Abstract
Autohydrolysis technology is widely used for extracting lignocellulose from wood chips. In this study, the flow through autohydrolysis of spruce wood chips was studied fundamentally. In addition to rising temperature to 185 °C and prolonging time of hydrolysis to 75 min, a flow rate of 2 L/min led to significant lignocellulose removal from wood chips. However, the lower temperature of 170 °C, 15 min and flow rate of 6 L/min led to lignocellulose with larger molecular weight in the hydrolysis liquor. Gel permeation chromatography analysis confirmed the existence of lignin–carbohydrate complexes (LCC) in the hydrolysis liquors. The compositions of lignin, hemicelluloses and LCC in hydrolysis liquors were related to autohydrolysis conditions. Nineteen percent of lignin moieties were in LCC form in the hydrolysis liquor produced under the conditions of liquid/solid (L/S) ratio of 10 (wt/wt), 180 °C and 15 min. Furthermore, the hydrolysis liquor produced under the conditions of L/S ratio of 5/1, 190 °C and 15 min contained 89% of lignin in LCC forms. Moreover, the efficiencies of membrane dialysis, acidification and ethanol extraction in extracting lignocellulose were different for different hydrolysis liquors implying that the properties of hydrolysis liquors (and thus the hydrolysis conditions) would significantly affect the performance of downstream processes for isolating lignocellulose from hydrolysis liquors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic materials consist mainly of cellulose, hemicellulose, lignin and extractives (Ayeni et al. 2015; Sarip et al. 2016). They have great potential for use in the production of value-added products. Cellulose is widely used for ethanol production as well as in pharmaceutical and papermaking industries (Garcia et al. 2011; Shokri and Adibkia 2013; Zheng et al. 2009). Hemicelluloses could be used for ethanol, xylitol and furfural productions (Chandel et al. 2011; Pothiraj et al. 2006). Lignin could be applied in the production of carbon fibers, car brakes, polyurethane foams and dispersants (Fatehi et al. 2016; Lora and Glasser 2002; Vishtal and Kraslawski 2011).
To use these lignocellulosic materials in value-added productions, they should be extracted from woody biomass effectively. Autohydrolysis technology is a chemical-free, efficient, cost-effective and commercially used method for extracting hemicelluloses from woody biomass (Carvalheiro et al. 2008; Sixta et al. 2013). The autohydrolysis process could be performed using hot water or steam (Galia et al. 2015; Pienkos and Zhang 2009; Santos Muguet et al. 2013). However, hemicellulose extraction is associated with lignin extraction in the autohydrolysis processes (Carvalheiro et al. 2008).
In the past, research on the autohydrolysis process was conducted in batch systems (Galia et al. 2015; Kumar and Christopher 2017). However, the flow through technology may have advantages over batch systems. For example, more lignin and hemicellulose extraction and less fermentation inhibitors were claimed to be produced in the flow through process compared to the batch process (Galia et al. 2015; Liu and Wyman 2003).
It was reported in the literature that autohydrolysis conditions impacted the isolation of lignocellulose from wood chips. The effect of temperature and residence time on autohydrolysis process were studied for batch systems (Kapu and Trajano 2014). Ando et al. (2000) stated that the majority of lignin and hemicelluloses could be extracted from bamboo and chinquapin in a flow through process in the temperature range of 180 and 285 °C. However, Japan cedar lignin showed high resistance against isolation from wood chips at the temperature range of 180 and 250 °C (Ando et al. 2000). It was reported that the flow through autohydrolysis of corn stover under the conditions of 0–10 mL/min flow rate, 180 °C and 16 min treatment time isolated 20–57.5 wt% of xylan and 14.7–34 wt% of lignin (Liu and Wyman 2003).
Water consumption is an important economical factor in the hydrolysis process (Jansson et al. 2014), and consequently liquid to solid (L/S) ratio is crucial at determining the feasibility of the autohydrolysis process. Tunc (2014) reported that an increase in the L/S from 3/1 to 50/1 resulted in an improvement in removing lignin from 2 to 4 wt% from Eucalyptus globulus in a batch system. The increase in L/S ratio also decreased the polysaccharide and monosaccharide extractions from 3 to 1.6 wt% and from 1.2 to 0.3 wt% from wood chips, respectively. In another batch system studied by Testova et al. (2009), lignin and xylan concentrations increased with the L/S ratio rise in the hydrolysis liquor for birch wood species. These results depicted that the amount of water was a major factor in separating lignocellulose in the autohydrolysis process. However, research on flow through autohydrolysis was mainly conducted on agro-based biomass. It is well known that softwood has more condensed structure than agro-based biomass (Fox and McDonald 2010). Thus, available literature results may not be conclusive for predicting the performance of flow through autohydrolysis of softwood species. The first objective of this study was to investigate the impact of flow through autohydrolysis process conditions on the extraction of lignin and hemicelluloses from spruce wood chips.
As stated above (Ando et al. 2000; Liu and Wyman 2003), the amounts of lignin and hemicellulose extractions in hydrolysis processes were correlated, implying that (1) the separation of lignocellulose from hydrolysis liquor may not produce pure hemicelluloses or lignin and (2) lignin and hemicelluloses may have chemical bonds and be present in lignin–carbohydrate complexes (LCC) in hydrolysis liquors. Lawoko et al. (2006) claimed that almost all of lignin and polysaccharides are bound in spruce wood species. In the autohydrolysis process, lignin and polysaccharides may be separated as individual segments or still present in LCC form in hydrolysis liquors. Recently, the presence of LCC in the hardwood-based hydrolysis liquor and neutral sulfite semichemical (NSSC) spent liquor were reported (Fatehi et al. 2016). The second objective of this study was to investigate the presence and properties of LCC in softwood-based hydrolysis liquor produced in an autohydrolysis process.
It is well known that the concentration of lignocellulose is low in hydrolysis liquors. To develop economically feasible process for the end-use application of lignocellulose, they should be extracted from the hydrolysis liquors. It was reported that lignin and hemicelluloses could be effectively isolated from prehydrolysis liquor (PHL) and black liquor of kraft pulping processes via acidification or organic solvent extraction (Liu et al. 2011a, b; Tarasov et al. 2015). Membrane technology has been widely used for isolating lignin and hemicelluloses from hydrolysis liquors (Oinonen et al. 2015). Du et al. (2013) applied membrane dialysis for the separation of LCC from a hydrolysis liquor. The third objective of this work was to assess the efficiency of different methods in isolating lignocellulose from hydrolysis liquors that were produced under different conditions.
In this research work, the autohydrolysis of softwood chips (spruce) was comprehensively studied in a flow through system. The main purpose of this work was to evaluate the effect of process conditions on the performance of autohydrolysis in extracting lignocellulose from spruce. The presence of LCC and the impact of autohydrolysis on the properties of LCC in the hydrolysis liquors was investigated for the first time. Based on the concentration, compositions and molecular weights of lignin and hemicelluloses in hydrolysis liquors, the properties of LCC in hydrolysis liquor was proposed. Furthermore, the efficiency of different extraction processes in isolating lignocellulose from hydrolysis liquors was investigated in detail.
Materials and methods
Materials
Spruce wood chips were obtained from a pulp and paper mill located in Northern Ontario, Canada. The wood chips were stored in sealed plastic bags at 4 °C prior to use. Ethanol (95 vol.%), sulfuric acid (98 wt%) and sodium hydroxide powder (97 wt%) were obtained from Fisher Scientific and used as received. NaNO3 powder, analytical grade, was purchased from Sigma Aldrich company. Cellulose acetate membrane tubes (molecular weight cut-off of 1000; 10,000 and 25,000 g/mol) were purchased from Wako Chemicals, Japan. Also, 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP) was purchased from Sigma Aldrich company.
Autohydrolysis
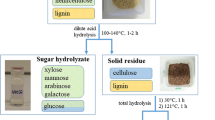
The process of autohydrolysis was conducted using a 2 L pulping digester (Greenwood Instruments, 2200). In this analysis, liquid is collected from the bottom of the digester and circulated to the top to provide a flow through process. In each experiment, 300 ± 5 g of wood chips were loaded in the pulping digester and distilled water was poured into the vessel. The impact of liquid/solid (L/S) ratio on the autohydrolysis of softwood chips is not reported in the literature for flow through systems. In the past, the autohydrolysis experiments of softwood species was only conducted in batch systems with the L/S ratio ranging 4/1 and 10/1 wt/wt (Kapu and Trajano 2014). In the present work, the L/S ratios of 4/1, 5/1, 6/1, 8/1 and 10/1 wt/wt (based on the dry weight of wood chips) were carried out.
Song et al. (2008) conducted hot water treatment of spruce chips and sawdust in a batch system. It was reported that the temperature rises from 160 to 180 °C resulting in a significant lignin extraction from both wood chips and sawdust. In the current study, the experiments were conducted at temperatures of 170, 175, 180, 185 and 190 °C for the process time range of 15 and 75 min. The residence time study was conducted after optimizing the hydrolysis temperature. In another work, the flow through autohydrolysis of spruce sawdust was conducted with only fresh water (without liquid circulation) for preventing hemicellulose degradation (Leppanen et al. 2011). In the present study, hydrolysis liquor was circulated in the digester to resemble the process applied in industry.
Furthermore, a liquid flow rate ranging between 2 and 6 L/min was used in this work. For temperature effect assessment, experiments were conducted at 15 min residence time; for residence time effect, they were carried out at 180 °C; both sets of experiments were carried out at the L/S ratio of 5 wt/wt and liquid flow rate of 6 L/min. The experiments for L/S ratio effect was conducted at 180 °C and 6 L/min flow rate for 15 min. The experiments for the flow rate effect were performed at 180 °C for 15 min residence time and L/S ratio of 5 wt/wt. After each experiment, the produced hydrolysis liquor was collected and kept at 4 °C in a refrigerator prior to analysis.
Separation of lignocellulose from hydrolysis liquors
The lignocellulose were separated from hydrolysis liquors using different methods. (1) The hydrolysis liquor was concentrated using membrane dialysis with three molecular weight cuts-off of 1000, 10,000 and 25,000 g/mol. The dialysis tubes were filled with approximately 50 ± 1 g of hydrolysis samples and placed into distilled water for 48 h. The distilled water was changed every 8–12 h. Then, the samples were transferred from dialysis tubes to plastic containers and stored at 4 °C as previously discussed. (2) Lignin isolation via acidification of spent liquors has been widely reported in the literature (Liu et al. 2011a; Shi et al. 2011). In one set of experiments, the pH of 100 ± 5 g hydrolysis liquor was decreased to 1.5 by adding 20 wt% sulfuric acid to hydrolysis liquor at room temperature (Liu et al. 2011a; Shi et al. 2011). After acidification, the acidified samples were placed in containers and centrifuged at 4000 rpm for 5 min. Afterward, the supernatants and the precipitants were separated. The supernatants were stored in a refrigerator at 4 °C. The precipitants were transferred to glass containers and placed in an oven at 60 °C for 48 h. (3) Hydrolysis liquors were mixed with ethanol at the ethanol/hydrolysis ratio of 4/1 wt/wt as described by Liu et al. (2011a). Due to limited solubility of lignocellulose in ethanol (Tarasov et al. 2015), lignocellulose aggregate via hydrogen bonding in the mixture and precipitate (Fatehi and Chen 2016).
Lignin, hemicelluloses, acetic acid and furfural analyses
The acid-insoluble and acid-soluble lignin contents of wood chips were analyzed in accordance with TAPPI 222 and TAPPI Method 250, respectively (Dashtban and Qin 2012). The cellulose content of wood chips was measured via applying the Kurschner-Hoffer method (Reid and Lynch 1937). The hemicellulose content of wood chips was determined in accordance with TAPPI T223. The extractive content of wood chips was determined via solvent (i.e., ethanol) extraction method following TAPPI T280.
The lignin content of hydrolysis liquor was analyzed in accordance with TAPPI UM 250 using UV spectrophotometry at 205 nm (GENESYS 10S UV/vis, Thermo Scientific) (Liu et al. 2011a). In the autohydrolysis process, monosaccharides and oligosaccharides isolate from biomass and dissolve in hydrolysis liquors (Leppanen et al. 2011). The amount of monosugars was measured using an ion chromatography (Dionex, ICS 5000, Thermofisher Scientific), which had a CarboPacTM SA10 column and an electrochemical detector (ED) (Dionex-300, Dionex Corporation, Canada). This instrument cannot detect polysugars. For determining polysugar concentration, the hydrolysis liquors were pretreated with 4 wt% sulfuric acid at 121 °C for 1 h in accordance with the procedure described by Liu (2008). This process leads to the conversion of polysugars to monosugars (Liu 2008), implying that all sugars in the hydrolysis liquors will be in monomeric forms. This analysis leads to the determination of total sugars (i.e., hemicelluloses) present in the hydrolysis liquor. The amount of monosugars in the hydrolysis liquor was determined using ion chromatography and the results were considered as total sugars of hydrolysis liquors. The concentrations of acetic acid (Ac) and furfural (Fur) in hydrolysis liquors were assessed using a nuclear magnetic resonance (NMR) spectroscopy (Varian Unity Inova 500 MHz) in accordance with the methods applied in the past (Saeed et al. 2012). The TSP was applied as internal standard for measuring the concentrations of acetic acid and furfural in the hydrolysis liquor.
To determine the mass of lignocellulose isolated from wood chips, the concentrations of lignocellulose in hydrolysis liquors were determined as described above. Considering the volume of hydrolysis liquors and the concentration of lignocellulose in hydrolysis liquors, the total mass of lignocellulose removed from wood chips were calculated. Based on the original content of lignocellulose in wood chips, the removal of lignocellulose from wood chips was identified for hydrolysis liquors produced under different conditions.
Molecular weight
Approximately, 5 g of the hydrolysis liquor samples were diluted in 0.1 mol/L NaNO3 to generate a 5 g/L concentration. Then, the measurements were conducted using a gel permeation chromatography, Malvern GPCmax VE2001 Module + Viscotek TDA305 with multi-detectors using PolyAnalytic PAA206 and PAA203 columns. A solution of 0.1 mol/L NaNO3 was utilized as solvent and eluent. In the measurements, column temperature was set at 35 °C and the flow rate was set at 0.70 mL/min. The molecular weight of lignin was analyzed via UV detector at 280 nm wavelength. The molecular weight of hemicelluloses was determined using reflective index (RI) and intrinsic-differential pressure (IV-DP) detectors (Fatehi et al. 2016). This technique was previously used for evaluating the molecular weight of lignin, hemicelluloses and LCC in various spent liquors (Fatehi et al. 2016; Lawoko et al. 2005).
Results and discussion
Wood compositions
The compositions of spruce wood chips are listed in Table 1. It contained 26 wt% acid insoluble lignin, 17 wt% hemicelluloses, 45 wt% cellulose and a balance of extractives. Mannose was the main sugar of spruce. These results are well in agreement with literature results (Sjostrom 1993).
Hydrolysis process
Figure 1 depicts isolation efficiency of lignin from wood chips obtained under different conditions of autohydrolysis. As seen, lignin was isolated more from wood chips at higher temperature and L/S ratio as well as longer residence time. Other researchers have observed similar trends in the hydrolysis of different biomass species (Leppanen et al. 2011; Song et al. 2008; Tunc 2014). Table 2 lists the properties of hydrolysis liquors produced under different conditions. At a higher temperature or longer residence time, more acetic acid was generated, which increased the acidity of the system, improving the lignin isolation from wood chips (Leppanen et al. 2011; Song et al. 2008). Leppanen et al. (2011) obtained 21% lignin isolation via flow through autohydrolysis of Norway spruce sawdust at 240 °C and noted that the amount of lignin in the hydrolysis liquor was increased at L/S ratio of larger than 6/1. Tunc (2014) and Testova et al. (2009) also noted that the higher ratio of L/S increased lignin isolation in the autohydrolysis of hardwood chips. The higher amount of hydrolysis liquor led to a higher possibility for lignin dissolution from wood chips in hydrolysis liquors (Tunc 2014). Flow rate showed insignificant effect on lignin extraction (Fig. 1).
Amount of lignin isolated from spruce wood chips under different autohydrolysis conditions. (experimental conditions a 15 min, L/S ratio of 5 wt/wt and flow rate of 6 L/min, b 180 °C, L/S ratio of 5 wt/wt and flow rate of 6 L/min; c 180 °C, 15 min and flow rate of 6 L/min; d 180 °C, 15 min and L/S ratio of 5 wt/wt)
Figure 2 shows the amounts of hemicelluloses in the hydrolysis liquors extracted from the wood chips under different conditions. The concentration of hemicelluloses in the hydrolysis liquor increased when the temperature increased to 185 °C and then decreased at a higher temperature. This reduction is attributed to the degradation of hemicelluloses into furfural and other products at elevated temperatures (Table 2) (Kapu and Trajano 2014; Song et al. 2008). The concentration of monosaccharides in the hydrolysis liquor was between 3.8 and 12.8% of total mass of hemicelluloses in wood chips. The removal of monosaccharides from wood chips increased with rising temperature and reached the maximum at the highest temperature studied (190 °C). Other researchers also observed a rise in the monosaccharides concentration with temperature increase in the autohydrolysis process (Leppanen et al. 2011; Song et al. 2008). This phenomenon can be explained by the acidity increase in the system as acetic acid was generated more greatly in the hydrolysis at a higher temperature (Table 2) (Song et al. 2008).
Total sugar and monosugars amounts isolated from spruce wood chips under different autohydrolysis conditions. (experimental conditions a 15 min, L/S ratio of 5 wt/wt and flow rate of 6 L/min, b 180 °C, L/S ratio of 5 wt/wt and flow rate of 6 L/min; c 180 °C, 15 min and flow rate of 6 L/min; d 180 °C, 15 min and L/S ratio of 5 wt/wt)
Furthermore, the hemicellulose concentration reached the maximum at 60 min residence time. Further time elongation did not affect the hemicelluloses extraction from wood chips. Leppanen et al. (2011) reported that half of hemicelluloses were extracted from biomass at 180 °C after 30 min of water hydrolysis. However, the mass of monosaccharides extracted from wood chips gradually increased with prolonging the residence time (Fig. 2), which is ascribed to the degradation of hemicelluloses to monosugars under acidic conditions at a prolonged time (Song et al. 2008). Song et al. (2008) conducted hot water hydrolysis of spruce in a batch system at 180 °C and obtained 39.7 and 65.4% removals for monosaccharides after 60 and 100 min of residence times, respectively.
It was observed that the extraction of total sugars (30%) and monosaccharides (7.5%) were insignificantly affected by L/S ratios (Fig. 2). Tunc (2014) and Testova et al. (2009) also reported an insignificant change in the hemicellulose extraction with changing the L/S ratio in the autohydrolysis of hardwood chips. This could be explained by better dissolution performance of carbohydrates at a higher liquid mass in the hydrolysis process (Testova et al. 2009).
Interestingly, the flow rate of liquid circulation affected the hemicellulose removals in that the maximum removal was obtained at the minimum liquid flow rate of 2 L/min. Monosugars also had the highest concentration in the hydrolysis liquor at the lowest flow rate of 2 L/min. This could be related to the higher acetic acid concentration in lower liquid flow rate as explained earlier (Table 2).
Table 2 also lists the compositions of hydrolysis liquors obtained under different conditions. It was observed that the amount of galactose and mannose in the hydrolysis liquor reached the maximum at 180 °C and 60 min. In the past, xylose and mannose represented major parts in the sugars of softwood species (Leppanen et al. 2011; Song et al. 2008). The glucose concentration in the hydrolysis liquor increased at a temperature above 180 °C. This phenomenon could be related to the cellulose degradation at this temperature (Thoorens et al. 2014; Yu and Wu 2010). The cleavage of bonds between galactose and galactoglucomannan backbone under acidic hydrolysis conditions may generate galactose, but harsh hydrolysis conditions may decompose it (Sjostrom 1993). This can be the reason for the maximum concentration of galactose at 185 °C. The maximum concentration of arabinose in hydrolysis liquors obtained at the lowest hydrolysis intensity (i.e., low temperature and time), which might be due to the liberation of arabinose linkage from arabinoglucuronoxylan chain under acidic conditions of hydrolysis (Sjostrom 1993). However, arabinose degraded under harsher experimental conditions due to its instability under acidic conditions (Song et al. 2008). It is seen in Table 2 that at temperature of 190 °C, furfural content is increased, while polysaccharides amount decreased. This behavior is attributed to the sugars decomposition to furfural and other by-products (Kapu and Trajano 2014; Song et al. 2008).
Impact of process conditions on molecular weight
Lignin with two different molecular weights of 800–1200 and 55,000 g/mol was found in hydrolysis liquors, and they remained unchanged, regardless of hydrolysis conditions (i.e., time, temperature, L/S and liquid flow rate). In another work, the molecular weight of lignin extracted from milled wood and enzyme treated Norway spruce were 23,500 and 53,850 g/mol, respectively (Tolbert et al. 2014).
Figure 3 presents the molecular weight of hemicelluloses in hydrolysis liquor. It is seen that the molecular weight of hemicelluloses decreased as temperature increased (Leppanen et al. 2011; Song et al. 2008), which provided evidence for the degradation of hemicellulose at an elevated temperature (Leppanen et al. 2011). Apparently, the molecular weight of hemicelluloses was higher at 180 °C than at 175 or 185 °C. It was reported that cellulose start to degrade at 180 °C (Yu and Wu 2010). It is possible that the molecular weight of cleaved glucan originated from cellulose was higher than other hemicelluloses available in the hydrolysis liquors and that contributed to the overall increase in the Mw of hemicelluloses at 180 °C. Santucci et al. (2015) observed a significant increase in the cellulose removal from sugarcane bagasse via autohydrolysis at 180 °C. However, further temperature increase (> 180 °C) led to the degradation of the dissolved lignin and hemicelluloses (Fig. 2 and Table 2) and thus a decrease in molecular weight (Fig. 3a).
Molecular weight of hemicelluloses in the hydrolysis liquors obtained under different conditions (experimental conditions a 15 min, L/S ratio of 5 wt/wt and flow rate of 6 L/min, b 180 °C, L/S ratio of 5 wt/wt and flow rate of 6 L/min; c 180 °C, 15 min and flow rate of 6 L/min; d 180 °C, 15 min and L/S ratio of 5 wt/wt)
The results also depict that the molecular weight of hemicelluloses in hydrolysis liquors decreased with time extension (Fig. 3b). A similar phenomenon was observed by Leppanen et al. (2011) on the flow through autohydrolysis of saw meal Norway spruce. This behavior is also related to the degradation of hemicelluloses at an extended time under acidic conditions. The L/S ratio insignificantly affected the molecular weight of hemicelluloses, which was also reported by Tunc (2014).
Interestingly, the molecular weight of hemicelluloses slightly increased as the liquid flow rate increased. Liu and Wyman (2003) hypothesized that long-chain LCC became isolated from biomass in a flow-through process and a high flow rate probably helped the isolation process.
At a higher flow rate, the chemistry of hydrolysis is not changed, thus the total amounts of hemicelluloses extracted would not change (Fig. 3 and Table 2), but a more turbulent system (and thus a higher shear rate) is provided at a higher flow rate within wood chips in the digester, which may accelerate the removal of larger hemicelluloses (than smaller ones) from wood chips.
Selection of hydrolysis liquor
Based on the results, four samples were chosen for further analysis. These samples were produced at the L/S ratio of 5/1, 180 °C and 15 min (sample 1); L/S ratio of 5/1, 180 °C and 45 min (sample 2); L/S ratio of 5/1, 190 °C and 15 min (sample 3); and L/S ratio of 10/1 and 180 °C for 15 min (sample 4). The flow rate was 6 L/min for producing these samples. Samples 1 and 2 had relatively high hemicellulose contents and Samples 3 and 4 had higher lignin concentrations.
Characteristics of lignin–carbohydrate complexes in hydrolysis liquors
Figure 4 shows the detectors’ responses in molecular weight analysis of sample 1. It is observable that the low molecular weight lignin (800–1200 g/mol) had a similar retention time with that of hemicelluloses. Similar results were observed for other samples (see supplementary materials). However, there was no RI and IV-DP responses for lignin with the higher molecular weight (Fig. 4). Similar retention times for UV, IR and IV-DP signals were reported as evidence of LCC’s presence in spent liquors in previous studies (Tunc et al. 2010; Fatehi et al. 2016). Therefore, it can be assumed that lignin moieties with low and high molecular weights were present in the hydrolysis liquors in LCC and carbohydrate-free forms, respectively. Fatehi et al. (2016) reported an LCC with the molecular weight of 1500 g/mol in hydrolysis liquor produced from mixed hardwood. Based on the area under the peaks in Fig. 4, it was proposed that 68, 59, 89 and 19% of lignin in hydrolysis liquors of samples 1, 2, 3 and 4 were in LCC forms, respectively. The rest of lignin moieties in the hydrolysis liquors were in carbohydrate-free forms.
Figure 5 shows the amount of lignin remained in hydrolysis liquors after membrane dialysis with different molecular weights. Interestingly, more than 85% of lignin was removed using membrane with the molecular weight cut off of 1000 g/mol, implying the significant removal of low molecular weight lignin compounds. Evidently, more than 80% of hemicelluloses were removed from hydrolysis liquors using membrane with the same molecular weight cut off (1000 g/mol), and these hemicelluloses were probably in monomeric and oligomeric forms. Logically, with using membrane with larger pore opening (e.g., 10,000 and 25,000 g/mol), less lignin and hemicellulose remained in the hydrolysis liquors, but some of these lignin and hemicelluloses were larger than the pore opening of the membranes.
As the molecular weight of hemicelluloses was smaller than 4000 g/mol (Fig. 3), they could be effectively removed from hydrolysis liquors when membrane with molecular weight cut off of 10,000 g/mol (or larger) was used. The presence of hemicelluloses in the hydrolysis liquors dialyzed with membranes with large pores (i.e., 10,000 and 25,000 g/mol) can indirectly illustrate that these hemicelluloses were attached to lignin in LCC form so that they were sufficiently large to remain in the hydrolysis liquors after dialysis.
The use of membrane with different sizes facilitated information about the size distribution of LCC in hydrolysis liquors. Samples 1 had more lignocellulose with molecular weights larger than 1000 g/mol. Also, samples 1–3 lost significant amounts of lignocellulose (probably in LCC forms) when the pore opening of the membrane increased to 25,000 g/mol. These results suggest that the samples 1, 2 and 3 contained a considerable amount of LCC with sizes smaller than 25,000 g/mol so that they were removed from the hydrolysis liquors via membrane dialysis. As stated earlier, more of lignin in hydrolysis liquors were in LCC forms in these samples, and these LCCs were smaller than carbohydrate free lignin (Fig. 4). Therefore, more of these LCCs were removed via dialysis of these samples than sample 4. The concentration of lignocellulose in sample 4 insignificantly dropped when the molecular weight of membrane increased to 25,000 g/mol, implying that this sample had more lignocellulose that were larger than 25,000 g/mol. The presence of hemicelluloses in samples dialyzed with the membrane with the pore opening of larger than 25,000 g/mol depicts that some LCC were larger than 25,000 g/mol.
The types of hemicellulose content of LCC can also be identified. Table 3 presents the compositions of hemicelluloses after membrane dialysis in the samples. It is apparent that xylan was significantly removed from hydrolysis liquor. Also, arabinose and rhaminose were removed from the samples, regardless of the pore opening of dialysis membrane. Lawoko et al. (2005) reported four LCC types of galactoglucomannan-lignin-pectin (GalGlcMan-L-P), glucan-lignin (Glu-L), glucomannan-lignin-xylan (GluMan-L-Xyl) and xylan-lignin- glucomannan (Xyl-L-GluMan), which contains 8, 4, 40 and 48% of total lignin of spruce wood species, respectively. Eriksson et al. (1980) proposed that lignin is bound to xylan with arabinose substituent groups in spruce wood species. Therefore, the removal of arabionose would result in the cleavage of xylose from LCC. Galactose, glucose and mannose were present after dialysis (Table 3). Therefore, it is suggested that LCC compounds were mainly GalGlcMan-L complexes.
Extraction via acidification and ethanol treatments
Figure 6 depicts lignin isolation from the hydrolysis liquor samples obtained via acidification and ethanol treatments. The acidification of hydrolysis liquors resulted in 16–21% lignin isolation from the liquors. In addition to protonation of lignocellulose under acidic conditions, lignin may be condensed under strong acidic conditions, which facilitates its removal.
Comparison of samples revealed that the acidification of the sample 4 produced at the higher L/S ratio led to slightly lower lignin extraction, which might be due to its higher acid-soluble lignin (Tunc 2014). It is also noted that ethanol treatment resulted in significantly lower lignin isolation compared to acidification.
Figure 7 shows the impact of acidification and ethanol treatment in isolating hemicelluloses from the hydrolysis liquors. It is apparent that the acidification led to 16–38% isolation of hemicelluloses. In the literature, the acidification of hardwood-based hydrolysis liquor resulted in 17 and 40% hemicellulose isolations at pH 2 and 1.5, respectively (Fatehi et al. 2016; Liu et al. 2011a). It is also possible that the hemicellulose reduction via acidification is attributed to sugar degradation, but further analyses are required for supporting this hypothesis, which is out of the scope of this work. Ethanol treatment significantly improved the isolation of hemicellulose (35–45% isolation of initial contents). The removal of hemicelluloses under acidic conditions is another indication of the linkage of hemicelluloses to lignin in LCC, as hemicelluloses are generally acid soluble, thus the ones that were linked to lignin should have been removed. Also, sample 1 had a higher lignin removal via ethanol treatment, which can be associated with the removal of LCC.
Comparison of Figs. 5, 6 and 7 depicts that membrane dialysis collected 10–16% of lignin and 8–20% of hemicelluloses. Acidification led to approximately 16–21% lignin removal and 16–38% hemicellulose removal for all sample. Ethanol treatment yielded in 35–45% of hemicellulose isolation and 10–14% of lignin isolation. The properties of samples also impacted the performance of purification process, as the efficiency of extraction processes were different for different samples. Lignocellulose was more efficiently isolated for sample 1 than other samples, regardless of the process. Sample 4 was less sensitive to the membrane pore opening, but more sensitive to acidification and ethanol treatment, than other samples, as it contained more lignin in carbohydrate free form.
Molecular weight of hydrolysis liquors after acidification and ethanol treatment
The separation methods insignificantly affected the molecular weight of lignin in the hydrolysis liquors. After acidification and ethanol treatments, the molecular weights of lignin were 50,000–54,000 and 51,000–60,000 g/mol, respectively. A slight increase in the molecular weight of lignin could be related to the removal of low molecular weight lignin and LCCs from the solution via acidification and ethanol treatment or condensation of lignin under acidic conditions, as stated earlier.
The molecular weight of hemicelluloses in the hydrolysis liquor after different treatment methods are presented in Fig. 8. The molecular weight of hemicellulose insignificantly reduced via acidification, implying that the hemicelluloses in lignin-free and LCC forms had similar molecular weights that the removal of LCC did not affect the overall molecular weight of hemicelluloses. Ethanol reduced the molecular weight of hemicelluloses in hydrolysis liquors implying that high molecular weight hemicelluloses were removed, and this removal reduced the overall molecular weight of hemicelluloses after ethanol extraction.
Application
Autohydrolysis is commercially used in industry. The results of this study showed that the yield and molecular weight of the extracted lignocellulose would be significantly affected by the conditions of autohydrolysis. If lignin were to be used for producing value-added products; higher temperature, longer time and larger liquid to wood ratio could be applied in the autohydrolysis process. Within the scope of this study, the extraction yield of hemicelluloses from wood chips seems to be affected less significantly than lignin by autohydrolysis process conditions. However, if hemicelluloses with larger molecular weight were to be used in value-added applications, the temperature of 170 °C and 15 min residence time and flow rate of 6 L/min might be used in the autohydrolysis process. The generation of LCC is unavoidable in the hydrolysis liquor, but the compositions of LCC can be affected by the hydrolysis conditions, which impacts the efficiency of acidification and ethanol treatment in isolating lignin and hemicelluloses from the hydrolysis liquor. As lignin and hemicelluloses cannot be produced in pure forms via the processes investigated, end-use applications that include both lignin and hemicelluloses as raw materials may be feasible options for the use of lignocellulose of autohydrolysis liquor. These applications can be the use of extracted lignin and hemicelluloses as additives in unbleached paper board, corrugated medium, composites and plywood productions.
Conclusions
Autohydrolysis conditions affected the amount and molecular weight of lignocellulose present in hydrolysis liquors. The molecular weight of lignin was stable regardless of hydrolysis conditions. The molecular weight of hemicelluloses decreased with increasing the severity of hydrolysis conditions (i.e., time and temperature) due to polysaccharide degradation. Based on GPC analysis, only 19% of lignin involved in LCC form in sample 4 that was produced at L/S ratio of 10, 180 °C and 15 min. In samples produced under the conditions of 180 °C, L/S ratio of 5 and residence times of 15 and 45 min, 68 and 59% of lignin seemed to be in LCC forms, respectively. Membrane dialysis assessment revealed that sample 4 contained more of high molecular weight lignin, but other samples had more of LCC with the molecular weight smaller than 25,000 g/mol. Acidification resulted in 16–21% of lignin and 16–40% of hemicellulose isolations, respectively. Acidification did not change the molecular weight of hemicelluloses significantly. The ethanol extracted 10–14% of lignin and 35–45% of hemicelluloses from hydrolysis liquors, and the molecular weight of hemicelluloses was slightly smaller in the ethanol pretreated hydrolysis liquor.
References
Ando H, Sakaki T, Kokusho T, Shibata M, Uemura Y, Hatate Y (2000) Decomposition behavior of plant biomass in hot-compressed water. Ind Eng Chem Res 39:3688–3693
Ayeni A, Adeeyo O, Oresegun O, Oladimeji T (2015) Compositional analysis of lignocellulosic materials: evaluation of an economically viable method suitable for woody and non-woody biomass. Am J Eng Res 4(4):14–19
Carvalheiro F, Duarte L, Girio F (2008) Hemicelluloses biorefineries: a review on biomass pretretments. J Sci Ind Res 67:849–864
Chandel A, Chandrasekjar G, Radhika K, Ravinder R, Ravindra P (2011) Bioconversion of pentose sugars into ethanol: a review and future directions. Biotechnol Mol Biol Rev 6(1):8–20
Dashtban M, Qin W (2012) Overexpression of an exotic thermotolerant β-glucosidase in trichoderma reesei and its significant increase in cellulolytic activity and saccharification of barley straw. Microb Cell Fact 11(63):1–15
Du X, Gellerstedt G, Li J (2013) Universal fractionation of lignin–carbohydrate complexes (LCC) from lignocellulosic biomass: an example using spruce wood. Plant J 74(2):328–338
Eriksson O, Goring DAI, Lindgren O (1980) Structural studies on the chemical bonds between lignin and carbohydrates in spruce wood. Wood Sci Technol 14:267–279
Fatehi P, Chen J (2016) Extraction of technical lignins from pulping spent liquors, challenges and opportunities. In: Fang Z, Smith RL Jr (eds) Production of biofuels and chemicals from lignin. Springer, Berlin, pp 33–54
Fatehi P, Gao W, Sun Y, Dashtban M (2016) Acidification of prehydrolysis liquor and spent liquor of natural sulfite semichemical pulping process. Bioresour Technol 218:518–525
Fox SC, McDonald AG (2010) Chemical and thermal characterization of three industrial lignins and their corresponding lignin esters. BioResources 5(2):990–1009
Galia A, Schiavo B, Antonetti C, Raspolli Galetti AM, Interrante L, Lessi M, Scildone O, Valenti MG (2015) Autohydrolysis pretreatment of Arundo donax: a comparison between microwave-assisted batch and fast heating rate flow-through reaction systems. Biotechnol Biofuels 8(218):1–18
Garcia J, Zamudio M, Perez A, Feria M, Gomide J, Coledette J, Lopez F (2011) Soda-AQ pulping of paulownia wood after hydrolysis treatment. BioResources 6(2):971–986
Jansson M, Danielsson S, Saadatmand S, Edlund U, Albertsson AC (2014) Upgrading of wood pre-hydrolysis liquor for renewable barrier design: a techno-economic consideration. Cellulose 21(3):2045–2062
Kapu NS, Trajano HL (2014) Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod Biorefin 8:857–870
Kumar H, Christopher LP (2017) Recent trends and developments in dissolving pulp production and application. Cellulose 24(6):2347–2365
Lawoko M, Henriksson G, Gellerstedt G (2005) Structural differences between the lignin–carbohydrate complexes present in wood and in chemical pulps. Biomacromolelules 6:3467–3473
Lawoko M, Henriksson G, Gellerstedt G (2006) Characterisation of lignin–carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods. Holzforschung 60:156–161
Leppanen K, Spetz P, Pranovich A, Hartonen K, Kitunen V, Ilvesniemi H (2011) Pressurized hot water extraction of Norway spruce hemicelluloses using a flow-through system. Wood Sci Technol 45:223–236
Liu S (2008) A kinetic model on autocatalytic reactions in woody biomass hydrolysis. J Biobased Mater Bioenergy 2(2):135–147
Liu C, Wyman CE (2003) The effect of flow rate of compressed hot water on xylan, lignin, and total mass removal from corn stover. Ind Eng Chem Res 42:5409–5416
Liu Z, Fatehi P, Jahan MS, Ni Y (2011a) Separation of lignocellulosic material by combined processes of pre-hydrolysis and ethanol extraction. Bioresour Technol 102:1264–1269
Liu Z, Fatehi P, Sadeghi S, Ni Y (2011b) Application of hemicelluloses precipitated via ethanol treatment of pre-hydrolysis liquor in high-yield pulp. Bioresour Technol 102:9613–9618
Lora J, Glasser W (2002) Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. J Polym Environ 10(112):39–48
Oinonen P, Zhang L, Lawoko M, Henriksson G (2015) On the formation of lignin polysaccharide networks in Norway spruce. Phytochemistry 111:177–184
Pienkos PT, Zhang M (2009) Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 16(4):743–762
Pothiraj C, Kanmani P, Balaji P (2006) Bioconversion of lignocellulose materials. Microbiology 34(4):159–165
Reid JD, Lynch DFJ (1937) Cellulose analysis. A comparison of three principal methods. Ind Eng Chem 9(12):570–573
Saeed A, Sarwar Jahan M, Li H, Liu Z, Ni Y, van Heiningen A (2012) Mass balances of components dissolved in the pre-hydrolysis liquor of kraft-based dissolving pulp production process from Canadian hardwood. Biomass Bioenergy 39:14–19
Santos Muguet MC, Ruuttunen K, Jaaskelainen AS, Colodette JL, Vuorinen T (2013) Defibration mechanisms of auto hydrolyzed Eucalyptus wood chips. Cellulose 20(5):2647–2654
Santucci B, Maziero P, Rabelo S, Curvelo A, Pimenta M (2015) Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermal pretreatment: a kinetic assessment. Bioenergy Res 8(4):1778–1787
Sarip H, Hossain S, Azeni M, Allaf K (2016) A review of the thermal pretreatment of lignocellulosic biomass towards glucose production: autohydrolysis with DIC technology. BioResources 11(4):10625–10653
Shi H, Fatehi P, Xiao H, Ni Y (2011) A combined acidification/PEO flocculation process to improve the lignin removal from the pre-hydrolysis liquor of kraft-based dissolving pulp production process. Bioresour Technol 102(8):5177–5182
Shokri J, Adibkia K (2013) Application of cellulose and cellulose derivatives in pharmaceutical industries. In: van de Ven T, Godbout L (eds) Cellulose—medical, pharmaceutical and electronic applications. InTech, London, pp 47–66
Sixta H, Iakovlev M, Testova L, Roselli A, Hummel M, Borrega M, van Heiningen A, Froschauer C, Schottenberger H (2013) Novel concepts of dissolving pulp production. Cellulose 20(4):1547–1561
Sjostrom E (1993) Wood chemistry. Fundamentals and Applications, 2nd edn. Academic press, San Diego
Song T, Pranovich A, Sumersky I, Holmbom B (2008) Extraction of galactoglucomannan from spruce wood with pressurized hot water. Holzforschung 62:659–666
Tarasov D, Leitch M, Fatehi P (2015) Production of lignosulfonate in NSSC-BASED BIOREfiNERY. Biotechnol Prog 31(6):1508–1514
Testova L, Vilonen KM, Pynnonen H, Tenkanen M, Sixta H (2009) Isolation of hemicelluloses from birch wood: distribution of wood components and preliminary trials in dehydration of hemicelluloses. Lenzing Berichte 87:58–65
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B (2014) Microcrystalline cellulose, a direct compression binder in a quality by design environment—a review. Int J Pharm 473:64–72
Tolbert A, Akinosho H, Khunsupat R (2014) Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod Biorefin 8:836–856
Tunc M (2014) Effect of liquid to solid ratio on autohydrolysis of Eucalyptus globulus wood meal. BioResources 9(2):3014–3024
Tunc M, Lawoko M, van Heiningen ARP (2010) Understanding the limitation of removal of hemicelluloses during autohydrolysis of a mixture of southern hardwoods. BioResources 5(1):356–371
Vishtal A, Kraslawski A (2011) Challenges in industrial applications of technical lignins. BioResources 6(3):3547–3568
Yu Y, Wu H (2010) Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind Eng Chem Res 49:3902–3909
Zheng Y, Pan Z, Zhang R (2009) Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng 2(3):51–68
Acknowledgments
The authors would like to thank the Early Researcher Award program of the Government of Ontario for funding this research. Also, support from Canada Foundation for Innovation, Ontario Research Fund, Canada Research Chair and Northern Ontario Heritage Fund Corporation is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tarasov, D., Leitch, M. & Fatehi, P. Flow through autohydrolysis of spruce wood chips and lignin carbohydrate complex formation. Cellulose 25, 1377–1393 (2018). https://doi.org/10.1007/s10570-017-1643-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1643-9