our experiments, we chose the fibers with long polymeric lengths of approximately 3,505 mm so that the chances of hydrolysis by sodium hydroxide were minimized. Moreover, the concentrations of sodium hydroxide used in these experiments are much lower than that required for the hydrolysis of cellulose fibers.
When we dipped cellulose fibers into 0.05 M sodium hydroxide solution and subsequently boiled with 25 mL of 1 mM HAuCl4, purple coloured fibers were obtained containing fairly uniform gold nanoparticles ranging from 15 to 20 nm in size as revealed by their FESEM micrographs (Fig. 3). It was also confirmed by EDX analysis (Figure S3) that there was much more loading of surface bound gold nanoparticles (34 wt% gold) as compared to other methods like sodium borohydride and lecithin reduction. The optimum concentration of sodium hydroxide solution was found to be 0.5 mM for treatment of cellulose fibers and the formation of silver nanoparticles with deep brown colour on its surface. From FESEM micrographs and EDX analysis (Figs. 4, S4), it is observed that particles are fairly spherical in shape and varied in size from 15 to 25 nm. The surface loading density of silver nanoparticles i.e., nanoparticles bound to the surface of cellulose fibers was found to be 26.61 wt%. Optimum conditions for synthesis of gold and silver nanoparticles using this method were established by soaking cellulose fibers in NaOH solutions, refluxing the reaction contents, and changing the molar concentrations of sodium hydroxide solutions. One possible explanation of the action of sodium hydroxide is that it would create negative charges on the surface of cellulose fibers by deprotonating the hydroxyl groups facilitating the electrostatic interaction of metal cations (Au and Ag). This improved interaction between the metal ions and negatively charged cellulose fibers would increase the surface loading density and also facilitate the reduction of gold and silver ions under boiling conditions. It was found through experiments that boiling of the negatively charged cellulose fibers in the presence of gold and silver ions is necessary to produce gold and silver nanoparticles as no such metal nanoparticles were produced at room temperature.
Lecithin is a group of fatty substances which occur in animals and plant tissues, and in egg yolk. It is composed of choline, phosphoric acid, glycerol, fatty acids, triglycerides, glycolipids and phospholipids (Jimenez et al. 1990; Iwata et al. 1993). The lecithin used in these experiments was of commercial grade with low solubility in water. Recently, lecithin has been reported to produce suspended gold nanoparticles (Hussain et al. 2010). It has also been used for producing gold nanoparticles on ink-jet printed patterns on different surfaces (Hussain et al. 2010). We have thus coated the cellulose fibers with lecithin and investigated its potential for producing metal nanoparticles. Gold and silver nanoparticles were formed on lecithin treated cellulose fibers at room temperature, as was evident by the change of colour of cellulose fibers (purple colour in case of gold nanoparticles and yellowish brown colour in case of silver nanoparticles). In case of gold nanoparticles, the cellulose fibers turned purple and reaction was completed within 1 h. However, silver nanoparticles were formed slowly as cellulose fibers turned light brown/yellowish after 1 h and the intensity of colour increased gradually over time. FESEM analysis of gold nanoparticles showed fairly spherical particles ranging in size from 20 to 30 nm with 15 wt% of gold (from EDX analysis) on these cellulose fibers as shown in Figs. 3 and S5. Silver nanoparticles formed by this approach were also spherical and in size range of 10–30 nm with 25 % surface loading density as shown by the FESEM and EDX results in Figs. 4 and S6.
Lecithin is a surfactant containing positively charged quaternary ammonium groups which interact strongly with the hydroxyl groups on cellulose fibers to form a fairly uniform surface coating. The negatively charged phosphate groups of lecithin then interact with the positively charged metal ions and result in their adsorption at the surface. The reduction of metal ions for producing metal nanoparticles using lecithin, however, involve a complex mechanism and needs further investigations for proposing a proper mechanism. The FTIR spectra (Figure S7) of lecithin, native cellulose, and cellulose treated with lecithin show that the lecithin is coated on cellulose fibers. This method provides a relatively environmentally benign approach for the production of nanoparticles on the surface of cellulose fibers without using any toxic reducing agents such as sodium borohydride, hydroxylamine hydrochloride, hydrazine, ascorbic acid, etc.
In thiol modification of the cellulose fibers, the surface hydroxyl groups are converted into mercaptoacetate groups as shown in a proposed reaction scheme in Fig. 5 and verified the degree of substitution of hydroxyl groups by mercaptoacetate groups (~6/cellubiose unit) by means of elemental analyzer. FTIR spectra (Figure S8) clearly shows the peaks of thioester at 1,731 cm−1 for the thiol modified cellulose fibers which are missing in the unmodified cellulose fibers. In this reaction scheme, para-toluene sulphonic acid acts as a catalyst and provides H+ ions for successful completion of the reaction. During thiol modification, cellulose fibers were hydrolyzed to some extent but did not lose their structures as the shape of fibers was retained at the end of the reaction. On dipping thiol modified cellulose fibers in 1.0 mM HAuCl4 and AgNO3 solutions, the fibers started to develop purple and yellowish brown colours respectively which changed to deep purple/blue and deep brown colours within 1 h of the mixing. The colour of the thiol modified fibers got intensified with time while the colour of gold salt solution gradually faded from light yellow to transparent. This indicated that thiol groups on the surface of cellulose fibers were attracting as well as reducing the gold ions on the surface of thiol-modified cellulose fibers on all possible available reaction sites through covalent linkage. Due to transparent solution, similar visual morphological change cannot be observed in case of silver nitrate solution. The reduction reaction was performed in the presence of excess of gold and silver ions to ensure maximum conversion of free gold and silver ions into gold and silver nanoparticles respectively. FESEM images of cellulose fibers coated with gold and silver nanoparticles are shown in Figs. 3 and 4. The metal nanoparticles are fairly spherical in shape and in the size range of 20–30 and 15–30 nm for gold and silver respectively. The maximum gold loading density was found to be 43.13 % by weight on the surface of thiol modified cellulose fibers as determined by EDX (Figure S9). However, in case of composites of cellulose–silver nanoparticles, 13 wt% of sliver was observed as determined by EDX studies (Figure S10). A possible explanation for low surface loading density of silver may be due to lesser affinity of thiol towards silver compared to gold under similar reaction conditions.
In order to evaluate the potential applications of cellulose–metal nanoparticles composites; thiol modified cellulose–nanoparticle composites were evaluated for the catalytic conversion of 4-nitrophenol into 4-aminophenol in the presence of sodium borohydride. Thiol modified cellulose–metal nanoparticle composites were chosen for this study because of higher metal content. When sodium borohydride was added in the solution of 4-nitrophenol in the absence (control) and presence of cellulose composites with gold and silver nanoparticles, 4-nitrophenolate ions were formed as indicated by the change in the colour of solution from faint yellow to dark yellow and the shift of absorption maxima from 317 to 400 nm. As the time lapsed, the colour of 4-nitrophenolate ions faded to transparent with subsequent decrease in the intensity of absorption at 400 nm and the appearance of a new peak corresponding to 4-aminophenol at 300 nm in the presence of thiol modified cellulose–gold nanoparticle composites (Fig. 6). The whole reaction took about 90 min to complete. No such complete reduction of 4-nitrophenol was observed even up to 72 h in the absence of composites (control) or the presence of thiol modified cellulose–silver nanoparticle composites (Fig. 6). As there was no catalyst in control reaction, no reduction was expected as observed even after 3 days. However, in the case of silver nanoparticles loaded cellulose composites, significant reduction was not observed probably due to the different nature of metal and its low metal content (~13 %) compared to the gold (~43 %). In the start of reaction within 3 min, intensity of absorption at 400 nm decreased slightly in the presence of thiol modified cellulose–silver nanoparticle composites and the colour of solution faded slightly but the reaction could not proceed to the completion.
Cellulose–metal nanoparticles composite materials with high metal content, smaller size, and fairly narrow size distribution may serve as potential substrates for catalysis and in electronics (after calcination conductive nano-patterns may develop/form on the patterns of composite materials). Cellulose–silver nanoparticle composites formed by above mentioned methodologies/treatments due to fairly high silver content may also potentially be used to produce antimicrobial bandages to suppress the growth of microbes/prevent infection in wounded area for extended periods of time. Rough metal surfaces formed on composite materials by above mentioned treatments may also serve as active substrates for enhancing the analytes signals in SERS.
Conclusion
We have used four different strategies to produce and immobilize gold and silver nanoparticles on cellulose fibers. They include the formation of gold/silver nanoparticles by sodium borohydride reduction, refluxing with NaOH, treatment of cellulose fibers with lecithin and incubating thiol modified cellulose fibers with the metal ions. These strategies can produce cellulose–metal nanoparticle composites with fairly high metal loading (15–43 wt%) and relatively narrow size distribution as compared to the well known borohydride reduction method. The treatment of cellulose fibers with NaOH results in the creation of negative charge at the surface by deprotonating the hydroxyl ions, which results in a better electrostatic attachment of metal cations on the surface of the fibers. Strong interaction of the positively charged quaternary ammonium groups on lecithin with the hydroxyl groups on cellulose fibers is responsible for the greater adsorption of metal ions on the surface of cellulose fibers, and relatively high loading density of metal nanoparticles. Thiol modification of cellulose substrate can also be used to improve metal nanoparticles adsorption on the surface of cellulose fibers. Thiol-modified cellulose–gold nanoparticle composites with high gold loading were found to be active catalysts for the conversion of 4-nitrophenol into 4-aminophenol. Due to high metal content in such cellulose–metal nanoparticle composites, we envision these composite materials to have potential applications in catalysis, electronics, antimicrobial products, and possibly as substrates for enhancing the signals in SERS, which we intend to explore in future.
References
Baker GA, Moore DS (2005) Progress in plasmonic engineering of surface-enhanced Raman-scattering substrates toward ultra-trace analysis. Anal Bioanal Chem 382(8):1751–1770
Barud HS, Barrios C, Regiani T, Marques RFC, Verelst M, Dexpert-Ghys J, Messaddeq Y, Ribeiro SJL (2008) Self-supported silver nanoparticles containing bacterial cellulose membranes. Mater Sci Eng C 28(4):515–518
Cai J, Kimura S, Wada M, Kuga S (2008) Nanoporous cellulose as metal nanoparticles support. Biomacromolecules 10(1):87–94
Chen J, Wang J, Zhang X, Jin Y (2008) Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater Chem Phys 108(2–3):421–424
de Santa Maria LC, Santos ALC, Oliveira PC, Barud HS, Messaddeq Y, Ribeiro SJL (2009) Synthesis and characterization of silver nanoparticles impregnated into bacterial cellulose. Mater Lett 63(9–10):797–799
Dong BH, Hinestroza JP (2009) Metal nanoparticles on natural cellulose fibers: electrostatic assembly and in situ synthesis. ACS Appl Mater Interface 1(4):797–803
Drogat N, Granet R, Sol V, Memmi A, Saad N, Klein Koerkamp C, Bressollier P, Krausz P (2011) Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J Nanopart Res 13(4):1557–1562
Egal M, Budtova T, Navard P (2008) The dissolution of microcrystalline cellulose in sodium hydroxide–urea aqueous solutions. Cellulose 15(3):361–370
El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18(1):75–82
Ferraria AM, Boufi S, Battaglini N, Botelho do Rego AM, ReiVilar M (2009) Hybrid systems of silver nanoparticles generated on cellulose surfaces. Langmuir 26(3):1996–2001
He J, Kunitake T, Nakao A (2003) Facile in situ synthesis of noble metal nanoparticles in porous cellulose fibers. Chem Mater 15(23):4401–4406
Hetrick EM, Schoenfisch MH (2006) Reducing implant-related infections: active release strategies. Chem Soc Rev 35(9):780–789
Huang H, Yuan Q, Yang X (2004) Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf B 39(1–2):31–37
Hussain I, Hussain SZ, Ihsan A, Rehman A, Khalid ZM, Brust M, Cooper AI (2010) In situ growth of gold nanoparticles on latent fingerprints—from forensic applications to inkjet printed nanoparticle patterns. Nanoscale 2(12):2575–2578
Iwata T, Kimura Y, Tsutsumi K, Furukawa Y, Kimura S (1993) The effect of various phospholipids on plasma lipoproteins and liver lipids in hypercholesterolemic rats. J Nutr Sci Vitaminol 39(1):63–71
Jimenez MA, Scarino ML, Vignolini F, Mengheri E (1990) Evidence that polyunsaturated lecithin induces a reduction in plasma cholesterol level and favorable changes in lipoprotein composition in hypercholesterolemic rats. J Nutr 120(7):659–667
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71(3):343–364
Kesting RE (1965) Semipermeable membranes of cellulose acetate for desalination in the process of reverse osmosis. I. Lyotropic swelling of secondary cellulose acetate. J Appl Polym Sci 9(2):663–688
Kotelnikova N, Panarin E, Shchukarev A, Serimaa R, Paakkari T, Jokela K, Shilov S, Kudina N, Wegener G, Windeisen E (1999) The effect of quaternary ammonium base adsorbates on the molecular and morphological structure of microcrystalline cellulose. Carbohydr Polym 38(3):239–246
Kwon JW, Yoon SH, Lee SS, Seo KW, Shim IW (2005) Preparation of silver nanoparticles in cellulose acetate polymer and the reaction chemistry of silver complexes in the polymer. Bull Korean Chem Soc 26(5):837–840
Lee HY, Park HK, Lee YM, Kim K, Park SB (2007) A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem Commun 28:2959–2961
Liu S, Ke D, Zeng J, Zhou J, Peng T, Zhang L (2011a) Construction of inorganic nanoparticles by micro–nano-porous structure of cellulose matrix. Cellulose 18(4):945–956
Liu S, Zhou J, Zhang L (2011b) In situ synthesis of plate-like Fe2O3 nanoparticles in porous cellulose films with obvious magnetic anisotropy. Cellulose 18(3):663–673
Lu Q, Gao F, Komarneni S (2006) Cellulose-directed growth of selenium nanobelts in solution. Chem Mater 18(1):159–163
Luong ND, Lee Y, Nam JD (2008) Highly-loaded silver nanoparticles in ultrafine cellulose acetate nanofibrillar aerogel. Eur Polym J 44(10):3116–3121
Mahmoud KA, Male KB, Hrapovic S, Luong JHT (2009) Cellulose nanocrystal/gold nanoparticle composite as a matrix for enzyme immobilization. ACS Appl Mater Interface 1(7):1383–1386
Rotello VM (2004) Nanoparticles: building blocks for nanotechnology. Springer, New York
Sarrazin P, Beneventi D, Chaussy D, Vurth L, Stephan O (2009) Adsorption of cationic photoluminescent nanoparticles on softwood cellulose fibres: effects of particles stabilization and fibres’ beating. Colloids Surf A 334(1–3):80–86
Sergeev GB (2006) Nanochemistry. Elsevier Science, Amsterdam
Serp P, Corrias M, Kalck P (2003) Carbon nanotubes and nanofibers in catalysis. Appl Catal A Gen 253(2):337–358
Shipway AN, Katz E, Willner I (2000) Nanoparticle arrays on surfaces for electronic, optical and sensor applications. ChemPhysChem 1(1):18–52
Son WK, Youk JH, Lee TS, Park WH (2004) Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol Rapid Commun 25(18):1632–1637
Tankhiwale R, Bajpai S (2009) Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf B 69(2):164–168
Taylor P, Omotoso O, Wiskel J, Mitlin D, Burrell R (2005a) Impact of heat on nanocrystalline silver dressings. Part II: physical properties. Biomaterials 26(35):7230–7240
Taylor P, Ussher A, Burrell R (2005b) Impact of heat on nanocrystalline silver dressings. Part I: chemical and biological properties. Biomaterials 26(35):7221–7229
Wang Y, Yang Q, Shan G, Wang C, Du J, Wang S, Li Y, Chen X, Jing X, Wei Y (2005) Preparation of silver nanoparticles dispersed in polyacrylonitrile nanofiber film spun by electrospinning. Mater Lett 59(24–25):3046–3049
Wu M, Kuga S, Huang Y (2008) Quasi-one-dimensional arrangement of silver nanoparticles templated by cellulose microfibrils. Langmuir 24(18):10494–10497
Wu J, Zhao N, Zhang X, Xu J (2012) Cellulose/silver nanoparticles composite microspheres: eco-friendly synthesis and catalytic application. Cellulose 19(4):1239–1249
Acknowledgments
We acknowledge the Higher Education Commission (HEC), Government of Pakistan, for financial support to Dr. Sumaira Ashraf for her PhD studies. We are also thankful to ex-National Commission on Nanoscience and Technology (NCNST) and the Ministry of Science and Technology (MoST), Government of Pakistan, for financial support to initiate nano-biotechnology research at NIBGE. IH thanks LUMS School of Science and Engineering (SSE), Lahore, Pakistan for providing start-up funds and supporting his research team. A part of this work was supported by DAAD project (ID 54372132) awarded to IH and Wolfgang J. Parak at Philipps University, Marburg, Germany. We are also thankful to Professor Wolfgang Parak for his support in characterizing the nanoparticle samples and intellectual discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ashraf, S., Saif-ur-Rehman, Sher, F. et al. Synthesis of cellulose–metal nanoparticle composites: development and comparison of different protocols. Cellulose 21, 395–405 (2014). https://doi.org/10.1007/s10570-013-0129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0129-7