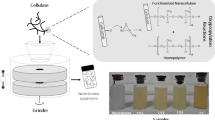
Abstract
Nanocellulose has attracted considerable attention in virtue of the unique mechanical and optical properties. There were less cost-efficient methods for its mass production. In this paper, a facile high yield procedure for cellulose nanofibrils consisting of sulfuric acid treatment followed by alkali neutralization and homogenization was developed. The product was termed “acid-assisted cellulose nanofibrils” (AACNF). AACNF yield was 91.6% from dissolving pulp when 55 wt% sulfuric acid was applied at 45 °C for 40 min. The diameter was 10–20 nm, while the length varied from 40 to 800 nm. AACNF aqueous suspension showed good transparency at 600 nm having rheological properties similar to cellulose nanocrystals. The crystallinity of AACNF was 81.3% and displayed thermal properties that in comparison to traditional nanocellulose were very attractive because of neutralization. This study provided a facile high yield procedure for the production of nanocellulose with similar characteristics to traditional nanocellulose, which was significant to commercialization of nanocellulose.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant natural biodegradable polymer that is composed of β-1,4-linked glycosides. It is the main component in pulp and paper, building materials, blown insulation, biofuels, and textiles (cotton) following chemical or biological conversions (Moon et al. 2011). Currently, increasing attention has been directed to forms of cellulose known as nanocelluloses. Because of their nanoscopic dimensions, large specific surface area, and high aspect ratios, they exhibit unique physical and optical properties (Habibi et al. 2010; Sturcová et al. 2005). They are classified into three categories: microfibrillated cellulose (MFC), cellulose nanocrystals (CNC), and bacterial nanocellulose. In general, diameters are a few tens of nanometers, while lengths of MFC and CNC may be several micrometers and 100–250 nm, respectively. These nanoscale materials can impart extraordinary properties to nanocomposites (Klemm et al. 2011).
MFC is produced from chemical pulp by mechanical treatment in which 5–10 passes through a homogenizer creates a gel-like MFC with pseudo-plastic and thixotropic properties (Klemm et al. 2011) that are assisted by hydrophilic polymers. However, there are several production drawbacks: specific electrical energy consumption is very high and the introduction of charged groups can facilitate fiber delamination. For example, completely defibrillated MFC is obtained through introduction of carboxymethyl groups (Wågberg et al. 1987). These groups enhance pulp fiber swelling and reduce cell wall cohesion resulting in reduced energy consumption. Furthermore, other treatments before homogenization such as refining and enzymatic treatment (Siqueira et al. 2010; Tian et al. 2017; Wang et al. 2015) can decrease energy consumption. (Saito et al. 2006, 2007) developed an efficient way to produce MFC by introducing carboxyl groups using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation. The primary hydroxyl groups at C6 are selectively oxidized to carboxylate groups to allow MFC to well disperse in water and form a transparent solution. CNCs are shorter and thinner than MFC prepared from various cellulosic raw materials. Rånby et al. (1949) first reported in the 1950s the production of nanoscopic suspensions of cellulose by sulfuric acid hydrolysis of cellulose fibers. Acid hydrolysis is the main process to produce CNC in which the amorphous and para-crystalline regions of cellulose are preferentially hydrolyzed to liberate rod-like crystalline cellulose. Sulfuric, hydrochloric, phosphoric and hydrobromic acids have been generally followed by a one-step mechanical treatment such as ultra-sonication. However, maximal yields are between 30 and 40% under standard hydrolysis conditions (64 wt% sulfuric acid concentration at ~ 45 °C for 60 min) (Chen et al. 2016).
Low CNC yield has been the main drawback using sulfuric acid-based hydrolysis (Chen et al. 2015). Efforts to increase yield to date negatively impact final cost. Bondeson et al. (2006) investigated optimizing hydrolysis by an experimental factorial design matrix and demonstrated yields only as high as 30% (based on initial weight). More recently, Hamad and Hu (2010) carried out a systematic investigation of the structure-process-yield interrelations for a commercial softwood kraft pulp in which they obtained high crystallinity (> 80%) but yields between 21 and 38%. Wang et al. (2012) reported CNC production from bleached kraft eucalyptus where cellulose loss approached zero. The process was combined with a recovery of a significant number of cellulosic solids residues (CSR) though a sulfuric acid (58 wt%) hydrolysis process in which the concentration was slightly lower. The recovered CSRs were used for production of nanocellulose through mechanical fibrillation. Later, Wang et al. (2014) demonstrated that acid concentration was the key parameter in controlling CNC production yield in which an abrupt ceiling occurs at acid concentrations of 56 ~ 58 wt%. In addition, Chen et al. (2015) obtained CNC yields ~ 70% by applying a similar procedure at 56 wt% sulfuric acid further illustrating the possibility of enhancing the yield of CNC production through lower sulfuric acid concentrations. Unfortunately, all of the latter work is marred by long times, high water consumption, and inefficient dialysis to remove residual acid.
The objective of this study was to produce nanocellulose with high yield in a short time using less water. The principle is that acid treatment may cause de-swelling by introducing sulfur groups on fibers surface throng esterification and provide a proton to the hydrogen bond between fibrils, but does not break down the β-1,4-glycoside linkages in cellulose molecule. The acid treatment can facilitate the separation of fibrils from fibers, which can be defibrillated to nanocellulose (AACNF) using a high-pressure homogenizer. Herein, when hydrolysis was terminated by water dilution and centrifugation, residual acid in the water-insoluble cellulosic residue (WCR) is neutralized by sodium hydroxide whose salt is removed by centrifugation. By virtue of high-pressure homogenization, the yield and size of AACNFs is tailored.
Materials and methods
Materials
Hardwood dissolving pulp (α-cellulose > 95%) was obtained from a paper mill (Shandong Huatai Paper Co., LTD, China). Sulfuric acid and sodium hydroxide were analytical reagent grade from Guangzhou chemical reagent factory.
AACNF production
All acid treatment experiments were conducted using a 1:8 (g/mL) ratio of fiber solids (oven dry weight) to acid. Sulfuric acid concentration, treatment temperature, and time were varied between 55 and 62 wt%, 35–65 °C, and 20–60 min, respectively. Each sample was immediately diluted sixfold by volume with deionized water to terminate reaction. The WCRs were separated and fully recovered by 3 centrifugations at 5500 rpm at 10 min each. The residual acid in WCRs was neutralized by 0.1 mol/L sodium hydroxide. The salt was removed by two centrifugations. The AACNF solutions were obtained after high-pressure homogenization (UH-60, Union-Biotech, China) at 600 bar over 5 passes. The AACNF yield was calculated by gravimetry.
Zeta potential and transparency measurements
AACNF particles were suspended in water and the surface potential of AACNF was characterized by zeta measurements using a Nanoparticle analyzer (SZ-100Z, Horiba, Japan). Measurements were performed in a cuvette consisting with 100 uL of a 0.5 wt% AACNF suspension. All samples were adjusted to pH 7. The transparency of AACNF suspensions (0.5 wt%) was measured by UV–Vis (Agilent Cary 60, Agilent, USA).
Elemental analysis
The content of S was measured using an elemental analyzer (Vario EL cube, Elementar, Germany). The number of surface sulfate half-ester groups per 100 bulk anhydroglucose units can be calculated based on the formula of C6H10O5-(SO3)n: n can be obtained using S (wt%) = 100n × S/[6C + 10H + (5 + 3n)O + nS], as described by Hamad and Hu (2010).
Atomic force microscope (AFM) characterization
The AACNF diluted suspension was dropped onto clean mica and dried at RT. The morphology of AACNF was observed by a Nanoscope V Multimode atomic force microscope (AFM) (Burker Corporation, Santa Barbara, USA). All samples were measured in tapping mode as 5 × 5 μm images. The length of AACNF was calculated by software (Nano measurer) based on > 100 samples.
Rheological measurements
Rheology measurements were carried out with a rheometer (AR-G2, TA, USA) using parallel plates of diameter = 25 mm. The steady state shear viscosity was measured for shear rate ranges of 0.01–100 rad/s. Isothermal dynamic frequency sweep was performed over the whole accessible frequency range. The strain amplitude used was 0.5–1% during all small-amplitude oscillation tests. To avoid sample evaporation, a small amount of silicone oil was placed on the periphery of samples with an evaporation blocker. All rheological measurements were performed at 25 °C.
X-ray diffraction
Wide-angle X-ray diffraction measurements of AACNF samples were made using a Bruker D8 Discover system with Cu-Kα radiation (Bruker Corp., Germany). The crystallinity was calculated according to the Segal Method (1959).
Thermal measurement
Thermal stabilities of AACNFs were determined using a thermo-gravimetric analyzer TGA (TA Q500 Instruments Inc., USA). Approximately 8 mg freeze-dried samples were heated at a uniform heating rate of 10 °C/min from 30 to 700 °C under a nitrogen flow of 25 mL/min.
Results and discussion
AACNF production
Dissolving pulp fibers having an average length ~ 0.8 mm were treated with sulfuric acid and homogenized to prepare nanocellulose (Rånby et al. 1949; Bondeson et al. 2006; Hamad and Hu 2010; Wang et al. 2012; Chen et al. 2015, 2016). However, low yields, long times, or multi-fractional separations were reported. Herein, AACNF is obtained with high yield by a modified sulfuric acid hydrolysis procedure as observed in Fig. 1. The yield of AACNFs is > 55% when 62 wt% sulfuric acid was used for 80 min at 45 °C. The yield of AACNFs was fairly easily controlled by changing treatment conditions (Fig. 1a–c). For example, a maximum yield of 91.6% was obtained at 55 wt% of sulfuric acid, 45 °C and 40 min (Fig. 1a). In fact, sizes of AACNFs depended on acid concentration consistent with a literature report that acid concentration was a key parameter for AACNF production (Chen et al. 2016).
The yield of AACNFs at different conditions (a the sulfuric acid concentration was 55 wt%, 57 wt%, 60 wt%, and 62 wt%, while the temperature was 45 °C and the reaction time was 40 min; b the temperature was 40–65 °C, while the sulfuric acid concentration was 62 wt% and the reaction time was 40 min; c the reaction time was 20–80 min, while the temperature was 45 °C and the sulfuric acid concentration was 62 wt%.)
In cellulosic plant fibers, cellulose is present in amorphous and crystalline states from both inter-molecular and intra-molecular hydrogen bonding (Wågberg et al. 1987). Acid treatment is a harsh process for obtaining CNCs. At milder treatment conditions such as 55 wt% and 57 wt%, partially amorphous regions yet remain in the raw material, which result in a higher yields as shown in Fig. 1a. Both low and high sulfuric acid concentrations damage hydrogen bonding and facilitate separation of the microfibrils under high-pressure homogenization. Acid treatment is not as faithful in terms of maintaining cellulose polymerization degree and sulfonation. This lack of fidelity depends on acid concentration (Bondeson et al. 2006; Hamad and Hu 2010). Wang studied the kinetics of acid hydrolysis of bleached kraft pulp and found that cellulose did not degrade to smaller molecular sugars when the concentration of sulfuric acid was < 58 wt% (Wang et al. 2014). Higher yields of AACNFs were obtained under these conditions demonstrating results similar to Wang.
Nanocellulose yield is ~ 30–40% from traditional acid hydrolysis of various cellulosic materials (Chen et al. 2016). The main reason is that 64 wt% sulfuric acid leads to excessive degradation and cellulosic solid residues from acid hydrolysis were underutilized. Thus, it may be possible that a milder acid-assisted treatment may be combined with a subsequent alkali neutralization process to overcome degradation and low yields. Hydrogen bonds between fibers and β-1,4 glycoside linkages can be hydrolyzed, but much less degradation occurs. In addition, WCR was collected through centrifugation with nearly zero loss. Neutralization removes the residual acid in WCR rapidly and effectively saving time and water as opposed to dialysis. In addition, neutralization converts the sulfonic acid groups (–SO3H) on the cellulose surface to sodium salts (–SO3Na) promoting re-dispersion of cellulose nanofibrils after fully drying (Beck et al. 2012).
A two-step centrifugation was performed after neutralization to remove the salt (Na2SO4). Oven dried AACNFs obtained from different acid concentrations were calcined in a muffle furnace at 550 °C for 6 h to calculate residual salt content. The results (Table 1) indicated that there was no salt when 55 wt% and 57 wt% sulfuric acid were employed, whereas only a small amount of salt remained at 60 wt% and 62 wt%. Therefore, centrifugation was an effective method to remove the salt after neutralization.
AFM characterization
In Fig. 2, different sizes of AACNFs were obtained after different acid concentration treatments. All AACNFs show needle shaped structures, with diameters of 10–20 nm and lengths of 40 ~ 800 nm that could be controlled by acid treatment. At low acid concentrations (55 wt%), cellulose was not fully depolymerized.
Software was used to calculate the length of the AACNFs shown in Fig. 3 in which more than 100 AACNFs for each sample were measured. The mean lengths of AACNFs obtained from different acid concentrations were 326 nm, 184 nm, 157 nm, and 122 nm whose cross sections were ~ 10–20 nm. This demonstrated that AACNFs were easier to separate through the same mechanical treatment at a higher concentration of sulfuric acid. The length of AACNFs obtained was similar to what was shown previously in which 100–300 nm CNCs were obtained from wood and non-wood cellulosic materials (64 wt% sulfuric acid, 45 °C and 40 min) (Wang et al. 2014).
Surface and optical properties
The treatment conditions affected the size and surface properties of AACNFs. Anionic sulfur groups were introduced on AACNFs particle surfaces during sulfuric acid treatment, which leads to electrostatically stabilized AACNFs aqueous suspensions (Dong et al. 1996; Shafeiei-Sabet et al. 2013). The degree of sulfation of AACNFs determined surface charge density, which in turn affected stability of the suspension. The concentration of sulfuric acid for AACNFs production was the main factor affecting degree of sulfation.
The nanoparticle analyzer measurements in Table 2 indicated that the ζ-potential of all AACNF aqueous suspensions were below − 20 mV verifying that these suspensions were stable. The highest absolute value was 60.1 mV at 57 wt%. Elemental analysis showed that the sulfur content increased rapidly when the sulfuric acid concentration was > 57 wt%. However, the absolute values of zeta potential did not increase with sulfuric acid concentration which was due to inorganic salts in AACNF aqueous suspensions at higher concentrations. There was little sodium salt in WCR after centrifugation because Na+ adsorbs onto negatively charged AACNF surfaces to compress the double layer, and lowering the absolute value of the zeta potential (Liu et al. 2014) similar to traditional methods.
The transparency of AACNF aqueous suspensions was affected by size and degree of sulfation (Isogai et al. 2011; Luo et al. 2018). Figure 4 shows the transparency of 0.5 wt% AACNF aqueous suspensions obtained from different concentrations of sulfuric acid treatment. Transparency of 55 wt%-AACNF aqueous suspensions at 600 nm was ~ 60%, while transparency was > 90% in more concentrated acid. There was little difference in the transparency between samples when > 57 wt% acid was applied. The AFM results demonstrated that the size of 55 wt%-AACNF was much larger. A fraction of the light was scattered as it passed through the AACNF aqueous suspension resulting in lower transparencies. Higher degrees of sulfation improved the stability of the AACNF aqueous suspension which benefited transparency.
Rheological properties
The macroscopic flow behavior is controlled by its complicated liquid crystalline ordered domains (González-Labrada and Gray 2012; Shafiei-Sabet et al. 2012). The phase separation of colloidal aqueous suspensions of nanocrystalline cellulose is a function of its concentration, whereas a chiral nematic ordered phase above a critical concentration and a birefringent gel-like material at even higher concentrations form (Hamad and Hu 2010; Hirai et al. 2009). In addition, phase separation is generally dominated by physical dimension, surface charge, and ionic strength (Hamad and Hu 2010; Shafeiei-Sabet et al. 2013; Xue et al. 1998). AACNF-57 wt% samples were chosen to conduct the rheological measurements and thus maintain the same properties.
The shear viscosity curves over a broad range of concentrations (1–11 wt%) were obtained. A Newtonian plateau at low shear rates appeared at concentrations of 1–3 wt% in which isotropic behaviors were observed due to the alignment of the nanocrystals along the shear direction (Shafiei-Sabet et al. 2014). Another plateau at a concentration of 1 wt% was the result of particle alignment. Nevertheless, only a single shear thinning behavior was observed over the whole shear rate range measurement when the suspension concentration was > 3 wt% mainly because the formation of chiral nematic ordered phases was inhibited by gel formation at high concentrations (Liu et al. 2011; Ureñabenavides et al. 2011).
Isothermal frequency sweep tests were performed to measure linear viscoelastic material functions. The storage modulus, G′, and loss modulus, G″, of AACNF suspensions at different concentrations are shown in Fig. 5. For the 1 wt% AACNF suspension sample, the loss moduli, G″ was higher than the storage moduli, G′, over the whole frequency range. The behavior of the sample was typical of a viscoelastic liquid. In the case of higher than 2 wt% AACNF suspensions, the behavior resembles that of a viscoelastic solid, where the G′ was significantly higher than G″ at all investigated frequency ranges and corresponding samples exhibit gel-like behavior.
XRD analysis
In Fig. 6, peaks were observed around 2θ = 15°, and 23°, corresponding to cellulose I structure, which indicates that the treatments did not significantly damage the crystal structure of the raw cellulose. The AACNFs samples were observed to be more crystalline than the raw material (Zhou et al. 2012). Indeed, the peak of native cellulose I did not shift because of neutralization of CNCs with NaOH (Li et al. 2014; Oun and Rhim 2016).
The crystallinity of AACNFs increased as a result of amorphous region disintegration under acid hydrolysis. The highest crystallinity (CrI) of AACNF was 81.3% when 60 wt% acid was applied, much higher than the CrI = 62% for CNCs isolated from rice straw by traditional methods (Nasri-Nasrabadi et al. 2014). The CrI decreased slightly at a higher acid concentration of 62 wt% due to crystalline degradation (Table 3).
Thermal properties
The thermal stability of AACNFs at different acid concentrations was shown in Fig. 7. The TGA thermograms of AACNFs and raw material showed two steps: all curves showed an initial weight loss at 50–110 °C, attributed to evaporation of free and bound water (Oun and Rhim 2016; Rhim et al. 2015) and a major weight loss over 30–600 °C. Compared with the raw material, decrease of major decomposition temperatures was observed, which might be attributed to increased heat transfer rate due to increased surface area at smaller particle sizes (Jiang and Hsieh 2013). The maximum degradation temperature range was 301–330 °C, much higher than the maximum degradation temperature of CNCs (234 °C) obtained from rice straw by traditional methods. The thermal stability of CNCs isolated by sulfuric acid hydrolysis decreased due to sulfate ester groups; however, the negative effect of sulfate ester groups diminished by neutralization with NaOH (Oun and Rhim 2015). The final residue was higher in AACNFs than in the raw material because the sulfate groups acted as a thermal insulator (Oun and Rhim 2016).
Conclusions
This study provided a facile protocol for AACNF under acidic conditions at high yield. The yield and morphology of AACNF was tailored by controlling treatment conditions, especially acid concentration. The AACNF aqueous suspensions showed transparency at 600 nm, whereas the rheological properties exhibited gel-like behavior when the concentrations were high than 2 wt%. The neutralization of residual acid in WCR for AACNF production greatly reduced the time and water consumption, and made the whole production process more effective as well. The crystalline structure of AACNFs was not affected by the neutralization process as validated by high crystallinity (> 80%). Lastly and most importantly, the neutralization eliminated the negative effect of sulfate ester groups on thermal stability evidenced by a higher maximum degradation temperature.
References
Beck S, Bouchard J, Berry R (2012) Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules 13:1486–1494
Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13:171–180
Chen L, Wang Q, Hirth K, Baez C, Agarwal UP, Zhu JY (2015) Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 22:1753–1762
Chen L, Zhu J, Baez C, Kitin P, Elder TJ (2016) Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem 18:3835–3843
Dong XM, Kimura T, Revol JF, Gray DG (1996) Effects of ionic strength on the isotropic—chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 12:2076–2082
González-Labrada E, Gray DG (2012) Viscosity measurements of dilute aqueous suspensions of cellulose nanocrystals using a rolling ball viscometer. Cellulose 19:1557–1565
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hamad WY, Hu TQ (2010) Structure–process–yield interrelations in nanocrystalline cellulose extraction. Can J Chem Eng 88:392–402
Hirai A, Inui O, Horii F, Tsuji M (2009) Phase separation behavior in aqueous suspensions of bacterial cellulose nanocrystals prepared by sulfuric acid treatment. Langmuir ACS J Surf Colloids 25:497–502
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Jiang F, Hsieh YL (2013) Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr Polym 95:32–40
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466
Li Y, Zhu H, Xu M, Zhuang Z, Xu M, Dai H (2014) High yield preparation method of thermally stable cellulose nanofibers. BioResources 9:1986–1997
Liu D, Chen X, Yue Y, Chen M, Wu Q (2011) Structure and rheology of nanocrystalline cellulose. Carbohydr Polym 84:316–322
Liu D, Li J, Sun F, Xiao R, Guo Y, Song J (2014) Liquid crystal microphase separation of cellulose nanocrystals in wet-spun PVA composite fibers. RSC Adv 4:30784–30789
Luo J, Semenikhin N, Chang H, Moon RJ, Kumar S (2018) Post-sulfonation of cellulose nanofibrils with a one-step reaction to improve dispersibility. Carbohydr Polym 181:247–255
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 42:3941–3994
Nasri-Nasrabadi B, Behzad T, Bagheri R (2014) Extraction and characterization of rice straw cellulose nanofibers by an optimized chemomechanical method. J Appl Polym Sci 131:2113–2124
Oun AA, Rhim JW (2015) Effect of post-treatments and concentration of cotton linter cellulose nanocrystals on the properties of agar-based nanocomposite films. Carbohydr Polym 134:20–29
Oun AA, Rhim JW (2016) Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr Polym 150:187–200
Rånby BG, Banderet A, Sillén LG (1949) Aqueous colloidal solutions of cellulose micelles. Acta Chem Scand 3:649–650
Rhim JW, Reddy JP, Luo X (2015) Isolation of cellulose nanocrystals from onion skin and their utilization for the preparation of agar-based bio-nanocomposites films. Cellulose 22:407–420
Saito T, Nishiyama Y, Putaux JL, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485
Segal L, Creely L, Martin AE, Conrad CM (1959) An empirical method forestimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Shafeiei-Sabet S, Hamad WY, Hatzikiriakos SG (2013) Influence of degree of sulfation on the rheology of cellulose nanocrystal suspensions. Rheol Acta 52:741–751
Shafiei-Sabet S, Hamad WY, Hatzikiriakos SG (2012) Rheology of nanocrystalline cellulose aqueous suspensions. Langmuir 28:17124–17133
Shafiei-Sabet S, Hamad WY, Hatzikiriakos SG (2014) Ionic strength effects on the microstructure and shear rheology of cellulose nanocrystal suspensions. Cellulose 21:3347–3359
Siqueira G, Tapin-Lingua S, Bras J, Perez DDS, Dufresne A (2010) Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 17:1147–1158
Sturcová A, Davies GR, Eichhorn SJ (2005) Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 6:1055–1061
Tian X, Lu P, Song X, Nie S, Liu Y, Liu M, Wang Z (2017) Enzyme-assisted mechanical production of microfibrillated cellulose from Northern Bleached Softwood Kraft pulp. Cellulose 24:3929–3942
Ureñabenavides EE, Ao G, Davis VA, Kitchens CL (2011) Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 44:8990–8998
Wågberg L, Winter L, Ödberg L, Lindström T (1987) On the charge stoichiometry upon adsorption of a cationic polyelectrolyte on cellulosic materials. Colloids Surf 27:163–173
Wang Q, Zhu JY, Reiner RS, Verrill SP, Baxa U, Mcneil SE (2012) Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 19:2033–2047
Wang Q, Zhao X, Zhu JY (2014) Kinetics of strong acid hydrolysis of a bleached kraft pulp for producing cellulose nanocrystals (CNCs). Ind Eng Chem Res 53:11007–11014
Wang W, Mozuch MD, Sabo RC, Kersten P, Zhu JY, Jin Y (2015) Production of cellulose nanofibrils from bleached eucalyptus fibers by hyperthermostable endoglucanase treatment and subsequent microfluidization. Cellulose 22:351–361
Xue MD, Revol JF, Gray DG (1998) Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5:19–32
Zhou YM, Fu SY, Zheng LM, Zhan HY (2012) Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polym Lett 6:794–804
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31570569), the Science and Technology Program of Guangzhou (201704020038), and the foundation of State Key Laboratory of Pulp and Paper Engineering (2017QN01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, Y., Duan, C., Elias, R. et al. A new protocol for efficient and high yield preparation of cellulose nanofibrils. Cellulose 26, 877–887 (2019). https://doi.org/10.1007/s10570-018-2112-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2112-9