Abstract
Traditionally, naturally extracted Indigo has been used for dyeing cotton. Amongst all the classes of dyes for cellulosic material, vat dyes are most widely used due to their excellent fastness properties. The vat dyeing process, depending upon the subclasses, has to go through reduction phase for solubilisation, dyeing and oxidation phases at specific conditions. Even with the advent of new techniques like the electrochemical and bacterial process, their industrial use is relatively limited. Prior investigation on bacterial reduction suggests only a few possible varieties of indigo-reducing bacteria, sources of most of which are still unknown. Also to implement this processes, they are required to be performed at higher temperatures. In the present study, we have developed a novel method of vatting and dyeing using bacterial cell lysate at room temperature followed by air oxidation. This paper also compares the newly proposed processing route with the existing conventional ones, and the experimental results have shown promising results regarding improvement in dye uptake, faster dyeing, and better levelness along with their fastness properties. Besides, the proposed process ensures energy saving, dye effluent load reduction and simplifies the existing process.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A recent archaeological research report (Splitstoser 2016) on the identification of extracted indigoid dye (indigotin) from a 6000 years old cotton fabrics found at Huaca Prieta, Peru invalidates the earlier scientific history on indigo dyeing from Fifth Dynasty Egypt (around 4400 years back) (Ferreira et al. 2004). They stated that “Indigo is one of the most valued and most globally widespread dyes of antiquity and of the present era (it being the blue of blue jeans)”. In India, as long as 5000 years ago, water-insoluble pigment, i.e. vat dyes (Indigo) dyeing methods were being used to dye natural cellulosic fibres (Aspland 1992). In fact, the fastness properties to light, washing etc. of vat dyes are unbeatable (Philips 1996; Zollinger 1991). In the colouration of cellulosic fibres, the vat (including Indigo) and sulphur dyes hold a large part of the dyestuff market (Brady 1992). They both, all together relatively represent about 31% dyestuff market (Brady 1992); among them, about 120,000 tons of vat dyes are being used annually in 2004 (Roessler and Jin 2003). The application of indigo for denim dyeing is almost unique (Bechtold et al. 1994).
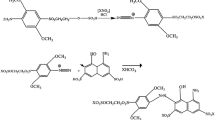
Nevertheless, the vat dyes have somewhat complicated application procedures, involving a reduction step with a strong reducing agent (Chakraborty and Chavan 2004) like sodium hydrosulphite to become a water-soluble form of the parent dye called salt of leuco-vat (Fig. 1). In this form, they have high substantivity towards the cellulosic fibre (Aspland 1992), and then the dyeing phase recommended to be continued till an equilibrium is achieved for dye adsorption and diffusion. After dyeing phase, it is oxidised back to the original water-insoluble form in situ in the fibre (Aspland 1992/2; Božic 2008). The current industrial practice of vat dyeing temperature ranges are from 50 to 80 °C depending upon the characteristic of vat subclass; classified into four groups as per application like Iw (warm), IN (normal), Ik (cool) and IN special (special) (Baumgarte 1987a, b; Shenai 1996).
From ancient times, the reduction of indigo could also be achieved by fermentation technique for the extended overnight period using ripe fruit and stale urine along with wood ash or lime as alkali, followed by repeated dipping and air exposure (Roessler and Jin 2003; John 2009). So far different methods have been reported for the reduction processes of vat dyes using electrochemical method, (Chavan et al. 1999) by bacteria (Nicholson and John 2005), or by indirect electrolysis using iron tri-ethanolamine complex as a reducing agent (Nicholson and John 2005), and other reducing agents such as sodium dithionite, salts of organic sulphonic acids, sodium borohydride, organic polyhydroxy compounds (glucose, hydroxyacetone), sulphides and polysulphides (Kulandainathan 2007; Bechtold et al. 1994; Baumgarte 1987a; Etters 1995). Also, many scientists have used various organic and inorganic compounds to reduce vat dyes in the absence of common reducing agent sodium hydrosulphite, such as carbohydrate, ferrous sulphate etc. (Baumgarte 1987b). However, the aim of the most of the different methods developed so far was to address the toxicity issue of sodium hydrosulphite by-products. None of them addresses the critical component of energy utilisation aspect of this reaction.
Importantly, the microbial reduction has been investigated, reported and patented frequently in early 2000; sooner or later for achieving an attractive alternative (specifically, oxidation at a mild temperature in less time (US 5948122 A.1999; Padden et al. 2000; Nicholson and John 2004, 2005; Pricelius 2007; Kim 2010; Božič et al. 2010; Park 2012). It is noteworthy to mention the Korean patent (KR101513853B1. 2015) that indicates these biological reduction methods using microorganisms are very complicated to identify (Božič et al. 2010). They are not only time consuming, but challenging to implement in a real industrial process; justifying the use of a potent reducing agent for industrial use (US20160222582). Currently, Milanović et al. (2017) reported, “Based on the current knowledge of indigo-reducing bacteria, it is considered that indigo-reducing bacteria constituted only a small fraction in the unique microcosm detected in the natural indigo dye vats.” Hence, it may be concluded that most of the novel vat dyeing applications are in development stage due to the limitations associated and more research directives are indicated for effluent colour removal rather (Bankole et al. 2017).
In fact, the dyeing phase of cotton by vat dyes requires a temperature of 50–80 °C depending on the different vat sub-classes. This process is an age-old technique, which is being followed by weavers, hand-dyers, and so as the dyeing factories. Specifically for traditional hand dyers, the most significant disadvantages of this process are the use of fossil fuels that resulted in high environmental load along with difficulties in precise temperature control (Jena 2015). For industrial dyeing, Rosa et al. 2014 estimated ecological cost of a typical vat dyeing; the consumption of calorific and electrical energy of the standard vat dyeing process was reported to be 1.58 × 106 J, 4.06 × 103 J respectively with gas fuel requirement 9.77 × 10−3 m3 and water consumption 50 L/Kg of cotton. In this age of energy crisis, it is highly desired to develop alternative methods to minimise energy requirement for reduction and dyeing. Though a precise temperature control may not be much essential for different vat dyes, however, a different category of dye requires precise temperature control to get better colour absorption (Baumgarte 1987a, b; Shenai 1996; Chavan et al. 1999; Chakraborty and Chavan 2004). In fact, improper temperature control results in losing significant dyeing cost for unutilized and expensive vat dyes those practised by non-industrial dyers like handloom dyers.
Alternatively, a method was developed to utilise the bacterial cell lysate, which, presumably a bio-reducer that helps in reduction of vat dyes. By reducing the particle size of the dye component, it facilitates the reduction and dyeing process at room temperature in less time. In search of a right kind of bacteria, we tried to analyse thermophilic bacteria, which has been isolated from hot spring. The bacteria were cultured and lysed. The lysate was used as a catalyst for the reduction of vat dye effectively at room temperature for successful dyeing of cotton textiles, and thus, the application of temperature (50–80 °C) is eliminated.
Material and experimental
Cotton
Lea’s of 2/120s semi-bleached mercerised cotton were taken for dyeing.
Dyes
The following 8 colours of vat dyes (Navinon) has been collected from IDI, Mumbai and used for 1% shade
-
1.
Golden Orange 3G (C.I. Vat orange 15).
-
2.
Brown R (C.I. Vat Brown 3).
-
3.
Pink (C.I. Vat Red 1).
-
4.
Violet RR (C.I. Vat Violet 1).
-
5.
Olive R (C.I. Vat Black 27).
-
6.
Black CH (C.I. Vat Black 16).
-
7.
Green FFB (C.I. Vat Green 1).
-
8.
Blue BC (C.I. Vat Blue 6).
Chemicals
Sodium hydrosulphide (Hydro:Na2S2O4) and sodium hydroxide flakes were collected from National Handloom Development Corporation (NHDC).
Preparation of bacterial cell lysate
Bacterial samples were collected from hot spring (Atri hot spring is located in the Khurda district of Odisha (20°09′N 85°18′E) in eastern India, Table 1), (Mohanty et al. 2014) and plated on LB-Agar plate without any antibiotics. Few single colonies were picked and were grown in LB medium supplemented with sodium thiosulphate at 55 °C. Colonies that survived the temperature were grown in the same condition overnight until they reached the stationary growth phase. At this point, the optical density @ 600 nm was 2.5, and the cell mass was 2.5 gm/100 ml of culture. The cells were harvested by centrifugation and lysed using sonicator and lysozyme. The lysate thus formed was taken for the experiment. The detail description on lysate and its composition is beyond the scope of this article, and the role of bacterial cell lysate on vat dyeing is discussed in the present study.
Dyeing process
1% shade (0.8 gm of dye) was taken for each dye on 80 gm of 2/120S semi-bleached mercerised cotton in lea form. Exhaust method yarn dyeing was carried out for both the cases with material to liquor ratio (M:L) 1:20. The amount of hydro and caustic was used as per the recommendation of dyestuff supplier. The processes adopted for comparisons are as follows:
-
(A)
Process 1 (new process)—dyeing at room temperature using required quantities of bacterial cell lysate, hydro and caustic i.e. dyes stuff + caustic + hydro + cell lysate(6% weight of the dyestuff) → vatting for 10 min → dyeing for 30 min → air oxidizing → soaping → air drying.
-
(B)
Process 2 (conventional process)—dyeing at required temperature (50–80 °C) in presence of required quantities of hydro and caustic i.e. dyes stuff + caustic + hydro → vatting for 15 min → dyeing for 40 min → air oxidizing → soaping → drying.
Machinery
Exhaust dyeing was carried out in superlab dyeing machine by Supertex instruments enterprises, Mumbai. The measurement of different fastness parameters and breaking strength were tested and collected from Textile Committee, Kolkata, which is a NABL accredited laboratory. The spectral reflectance and colour parameters were measured in dual beam visible spectrophotometer, at CET, Bhubaneswar.
-
Fastness properties:
Colour fastness to artificial light with Xenon Arc Lamp, standard: IS 2454:1985-RA 2006.
Colour fastness to washing at 40 °C was tested with IS/ISO 105-C10-A1-2006.
Colour fastness to washing at 50 °C was tested with IS/ISO 105-C10-B2-2006.
Colour fastness to rubbing was tested with IS766:1988 RA 2009.
-
Breaking Strength of Single yarn was tested in Newton with IS 1670:1991 RA 2007.
-
Colour strength as K/S value was measured for colour difference as DE, dL*, da*, db* in computer colour matching m/c (Model SS5100H by Premier colourscan ltd., Mumbai).
-
XRD: LabX XRD- 6100, X-ray Diffractometer, Shimadzu.
-
Particle size analyser: determination of hydrodynamic diameter and zeta potential were evaluated by Zetasizer (Malvern, UK).
Results and discussion
Mechanism
In fact, there are four multiple stages in vat dyeing; reduction to water-soluble form (salt of leuco vat dye), then dyeing stage (adsorption and diffusion into the fibres till equilibrium is attended), oxidation phase to water-insoluble form again followed by after treatment (Fig. 1). As discussed earlier, the current industrial vat dyeing practice, the temperature is recommended to be in the range of 50–80 °C, depending upon application sub-classes like Iw, IN, Ik and IN special (Baumgarte 1987a, b; Shenai 1996; Chavan et al. 1999; Horne 1995; Chakraborty and Chavan 2004). Earlier studies (Valko 1941) has shown the importance and role of particle size in the vat dyeing of cellulose for their dye adsorption, diffusion and uptake. It has been widely recognised later that the reduction of smaller vat particles are more accessible than the larger ones (Vickerstaff 1954; Roessler and Jin 2003; Nicholson and John 2004, 2005) studied carefully the size reduction with C. isatidis and others that revealed the average diameter of the indigo particles could drop from 35 to 3 μm. The particle size has been derived as hydrodynamic diameter measured by dynamic light scattering technique. Initial decreased size followed by increased trend can be attributed to the thermal expansion of dye molecule followed by their aggregation. The analysis of zeta potential supports the data (Fig. 2). It was observed that the particle size (hydrodynamic diameter) of Vat blue had been reduced from 5200 to 600 nm and that of the Vat Pink from 2260 to 245 nm. However, in both cases, the particles underwent aggregation phases before particle size reduction. The Vat pink has shown a distinct trend for initial aggregation and drastic reduction further to smallest size with the rise of zeta potential after undergoing minimally. This phenomenon may be responsible for the greatest dye uptake (K/S) for pink dyes (Fig. 4d) and the highest colour difference (DE). It is attributed to their lowest particle size among the eight (significant size reduction; estimated hydrodynamic diameter from 2060 to 245 nm).
The novelty in our case is that this new bacterial vatting and dyeing process has shown superior performance in terms of quality at room temperature in less time that ensures lower energy demand with much simplified technical processing route. The details of the dyestuffs structure, colour index no. (C.I. No.), their specified vatting and dyeing temperature and achieved colouration effects has been summarised in Table 2.
The bacterial cell lysate is composed of proteins, enzymes, lipids as well as various cofactors. As discussed earlier, their constitution and mechanism are complex (Milanović et al. 2017), and cell lysate is probably acting as catalysts to reduce vat dyes to leuco form along with scaling down the particle size. It is important to mention here that the lysate does not assist in the reduction of vat dyes without the addition of sodium hydrosulphite even at high temperature.
We analysed a typical XRD for VAT violet 1 (Fig. 3) for comparing both processes; the three major peaks were recorded in Table 3 and the detailed magnified profiles were provided in supplementary file.
From the Table 3 for three strongest peaks and magnified peak profile with a subtracted background, an asymmetrical shift, peak broadening and lower full width at half maximum (FWHM) in case of new process (E) were observed. This macro strain broadening phenomenon indicates that the unit cells displacements or dislocation about their normal positions or domain boundaries, surfaces etc. It could be described as “Grain Surface Relaxation” (http://prism.mit.edu/xray), which may be attributed to the reduction reaction mechanics in inhomogeneity solution and temperature factors. Importantly, the XRD analysis only could infer more on the crystallite size (different than particle size), and we found that particle size has been reduced. It is noteworthy to mention here the study on morphological details of indigo dye crystals on reduction by SEM analysis (Vuorema 2008); specifically the delamination and disintegration of the indigo crystal sheets upon reduction. The XRD of new process with broader peaks indicates the crystallite size getting smaller as compared to traditional one.
Dye uptake, colour parameters and chromaticity
The K/S values over visible wavelength for each 1% shade of eight different vat colours developed by recommended conventional process and our new process at room temperature was measured in the spectrophotometer. The comparative K/S plots (dye uptake comparison of conventional and proposed) and their respective CIE L*a*b*c*h* [L*(Lightness/darkness) a* (redder/greener) b*(bluer/yellower) c*(purity) h* (hue angle)] values are shown in Fig. 4. Higher dye uptake with better levelness is achieved in each case of the newly proposed process than the conventional one. It is imperative that the particle size reduction capability of lysate resulted in higher adsorption and diffusion of the dyes even at room temperature; their colour difference values are also prominent visually (DE > 0.5). This is highest in case of pink as discussed earlier. In industrial practice, it is a well-known difficulty to dye specifically black and pink very often, but they were dyed successfully at room temperature in the presence of bacterial cell lysate (Fig. 5).
Their detailed spectral readings are provided in the supplementary file. Besides, the chroma (purity of colour), hue angle (attribute) readings and the CIE plot (Fig. 6) were given for better perception and prediction (Barik et al. 2017). The visual shade matching/difference, the minimal changes in purity direction ensures the colour tone of dyeing has not significantly different from the parent dye though K/S or dye update has been enhanced. The statistical analysis has been provided in the supplementary file.
Fastness properties
We further tested the fastness properties of vat-dyed samples with this new process (process 1) and conventional process (process 2). The results are given in Table 4. It was observed that the washing and light fastness results were same in case of both the processes and the rubbing fastness results were almost comparable.
Strength
Further, the lea breaking strength was analysed for two processes. The mean standard error for breaking strength test of process 1 was found to be 4.57%, and in case of process 2, it was 3.8%. As indicated in Table 4. there is no significant difference in the breaking strength of the yarns processed by two methods, however, in some case, the breaking strength of yarn lea dyed with the new process is better than the breaking strength of yarn lea dyed with the conventional method.
Conclusion
A simple, faster and sustainable alternate process for vat dying at room temperature is established by using bacterial cell lysate that ensures superior performances concerning dye uptake, fastness properties and levelness. In addition, the wastage of dye in dye bath is reduced significantly with much-simplified application protocol. The vat dye particle size reduction by the application of cell lysate has been confirmed from hydrodynamic diameter estimation, zeta potential analysis, XRD study and colour analysis of eight typically selected vat dyes. It is evident that the said room temperature process has potential industrial application in reducing energy. In fact, vat dyes being used majorly in rural handloom sectors and they use dry woods, coal and other environment polluting sources for conventional dyeing. More natural control at room temperature with quality dyeing would be helpful for a sustainable socioeconomic growth with the reduced environmental load.
References
Aspland JR (1992) Vat dyes and their application. Text Chem Color 24(1):22–24
Bankole PO et al (2017) Degradation of indigo dye by a newly isolated yeast, Diutina rugosa from dye wastewater polluted soil. J Environ Chem Eng 5(5):4639–4648
Barik S et al (2017) Nano-Mg–Al-layered double hydroxide application to cotton for enhancing mechanical, UV protection and flame retardancy at low cytotoxicity level. Cellulose 24(2):1107–1120
Baumgarte U (1987a) Redukions-und Oxidations-Prozessebeim Färbenmit Küpenfarbbstoffen. Melliand Textilber 189(68):276–281
Baumgarte U (1987b) Developments in vat dyes and their application 1974–1986. Rev Prog Color 17(1):29–38
Bechtold T et al (1994) The reduction of vat dyes by indirect electrolysis. Color Technol 110(1):14–19
Božič D (2008) Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigments 76(2):299–309
Božič M et al (2010) Enzymatic reduction of complex redox dyes using NADH-dependent reductase from Bacillus subtilis coupled with cofactor regeneration. Appl Microbiol Biotechnol 85(8):563–571
Brady PR (1992) Diffusion of dyes in natural fibres. Color Technol 22(1):58–78
Chakraborty J, Chavan RB (2004) Dyeing of denim with indigo. Ind J Fib Text Res 29(3):100–109
Chavan RB et al (1999) Chemical processing of handloom yarns and fabrics, 1st edn. Department of Textile Technology IIT, New Delhi
Etters JN (1995) Advances in indigo dyeing: implications for the dyer, apparel manufacturer and environment. Text Chem Color 27(2):17–22
Ferreira ES et al (2004) The natural constituents of historical textile dyes. Chem Soc Rev 33(6):329–336
Horne CM (1995) A review of vat dyeing on cotton yarns. Text Chem Color 27(12):27–30
Jena B (2015) Eco-friendly processing of textiles. Mater Today Proc 2(4–5):1776–1791
John P (2009) “Indigo-extraction” Handbook of natural colorants. Wiley, New York, pp 105–133
Kim MK (2010) A study on the dyeing conditions and properties of cotton fabric dyed with natural polygoum tinctoria. Unpublished master’s thesis, Ewha Woman’s University, Seoul
Kulandainathan MA (2007) Potentiostatic studies on indirect electrochemical reduction of vat dyes. Dyes Pigments 73(1):47–54
Milanović V et al (2017) Insight into the bacterial diversity of fermentation woad dye vats as revealed by PCR-DGGE and pyrosequencing. J Ind Microbiol Biotechnol 44(7):997–1004
Mohanty S et al (2014) The Atri hot spring in Odisha—a natural ecosystem for global warming research. Int J Geol Earth Environ Sci 4(1):85–90
Nicholson SK, John P (2004) Bacterial indigo reduction. Biocatal Biotransform 22(5–6):397–400
Nicholson SK, John P (2005) The mechanism of bacterial indigo reduction. Appl Microbiol Biotechnol 68(1):117–123
Padden AN et al (2000) Indigo-reducing Clostridium isatidis isolated from a variety of sources, including a 10th-century viking dye vat. J Archaeol Sci 27(10):953–956
Park S (2012) Isolation and characterization of alkaliphilic and thermotolerant bacteria that reduce insoluble indigo to soluble leuco-indigo from indigo dye vat. J Korean Soc Appl Biol Chem 55(1):83–88
Philips D (1996) Environmentally friendly, productive and reliable: priorities for cotton dyes and dyeing processes. Color Technol 112(7–8):183–186
Pricelius S (2007) Enzymatic reduction of azo and indigoid compounds. Appl Microbiol Biotechnol 77(2):321–327
Roessler A, Jin X (2003) State of the art technologies and new electrochemical methods for the reduction of vat dyes. Dyes Pigments 59(3):223–235
Rosa JM et al (2014) Development of colors with sustainability: a comparative study between dyeing of cotton with reactive and vat dyestuffs. Text Res J 84(10):1009–1017
Shenai VA (1996) Technology of textile processing volume-VI, technology of dyeing, 7th edn. Sevak Publications, Bombay, p 211
Splitstoser JC (2016) Early pre-Hispanic use of indigo blue in Peru. Sci Adv 2(9):e1501623
Valko EI (1941) Particle size in the vat dyeing of cellulose. J Am Chem Soc 63(5):1433–1437
Vickerstaff T (1954) Physical chemistry of dyeing. Oliver and Boyd, London
Vuorema A (2008) Reduction and analysis Methods of indigo. Thesis, Department of Chemistry, University of Turku, Finland
Zollinger Z (1991) Color chemistry: syntheses, properties and applications on organic dyes and pigments, 2nd edn. VCH Publishers Inc, New York, p 496
Acknowledgments
Our sincere thanks to D.C. Handloom, Govt. of India, Ministry of Textiles for funding this research project. We express our gratitude to the textile committee, ICT, Mumbai, College of Engineering and Technology (CET, TEQIP-II), KIIT University for their kind support for testing. A Patent application titled “Improved Process for Dyeing of Textiles” has been filed at the patent office on February 16, 2015; application number ‘186/KOL/2015’.
Author information
Authors and Affiliations
Contributions
SKP, AKP and PO developed the Cell lystate bacteria for vat dyeing and planned experiments. NSS and AK also planned some experiments, conducted and prepared the manuscript draft. All authors contributed their suggestions for editing the final manuscript.
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patra, S.K., Patra, A.K., Ojha, P. et al. Vat dyeing at room temperature. Cellulose 25, 5349–5359 (2018). https://doi.org/10.1007/s10570-018-1901-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1901-5