Abstract
This paper examines the mechanism of an enzyme pre-treatment on mechanical preparation of microfibrillated cellulose (MFC), the effects of hemicellulase and cellulase, on enhancing of the PFI refining efficiency of Northern Bleached Softwood Kraft pulp for fiber’s devillicate, and changing of morphologies and physical characteristics on fibers, such as specific surface area were investigated, respectively. It was revealed that the enzyme pre-treatment could promote (1) an acceleration of fine productivity from the fibers, (2) intensive reduction of the size of the fibers through mechanical cutting and (or) fibrillation, and (3) energy efficiency in the reduction of fiber length without productivity impairment. However, distinct mechanical actions on the fibers pre-treated with hemicellulase and cellulase were indicated, according to the dissimilar fibrillation patterns and morphological properties found in the MFC product through intensive mechanical refining.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Micro-fibrillated cellulose (MFC) is mainly composed of cellulose nanofibrils (CNF), with other small portions of fibrillar fines, fibers and fiber fragments (Chinga-Carrasco 2011). As a type of raw material artificially prepared from natural cellulosic biomass, the MFC combines the desirable mechanical properties of high strength, stiffness and low weight with a biodegradable, recyclable and renewable resource (Siro and Plackett 2010; Shatkin et al.2014; Osong et al. 2016). A growing interest of applying it in fabrication of composite materials has led to increasing demand for production of MFC.
In natural cellulosic fibers, cellulose micro-fibrils are gathered laterally by means of hydrogen bonding strength (Janardhnan and Sain 2011). To produce MFC, the patterns need to be chemically or mechanically broken down by fiber delamination and fibrillation using technical methods that are conducted or in combination (Nakagaito and Yano 2004; Isogai et al. 2011; Espinosa et al. 2017). The mechanical fabrication and shearing methods that have been popularly applied can be easily scaled for industrial, large-scale use; however, these methods still face certain challenges: (1) high energy consumption (>25,000–70k kWh/ton); and (2) an unstable machining process caused by the clogging or blocking of the micro cells or channels in the equipment by fragments of fiber (Spence et al. 2011; Klemm et al. 2011; Lavoine et al. 2012).
For this reason, comprehensive tests have been performed that combine the mechanical process with prior enzymatic, chemical or mechanical treatments of the fibers (Henriksson et al. 2007, 2008; Paakko et al. 2007, 2008; Zhu et al. 2011; Ankerfors 2012; Qing et al. 2013). The pre-treatment step that provided fiber with less stiffness and cohesive properties proved beneficial to reducing the size of fibers and lowering the fibrillating intensity in the subsequent mechanical process. With a lower energy consumption associated, the processing efficiencies were greatly improved (Siro and Plackett 2010; Lavoine et al. 2012; Chen et al. 2016).
Some previous work has shown the success of employing endoglucanase or cellulase cocktail in the enzyme pre-treatment process to assist the mechanical refining or homogenization of pulp for MFC or CNF production. (Henriksson et al. 2007, 2008; Paakko et al. 2007, 2008; Zhu et al. 2011; Ankerfors 2012; Qing et al. 2013). With high efficiency and selectivity in the cleavage of specific chemical bonds, the enzyme targeted internal bonds and led to a controllable degradation of amorphous cellulose composition in the pulp, yielding smaller fiber fragments or increased cellulosic fiber swelling in water (Henriksson et al. 2007; Henriksson and Teeri 2009). Also, endoglucanase has been used to break down the hydrogen bonding linkages between the microfibrils (Morgado et al. 2000; Perez et al. 2002), which had a positive influence on the release of fibrils from the fiber surface.
Due to high purity and content of cellulosic fibers, bleached kraft pulp and bleached sulfite pulp have become the industrial source materials for MFC production. (Saito et al. 2007; Paakko et al. 2007; Henriksson et al. 2007; Spence et al. 2011; Zhu et al. 2011; Ankerfors 2012). In these materials, the hemicellulose composition took the form of highly branched amorous polysaccharides that strengthen the cell wall by binding neighboring cellulose micro-fibrils via hydrogen bonding (Sarkar and Manfred 2016). A change in the amount and composition of hemicellulose branches significantly altered cell wall strength and microstructure (Silveira et al. 2013). To use of the hemicellulase by which the hemicellulose composition in the devillicate of pulp fibers could be naturally de-structured (Shallom and Shoham 2003) may benefit the fiber fibrillation and micro- or nano- fibril formation.
Rather than cellulase that mainly caused fiber swelling, hemicellulase acts in a different way on fibers by helping the removal of hemicellulose composition which can form a network with microfibrils within the fibers (Cosgrove 2005). Through destruction of the network connectivity of hemicellulose, the role of applying hemicellulase on fiber fibrillation and micro- or nano- fibril formation for mechanical preparation of MFC were still not well known. In exploiting an efficient method for MFC fabrication from woody pulp, it would be of great significance to have the hemicellulase enzyme introduced to improve the mechanical refining efficiency.
In this study, the integration of an enzyme pretreatment of NBSK pulp as well as an intensive PFI mechanical shearing treatment for MFC fabrication was performed. Compared with the more commonly used cellulase enzyme, the different performance of applying hemicellulase on reducing fiber size, promoting fiber fibrillation and releasing fibrils was evaluated. Changes in the cellulosic structures as well as lowered energy consumption were also investigated.
Materials and methods
Materials
The NBSK pulp was obtained from commercial pulp board (Irving Pulp & Paper, NB, Canada). A 25 g pulp board (oven-dry mass weight, ODMW) was pre-soaked in 500 mL distillated water at 25 °C for 4 h. Then the pulp was disintegrated using a British standard disintegrator (Labtech, Laval, Canada), and finally thickening to a total mass of 250 g for use. Three different commercial enzyme preparations (Table 1) were obtained from Novozyme™ North American Inc. (Franklinton, NC, USA). The determination of the activities was following the International Union of Pure and Applied Chemistry recommended procedures. A Noram PFI (Cat No. CA 318, Noram quality control & research equipment Limited, Quebec, Canada) with variable revolution settings was employed to conduct the pre-refining and the secondary refining of the pulp used in this work.
Preparation of MFC
Pre-refining of pulp
The PFI refining is a standard method that achieves the mechanical action applied to pulp between two parallel surfaces that move differentially relative to one another (TAPPI 2008). A 250 g sample of disintegrated pulp with 10% solid consistency (w/w, ODMW) was submitted to the PFI refining. Clearance between the roll and bedplate was 100 µm. After 10k total revolutions, all of the refined pulp was collected and transferred into a 1000-mL plastic beaker (Fisher, Canada). The consistency was adjusted to 2% (w/w) using a 0.05 M sodium citrate buffer for the subsequent enzyme process.
Enzyme hydrolysis of the pulp
Enzyme hydrolysis was catalyzed using two types of enzyme formulas. Dosage of each enzyme in the solutions and parameters in the enzyme process are shown in Table 2. Pulp substrates and the buffer were mixed and preheated to the reaction temperature. Then the enzyme solutions were added into the suspension to initiative the hydrolysis reaction which was carried out in sealed plastic Ziploc® Freezer Bags (Smart Zip Plus® Seal, S.C. Johnson & Son, Inc., USA) using a shaking incubator (89032-226, VWR international, USA) at 200 rpm.
After defined reaction times, the mixture was filtered to remove the liquid using a Buchner funnel with filter paper (Fisherbrand™, Cat No. 09-801E, USA). Then the enzyme treated chemical pulp (ETCP) was thoroughly washed 3 times with 2 L of water to remove any soluble residuals. The ETCP samples were held at 4 °C for 6 h for moisture balancing and then the biomass consistency was further analyzed.
Secondary PFI beating and sampling
A 200 g wet ETCP sample with 10% consistency (containing 20 g dry mass, w/w) was submitted to the PFI beating. All of the secondary PFI-refining was carried out with 10–200k revolutions with a clearance of 50 µm. A 0.5 g wet sample was collected from the PFI chamber after each 50k revolutions and stored at 4 °C until analysis was performed, within 24 h.
Composition analysis
Determination of glucan, xylan and mannan composition was conducted to indicate the positive efficiency of xylanase and mannanase on hemicelluloses removal in the pulp and the MFC product substrate; this determination was done in accordance with the NERL/TP-510-42618 procedure using an ion chromatography (IC) equipped with a pulsed amperometric detector (PAD) and a CarboPacTM PA1 column (Dionex-300, Dionex Corporation, Canada). The hydrolysates were diluted with a factor of 50 and then filtered through a cellulose acetate membrane with pore size of 0.2 µm (VWR, Canada). A 25 µm dilution was loaded on the column for carbohydrate analysis. A 0.1 N NaOH solution was used as the flowing phase at the flowing rate of 0.5 mL/min. Determination of the chemical composition was performed in duplicate.
Changes in morphology of fibers and the fiber contents
The pulp sample was diluted to 0.05% (w/w) by complete blending. 100 µL of suspension was dropped on the silica slide and covered with an overslip for optical microscope examination under 10× amplification (DM 4000M, Leica microsystem Wetzlar GmbH, Germany). Changes in the morphology of the pulp fibers and the content of remaining fiber were captured using Autospec software and processed by ImageJ (v 1.48, National Institute of Health, USA) to have the scale bars attached. Selected photos were regarded to be representative of the fibrils as well as the patterns of the devillicated fibers after the enzyme or PFI processes. At least 3 different fields were randomly photographed on each slide for evaluation of the area proportions of the remaining fibers, including fiber segments and fiber fragments (with diameters > 20 µm) in the range of the total area covered by all sizes of fibers and fibrils (diameter < 1 µm). Photos were processed for evaluation of fiber area percentage using software Image-Pro plus 6.0 (Media Cybernetics, Inc. MD, USA). Duplicated measurements were conducted at each image location, and the average data was reported.
The content of the fibers remaining (RF) in the pulp sample was calculated by Eq. 1:
where RF (%) was the percentage of the fibers, Af (mm2) was the area covered by whole fibers, fiber segments and fiber fragments with diameters greater than 20 µm, and Ap (mm2) was the total area covered by the pulp.
Fiber length and specific surface area of the MFC product
A fiber quality analyzer (FQA, LDA 96074, by OpTest Equipment, Canada) was used in this study to determine the change in fiber length before and after the enzyme treatment. The processed pulp samples were first diluted with distilled water to 0.1% (w/w) and then submitted to the test in duplicate. Determination of the specific surface area (SSA) was conducted according to the method described by Ougiya et al. (1998). Each sample was measured three times.
AFM and SEM morphologies of the fibers and fibrils in the MFC product
The surface topography of the fibers and fibrils in the MFC product was characterized using SEM (JEOL6400SEM, JEOL Ltd., Japan) at an accelerating voltage of 15 kV. Samples were mounted on aluminum stubs using double-sided scotch tape and then coated with approximately 10 nm gold (S150A Sputter coater, Edwards, Crawley, UK). At least three different areas where MFC gathered were examined and photographed. An atomic force microscopy (AFM, MFP-3D™, Santa Barbara, CA, USA) system equipped with a standard silicon cantilever (Asylum research, Santa Barbara, CA, USA) was used to visualize the changes in the patterns on the fiber surface against the enzyme treatment and PFI refining. Phase and topological images were obtained using the non-contact tapping mode in the air with a real-time scanning rate of 1.0 Hz. A set point of 800 mV and an integrated gain of 2.0 were chosen for optimizing the images that were used.
Results and discussion
Enzyme treatment of the pulp
To remove the natural recalcitrance of cellulose to allow better attachment of the enzymes to the biomass surface (Ankerfors 2012), pre-refining was carried out before the enzyme pre-treatment in this study. The effects of the enzyme treatment on the fiber characteristics with respect to pulp recovery, fiber length change and morphology were examined. During the pre-treatment, mass loss was caused mainly by the release of soluble sugars from the cellulose or hemicellulose components in the pulp through enzymatic hydrolysis.
Change in the pulp chemical compositions
Both the hemicellulase and the cellulase slightly modified the chemical composition of the pulp materials (Table 3). The glucan component in the hemicellulase-treated pulp was slightly increased by 0.5%, while the composition of xylan and mannan declined slightly by 0.7 and 0.2%, respectively. Enzyme treatment is a green technology that does not significantly change the chemical composition. In the meantime, the average pulp recovery after hemicellulase and cellulase treatment was 95.7 and 96.4%, respectively. Higher recovery of biomass would be beneficial for providing sufficient necessary substrate for further processing with high productivity.
Effect of enzyme pre-treatment on fiber properties
Compared to the original pulp, the length-weighted fiber length of the samples after cellulase treatment was slightly reduced by 22.8%, with a 29.7% reduction in the mean curl index (Table 3). It was reported that cellobiohydrolase, an important ingredient in the cellulase cocktail, could attack the reducing or non-reducing ends of fibril chains and thus reduce the fiber length (Kumar and Murthy 2013). The 1.3-time reduction of the mean curl index versus that of the length revealed a loosened fiber structure, which may have been caused by the weakened internal connecting forces along the fibril chains; the cleavage of the internal β-1,4-glycosidic bonds in the cellulosic amorphous region induced by the endoglucanases in the cellulase cocktail may also have contributed to the loosened fiber structure. However, pre-treatments with hemicellulase did not appear to modify the fiber properties significantly (Oksanen et al. 1997), although they did cause a slight promotion in the mean curl index and also reduced the fiber length, indicating a transformation in the tense structure of the pulp fiber.
Enzymatic modification on the fiber morphology
Figure 1a-1–a-3 shows that the linear dimensions of the fibers were not changed appreciably after treatment by the enzymes. The formation of small peered cellulose fibrils released from or attached to the surface of the fiber, controlled through the fibrillation of the pre-refining process, was observed (Fig. 1a-1). After pre-treatment with cellulase, the peered fibrils disappeared, leaving a glabrate surface (Fig. 1a-3). In contrast, these fibrils were not sensitive to the treatment with hemicellulose, but remained on the fibers (Fig. 1a-2). This suggests that the hemicellulase had better selectivity in specifically hydrolyzing hemicellulose that did not have a role in the structural composition of the fibers and fibrils, except the role of linkage.
The action of enzymes on the NBSK pulp was observed by means of a scanning electronic microscope (SEM) (Fig. 2). The SEM images of the untreated, hemicellulase- and cellulase-treated fibers are presented in Fig. 2a-1, b-1, c-1, respectively. Compared with the untreated pulp, the hemicellulase only slightly modified the surface of the pulp fibers, leaving similar residuals of primary cell wall and tiny fibril-branches attached to the fiber surface (Fig. 2b-1). This result was consistent with the reports of Liu et al. (2012). However, cellulase-modified fibers provided a more glabrous surface, which agreed with the report of Garcia-Ubasart et al. (2013).
AFM phase images show there was a well-proportioned distribution of the higher phase (>80°) in the pulp without enzyme treatment (Fig. 1b-1). After pre-treatment with hemicellulase, the portions of higher phase distribution declined (Fig. 1b-2). Hemicellulase treatment seemed to have modified the fiber surface by reducing the higher-phase component that coated the fiber’s surface. Because cellulose, hemicelluloses and lignin are the main components in NBSK pulp, the high phase component was recognized as the hemicellulose composition due to its specific sensitivity against the hemicellulase treatment. However, the high phase component may also be relevant to lignin residual, as hemicellulose polymers also bind to lignin in the fiber’s secondary cell wall (Scheller and Ulvskov 2010; Vanholme et al. 2010). Therefore, hemicellulase treatment may remove hemicellulose composition and (or) lignin residuals to clearly expose the fibrils network of the cell wall (Fig. 1c-2). In contrast, pre-treatment with cellulase caused the higher-phase component to be redistributed or concentrated without a remarkable reduction in its content (Fig. 1b-3). As described above, cellulase has strong accessibility to the cellulose fibers and breaks down the surface fibrils by hydrolyzing the internal or external glucosidic bonds in the cellulosic amorphous region. The distribution patterns may be attributed to fibril degradation on the fiber’s surface layer.
Impact of mechanical refining on fiber morphology
PFI beating is a technique that has been widely applied in the mechanical refining of lignocellulosic fiber materials. An intensive PFI beating would be an alternative application in MFC fabrication, as it has been shown to have remarkable effects on fiber in many ways, such as cutting and shortening, external fibrillation, cell-wall delamination, internal fibrillation or swelling, redistribution of hemicelluloses from the interior of the fiber to the exterior, abrasion of the surface at the molecular level to produce a more gelatinous surface, etc. (Lumiainen 2000). In this study, application of intensive PFI refining significantly modified the fibers after enzyme pre-treatment for MFC fabrication in different ways.
Through the cutting and fibrillation of the PFI, the prepared MFC in this study contained several portions: (1) fibers, fiber segments or fiber fragments with cell walls that were not visibly de-structured, with diameters of 20–60 µm; (2) fines with diameters <20 µm, which were defined into three portions: flakes (size < 20 µm, irregular shape), fibril bundles (diameter = 1–20 µm) and fibrils (diameter < 1 µm); and (3) CNF (diameter < 100 nm). To clearly reveal the fibrillation patterns of intensive PFI refining on ETCP treated with hemicellulase and cellulase, we focused on monitoring the dynamics of any changes in length, content and morphology of the fiber portion of the MFC products. As the compositions were heterogeneous in size and dimension, we also evaluated changes in the prepared MFC product by means of the physical properties of the specific surface area, hydrogen bonds and cellulose crystallinity.
Decrease of fiber content in pulp
Figure 3a shows a deceleration trend of the fiber portion when intensive refining was applied with an increase in PFI revolutions. According to a fast or slow decreasing rate, all three dynamic curves could be approximately divided into two phases. Before mechanical refining, fiber content remained at 100, 86.3 and 90.5% in the control sample and the ETCP after pre-treatment with hemicellulase and cellulase, respectively. When the PFI revolutions reached 100k, the fiber content declined by 57.9, 47.2 and 68.5% to 42.1, 45.6 and 28.5%, respectively. After that, the rate of fiber reduction slowed. The control sample and ETCP after pre-treatment with hemicellulase and cellulase lost only 24.1, 29.7 and 20.4% of their fiber content. Compared to the control sample and ETCP pre-treated with cellulase, hemicellulase pre-treatment slowed the rate of the fiber de-structuring in the ETCP.
In Fig. 3a, the two cross points reveals a gentler decline of the fiber content in ETCP after hemicellulase treatment, compared to that in the ETCP treated by cellulase or without any treatment. Cellulose degradation by the cellulase treatment had directly reduced the weighted mean length of the ETCP fibers, which subsequently contributed to a rapid decrease of the fiber content once the PFI refining was applied. By means of hemicellulase treatment, release of the hemicellulose associated with the major cellulose composition could have the fiber structure slightly loosened (Przybysz Buzała et al. 2016). Although the loosen structure did not promote a further increase of the fiber susceptibility to PFI refining, it could protect the fiber from an intensive mechanical breakage due to an improved flexibilization or better lubrication between fibers and fines (Liu et al. 2012; Oksanen et al. 1997).
Change in morphological characteristics of fibers
Figure 3b shows the simultaneous realization of the reduction of the fiber length in all samples accompanying the decrease in fiber content when intensive PFI refining was applied (10–200k revolutions). Cellulase pre-treatment led to the highest rate of the decline in fiber length. This can be explained by the ETCP fiber with cellulase treatment possessing a distinct way to be split or disintegrated during the mechanical refining. The fibers were dramatically cut apart into smaller fiber fragments and small flakes (Figs. 2c-2, 4c-2). With intensive refining, distribution of the pieces was expanded, creating smaller aggregates around the fiber fragments (Fig. 4c-3, c-4). The average fiber length decreased from over 1.45 mm in the original NBSK fibers to 0.57 mm at 10k revolutions, decreased to 0.27 mm at 50k revolutions, and finally was 0.19 mm at 200k revolutions. This reveals that treatment with cellulase allowed sites with loosened or weakened intermolecular connecting forces to be easily cut by mechanical refining. In cellulose amorphous regions, cellulase efficiently hydrolyzed the glucosidic bonds that acted as the linkages of the cellulosic crystal rods along the axis of the cellulose chains. A partial breakdown of the linkages would not affect the fiber length after the treatment with cellulase, but rendered the sites in the fiber more susceptible to mechanical refining. The accumulation of fiber segments or fragments led to the acceleration of mechanical fibrillation from the fiber terminals that in turn led to the remarkably rapid decrease in the fiber content remaining in the pulp.
The ETCP with hemicellulase treatment and the control sample showed dissimilar patterns in length reduction versus that of the fiber treated with cellulase. The average fiber lengths in both decreased gradually from 1.8–1.9 mm to 0.26–0.41 mm (Fig. 3b). Even after 200k revolutions, fibers with lengths of over 1 mm were found in the prepared MFC product (Fig. 4b-4). With the increase in the PFI revolutions, more fibrils were observed peeling off from the surface of the fiber cell wall in the ETCP with hemicellulase treatment compared to the control sample; nevertheless, the dimensions of the single fibers in both samples remained roughly the same (Fig. 4a-3, a-4, b-3, b-4).
Through morphological comparison, different mechanisms of fibrillation were indicated according to the alternative locations where fibrillation tended to occur. For untreated NBSK fibers, the fibers could be split apart into smaller fiber segments or fragments that were further disintegrated into thinner fibril bundles (Fig. 4a-2). Fibril branches with further fibrillation readily appeared at the fiber ends rather than on the fiber surface (Fig. 4a-3). For the ETCP pre-treated with hemicellulase, more side-branches were peeled away in multiple sites along the fiber’s axis, and they were fibrillated afterward by the increasing of the revolutions (Fig. 4b-2–b-4). These multi-branches seemed to be of importance for the acceleration of the fibrillation, and thus the increasing of the content of fibrils in the pulp. To further explore the differences in fibrillation mechanisms between untreated fibers and ETCP with hemicellulase treatment, we preformed a screening of the changes in the surface morphologies of the fibers and fibrils using SEM and AFM imaging methods, respectively. The fibrillation of the un-treated NBSK pulp showed a split of the entire fiber into fibril bundles (Fig. 4a-2) followed by a peeling of the fibrils from the surface of the bundles (Figs. 2a-3, a-4, 5b-1). In contrast, the hemicellulase pre-treatment promoted a relaxation of the helical twine-like structure (Fig. 2b-2–b-4) that led to a gradual uncoiling of the fibrils. AFM observation clearly showed an uncoiling pattern in the fibrils in the ETCP with hemicellulase treatment (Fig. 5b-2). As the hemicellulase may significantly hydrolyze the hemicellulose composition in the pulp, the distinct patterns in the fibrillation of the ETCP with hemicellulase treatment may be caused by the breakdown of the linkages in the hemicellulose network within the cellulose fibers. Furthermore, some research has indicated that small molecules, like oligosaccharides, may promote fibrillation by acting as lubricants during mechanical refining. However, explaining the differences in fibrillation patterns requires further investigation.
Morphology and diameter distributions of fibrils and CNF
Fibrils with visible diameters of less than 1 µm in the MFC products were observed by means of optical microscopy (Fig. 5a-1–a-3). The shift in the patterns on the fiber surface layer after either hemicellulase or cellulase treatment showed that different mechanisms were causing the release of microfibrils from the fiber surface. Hemicellulase treatment caused partial destruction of the hemicellulose component, and caused the microfibrils to loosen up from the cell wall at multiple sites more easily (Fig. 5a-2). Pulp fiber after cellulase treatment was more easily cut into shorter fiber segments (Fig. 5a-3, b-3) through an intensive PFI refining with over 100k revolutions.
The ETCP with cellulase treatment contained large amount of fibrils with a reduction in length (around 5 µm), while fibrils with an estimated length >100 µm dominated in the MFC products made from ETCP with hemicellulase treatment (Fig. 5a-2, a-3). Therefore, fibrils from the ETCP with cellulase treatment had a lower aspect radio (>5), but a crystalline structure dominated in the fibrils due to a hydrolysis of the amorphous region during the cellulase treatment. However, the fibrils showed a high aspect radio (>100) in the MFC products made from ETCP with hemicellulase treatment.
With 200k revolutions, diameters of CNF produced from the ETCP with hemicellulase treatment were between 50 and 100 nm, similar to the untreated pulp as seen through analysis of the SEM images (Fig. 2a-4, b-4). However, the appearance of the CNF with diameters of less than 100 nm was very rare in the MFC product made from ETCP with cellulase treatment (Fig. 2c-4).
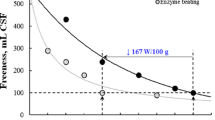
Electrical energy-saving efficiency and characterization of specific surface area of the MFC product
Considering the similar features of the MFC product from the untreated pulp and the ETCP with hemicellulase treatment, the effect of the hemicellulase pre-treatment on the energy consumption of mechanical refining was evaluated according to the decreased rate of fiber content, which was accomplished through PFI refining with a controllable clearance and countable revolutions, which may be equivalently calculated to energy consumption. As shown in Table 4, applying either hemicellulase or cellulase achieved a 75–33.3% reduction in energy consumption. As the PFI refining in this study was a batch process without a supply of fresh raw materials, the energy consumption in fiber fibrillation might be exponentially increased with a further accumulation of the smaller fines that diluted the fiber concentrations. The contribution of both enzyme pre-treatments to energy saving gradually declined with the intensive refining, especially for the hemicellulose, for which the electrical energy-saving efficiency dropped from 75% down to 0%.
For an indirect prediction based on an equivalent reduction of fiber content achieved, applying enzyme pre-treatment to the downstream mechanical fabrication of MFC production showed a positive prospect in improving energy efficiency. It was consistent with previous reports suggesting the significant electrical energy-saving effect of applying hemicellulase in mechanical refining (Bajpai 2011; Przybysz Buzala et al. 2016).
Moreover, this study revealed the positive role of hemicellulase pre-treatment in the remarkable promotion of changes in the pulp’s specific surface area, which may greatly improve the properties of the MFC product for high-quality applications (Fu et al. 2016). As shown in Fig. 3c, the SSA of all of the samples increased gradually with the increase of the refining intensity. However, the ETCP with cellulase treatment and the NBSK pulp showed a dramatic decline in their SSA at 200k revolutions. In contrast, the SSA of the ETCP with hemicellulase treatment continued to grow and reached 175.93, which was the 2.36 and 2.89 times over the values for the ETCP with cellulase treatment and the NBSK pulp, respectively. This indicates the positive effects of hemicellulase treatment on the ETCP fibers toward preventing the random kinking and aggregation of the fines when they became smaller in size. The better lubrication effect may be caused by loosened hydrogen bonds within the cellulose chains and the addition of the repulsive charges of the carboxyl group (Liu et al. 2012); however, this is a hypothesis that requires further investigation.
Conclusions
The hemicellulase and cellulase contributed distinct mechanisms of action while they were applied in assisting the mechanical refining of fiber cell walls for MFC production. Rather than cutting fiber apart into smaller fiber fragments and flakes by the use of cellulase, hemicellulase led to a gradual uncoiling of the fibrils from the fiber surface in multiple sites along the fiber’s axis. Relied on benefiting the intensive fibrillation, hemicellulase shows a promising prospect to be used in pre-treating the chemical pulp for MFC production but still needs to be further evaluated for scaled-up applications.
Abbreviations
- AFM:
-
Atomic force microscopy
- ETCP:
-
Enzyme treated chemical pulp
- FQA:
-
Fiber quality analyzer
- IC:
-
Ion chromatography
- MFC:
-
Microfibrillated cellulose
- NBSK:
-
Northern Bleached Softwood Kraft
- ODMW:
-
Oven-dry mass weight
- PAD:
-
Pulsed amperometric detector
- SEM:
-
Scanning electronic microscope
- SSA:
-
Specific surface area
References
Ankerfors M (2012) Microfibrillated cellulose: energy-efficient preparation techniques and key properties. Dissertation, KTH Royal Institute of Technology
Bajpai PK (2011) Emerging applications of enzymes for energy saving ln pulp and paper industry. Ippta J 23:181–186
Chen N, Zhu JY, Tong Z (2016) Fabrication of microfibrillated cellulose gel from waste pulp sludge via mild maceration combined with mechanical shearing. Cellulose 23:2573–2583. doi:10.1007/s10570-016-0959-1
Chinga-Carrasco G (2011) Cellulose fibres, nanofibrils and microfibrils: the morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res Lett 6:417
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Espinosa E, Domínguez-Robles J, Sánchez R, Tarrés Q, Rodríguez A (2017) The effect of pre-treatment on the production of lignocellulosic nanofibers and their application as a reinforcing agent in paper. Cellulose. doi:10.1007/s10570-017-1281-2
Fu J et al (2016) A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc-air batteries. Energy Environ Sci 9:663–670. doi:10.1039/C5EE03404C
Garcia-Ubasart J, Torres AL, Vila C, Pastor FIJ, Vidal T (2013) Biomodification of cellulose flax fibers by a new cellulase. Ind Crop Prod 44:71–76. doi:10.1016/j.indcrop.2012.10.019
Henriksson G, Teeri T (2009) Biotechnology in the forest industry. In: Ek M, Gellerstedt G, Henriksson G (eds) Wood chemistry and biotechnology. Walter de Gruyter GmbH &Co. KG, Berlin, pp 273–300
Henriksson M, Henriksson G, Berglund LA, Lindstrom T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J 43:3434–3441. doi:10.1016/j.eurpolymj.2007.05.038
Henriksson M, Berglund LA, Isaksson P, Lindstrom T, Nishino T (2008) Cellulose nanopaper structures of high toughness. Biomacromolecules 9:1579–1585. doi:10.1021/bm800038n
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85. doi:10.1039/c0nr00583e
Janardhnan S, Sain M (2011) Isolation of cellulose nanofibers: effect of biotreatment on hydrogen bonding network in wood fibers. Int J of Polym Sci 2011:6. doi:10.1155/2011/279610
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Edn 50:5438–5466
Kumar D, Murthy GS (2013) Stochastic molecular model of enzymatic hydrolysis of cellulose for ethanol production. Biotechnol Biofuels 6:63. doi:10.1186/1754-6834-6-63
Lavoine N, Desloges I, Dufresne A, Bras J (2012) Microfibrillated cellulose—its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym 90:735–764. doi:10.1016/j.carbpol.2012.05.026
Liu N, Qin Menghua, Gao Y, Li Z, Fu Y, Xu Q (2012) Pulp properties and fiber characteristics of xylanase-treated Aspen APMP. BioResources 7:3367–3377
Lumiainen J (2000) Refining of chemical pulp. In: Paulapuro H (ed) Papermaking science and technology book 8: papermaking part 1, stock preparation and wet end, 1st edn. Fapet Oy, Helsinki, pp 87–122
Morgado J, Cavaco-Paulo A, Rousselle MA (2000) Enzyme pre-treatment of lyocell-clarification of depilling mechanisms. Text Res J 70:696–699
Nakagaito AN, Yano H (2004) The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl Phys A 78:547–552. doi:10.1007/s00339-003-2453-5
Oksanen T, Pere J, Buchert J, Viikari L (1997) The effect of trichoderma reesei cellulases and hemicellulases on the paper technical properties of never-dried bleached kraft pulp. Cellulose 4:329–339. doi:10.1023/A:1018456411031
Osong SH, Norgren S, Engstrand P (2016) Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23:93–123. doi:10.1007/s10570-015-0798-5
Ougiya H, Hioki N, Watanabe K, Morinaga Y, Yoshinaga F, Samejima M (1998) Relationship between the physical properties and surface area of cellulose derived from adsorbates of various molecular sizes. Biosci Biotechnol Biochem 62:1880–1884. doi:10.1271/bbb.62.1880
Paakko M et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941. doi:10.1021/bm061215p
Paakko M et al (2008) Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 4:2492–2499. doi:10.1039/b810371b
Perez J, Munoz-Dorado J, de la Rubia T, Martinez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Przybysz Buzała K, Przybysz P, Kalinowska H, Derkowska M (2016) Effect of cellulases and xylanases on refining process and kraft pulp properties. PLoS One 11:e0161575. doi:10.1371/journal.pone.0161575
Qing Y, Sabo R, Zhu JY, Agarwal U, Cai Z, Wu Y (2013) A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr Polym 97:226–234. doi:10.1016/j.carbpol.2013.04.086
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Sarkar P, Manfred A (2016) Organization of the plant cell wall. In: Ray JR (ed) Molecular cell biology of the growth and differentiation of plant cells. CRC Press, Boca Raton, pp 101–119
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61(1):263–289
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6(3):219–228
Shatkin JA, Wegne TH, Bilek EMT, Cowie J (2014) Market projections of cellulose nanomaterial-enabled products—Part 1: applications. Tappi J 13:9–16
Silveira RL, Stoyanov SR, Gusarov S, Skaf MS, Kovalenko A (2013) Plant biomass recalcitrance: effect of hemicellulose composition on nanoscale forces that control cell wall strength. J Am Chem Soc 135:19048–19051. doi:10.1021/ja405634k
Siro I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494. doi:10.1007/s10570-010-9405-y
Spence K, Venditti R, Rojas O, Habibi Y, Pawlak J (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18:1097–1111. doi:10.1007/s10570-011-9533-z
Technical Association of the Pulp and Paper Industry (2008) Laboratory beating of pulp (PFI mill method), Test Method T 248 sp-08. TAPPI, Peachtree Corners
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153(3):895–905
Zhu JY, Sabo R, Luo X (2011) Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem 13:1339–1344. doi:10.1039/c1gc15103g
Acknowledgments
The authors acknowledge the financial support of Scientific Research Foundation of Guangxi University (XGZ160294), the Research Fund of State Key Laboratory of Pulp and Paper Engineering (201351), and the National Science Foundation of Guangxi (2015GXNSFBA139042), Dean Project of Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control (ZR201607), as well as the Opening Project of Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control (KF201603).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, X., Lu, P., Song, X. et al. Enzyme-assisted mechanical production of microfibrillated cellulose from Northern Bleached Softwood Kraft pulp. Cellulose 24, 3929–3942 (2017). https://doi.org/10.1007/s10570-017-1382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1382-y