Abstract
In response to the shortage of petroleum resources and the growing need for sustainable development, cellulose-based amphiphilic copolymers have emerged as a new generation of value-added functional nanostructures from biomass resources. In this article, 17 amphiphilic hydroxyethyl cellulose-based graft copolymers with different side chains, including poly(lactide), poly(ε-caprolactone) and poly(p-dioxanone), were synthesized via homogeneous ring opening polymerization in ionic liquid 1-butyl-3-methylimidazolium chloride and characterized by FT-IR, 1H NMR, thermogravimetric analysis and gel permeation chromatography. The resultant copolymers can self-assemble into micelles with a low critical micelle concentration that varies in the range of 0.03–0.24 mg/ml. TEM observations revealed the obtained micelles had a spherical and well-distributed morphology, and DLS analysis showed the nanoscaled sizes were between 40 and 150 nm. These HEC-based micelles can be used as nano-sized vesicles and have great latent forces in drug delivery systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphiphilic polymers can self-assemble into special nanostructures with a hydrophobic core and hydrophilic shell in aqueous solution (Jones and Leroux 2010; Li et al. 2008). The self-assemblies based on amphiphilic polymers are excellent carriers of water-insoluble drugs and functional materials (Guo et al. 2012). To date, the amphiphilic polymeric materials have been applied in a broad range of fields, such as drug delivery (Chang et al. 2008; Chen et al. 2011b; Miller et al. 2013; Wang et al. 2014b), bioimaging (Lu et al. 2012; Zhang et al. 2013) and sensing (Gong et al. 2014; Wang et al. 2012a). Lately, in response to the shortage of petroleum resources and the growing need for sustainable development, the natural polysaccharide-based amphiphiles have received a great deal of interest because of their promising properties, e.g., ready availability, good biocompatibility, biodegradability and nontoxicity (Hassani et al. 2012). As the most abundant biomass resource in nature, cellulose and its derivatives are especially desirable as precursors of amphiphilic polymeric assemblies.
Cellulose has a linear β-(1–4) linked glucan structure, which enables strong cooperative intra- and intermolecular hydrogen bond patterns and also stiffens the chain (Guo et al. 2013a; Klemm et al. 2005; Moghaddam et al. 2014). As a result, cellulose cannot be dissolved in normal organic solvents, while most amphiphilic modifications of cellulose need to introduce liposoluble side chains. Fortunately, ionic liquids (ILs) have been discovered to be excellent solvents for cellulose and its derivatives to solve the problem of poor solubility (Pinkert et al. 2009; Wang et al. 2014a; Weerachanchai et al. 2014). In general, ILs are defined as salts that melt at or below 100 °C to afford liquids (Pinkert et al. 2009; Quan et al. 2010). As good hydrogen bond acceptors, ILs can disrupt the interpolymer attractions in cellulose, resulting in efficient dissolving under relatively mild conditions (Sun et al. 2011). Moreover, ILs also can readily dissolve the hydrophobic molecules and then provide a homogeneous reaction system (Guo et al. 2012).
In recent years, many amphiphilic modifications of cellulose have been performed homogeneously and efficiently in ILs by various techniques, such as reversible addition-fragmentation chain transfer (RAFT) (Liu et al. 2010), atom transfer radical polymerization (ATRP) (Kang et al. 2013) and ring opening polymerization (ROP) (Labet and Thielemans 2011). Lin et al. (2013) reported the first illustration for the graft copolymerization of methylmethacrylate onto cellulose by RAFT in 1-butyl-3-methylimidazolium chloride (BmimCl) and proved a more controlled/living polymerization character. Another group successfully prepared microcrystalline cellulose-graft-poly(N-isopropylacrylamide) in BmimCl IL using a γ-ray irradiation technique at room temperature (Hao et al. 2009). The resulting copolymers exhibited obvious thermal sensitivity at about 35 °C. In our previous work, a series of amphiphilic cellulose graft copolymers were successfully synthesized via ROP in BmimCl. These cellulose-based amphiphiles, including cellulose-graft-poly(ε-caprolactone) (PCL) (Guo et al. 2013b), microcrystalline cellulose-graft-poly(lactide) (PLA) (Guo et al. 2012) and pulp-graft-PLA (Guo et al. 2013a), could self-assemble into nanosized spherical micelles in aqueous media and are suitable for use as vehicles for antitumor drug delivery. However, the above amphiphilic cellulose derivatives show a limited controllable hydrophilic-hydrophobic balance because of the fundamental hydrophilicity of cellulose. A possible route to enhance the hydrophilicity of amphiphilic cellulose is to use hydrophilic modified cellulose derivatives as the starting material.
In this work, a nonionic water-soluble derivative of cellulose-hydroxyethyl cellulose (HEC) was chosen as the starting material for preparing amphiphilic graft copolymers of cellulose in IL. Three different hydrophobic segments including PLA, PCL and poly(p-dioxanone) (PDO) were introduced onto the HEC backbone via homogeneous ROP in BmimCl, respectively. PLA, PCL and PDO are FDA-approved hydrophobic aliphatic polyesters, which are known for their good biodegradability, biocompatibility and mechanical properties (Jacobsen et al. 1999). Although they are all aliphatic polyesters, some differences can be found in their structure. For example, PCL possesses a longer carbon chain of repeat units than PLA and thus more easily forms a long chain polymer via ROP. PDO has special ether bonds providing it with excellent flexibility and a fast hydrolysis rate (Wang et al. 2012b). Therefore, the parallel synthesis of HEC-graft-PLA, PCL and PDO would certainly provide information about the effect of the polyester structure on the properties and micellization of amphiphilic HEC, which has not been reported previously. Moreover, the effect of the molecular weight of HEC, the initial dosage of the hydrophobic monomer and the other reaction conditions should also be considered.
Materials and methods
Materials
HEC, with M w of 250 kDa, D S of 3.0, purchased from Aladdin Reagent Inc., and M w of 50 kDa, D S of 1.8–2.0, obtained from Shandong Ruitai Industry Co., Ltd. (China), were dried at 45 °C for 48 h in vacuum before use. The monomer of l-lactide (l-LA, 99.5 % purity) was supplied by Jinan Daigang Biomaterial Co., Ltd. (China). ε-Caprolactone (ε-CL) with a purity of 99.5 % was purchased from Aladdin Reagent Inc. p-Dioxanone (p-DO) with a purity of 99.0 % was obtained from Jiaxing Light Chemicals Co., Ltd. (Zhejiang, China); it was dried over CaH2 for 48 h and distilled twice under reduced pressure immediately before use. BmimCl (99 % purity), from Chengjie Chemical Co., Ltd. (Shanghai, China), was kept in a vacuum oven for 48 h at 50 °C prior to use. 4-Dimethylaminopyridine (DMAP) with a purity of 99.0 % was obtained from Saen Chemical Technic Co., Ltd. All other chemicals and solvents were of analytical grade and used as received.
Synthesis of HEC-based amphiphilies
The preparation of HEC-based amphiphilies was performed according to a typical polymerization process. Briefly, a certain amount of HEC was added to BmimCl IL to give a final concentration of 3.3 % (w/w). The HEC/BmimCl mixture was magnetically stirred at 80 °C for 3 h. Then l-LA or ε-CL or p-DO monomer and catalyst DMAP (2 wt%) were slowly added into the above solution. The ROP reaction was carried out at 100 °C for 8 h with the protection of nitrogen. Then the reaction mixtures were precipitated with excess ethanol. The precipitates were further stirred in dichloromethane for 72 h to obtain the purified graft copolymers. The final products were dried in a vacuum oven at 50 °C for 24 h.
Characterization of HEC-based amphiphilies
After being dried under an infrared lamp for half an hour, the hydroxyethyl cellulose-based amphiphilies were characterized with the following protocols.
The weight percentage gain (WPG) was calculated according to the following equation:
where W G is the vacuum-dry weight of amphiphilic grafted HEC copolymers and W HEC is the corresponding vacuum dry weight of the unmodified HEC.
The FT-IR spectra were collected from a Bruker spectrometer (TENSOR 27, Switzerland) in KBr discs at wavelengths in the range of 500–4000 cm−1 32 times. 1H-NMR spectra were analyzed on a Bruker AV-III 400M spectrometer (Germany) at room temperature using DMSO-d6 as solvent and tetramethysilane (TMS) as internal standard.
The TGA (TGA Q500, TA, USA) of graft copolymers was carried out in an aluminum crucible under nitrogen atmosphere with the heating temperature ranging from 50 to 600 °C. The heating rate was 10 °C/min, while the nitrogen flow was 30 ml/min.
The molecular weight and molecular weight distribution of HEC-based copolymers were determined by gel permeation chromatography (GPC) on a dual-detector system with a MALLS device and an interferometric refractometer. Each sample was dissolved in water with a concentration of 5 mg/ml and then filtered through a 0.22-μm membrane. The flow rate of the eluent was 0.5 ml/min, while the laser wavelength of the MALLS detector was 690 nm. The d n /d c value of HEC in aqueous water was 0.1396 ± 0.0024 ml/g.
Self-assembly behavior of HEC-based amphiphilic copolymers
The critical micelle concentration (CMC) of the amphiphilic copolymers was established by a fluorescence spectrometer (Jobin-Yvon Fluorolog Tau-3 system) with a fluorescence probe of pyrene. In brief, the pyrene solution in acetone (6.0 × 10−5 M) was added into the test tubes. Acetone was evaporated by a stream of nitrogen gas. Then, the amphiphilic copolymer aqueous solutions of various concentrations (0.001–2 mg/ml) were added into the test tubes containing pyrene with a final concentration of 6.0 × 10−7 M. Then the mixtures were sonicated for 30 min at room temperature and further shaken in a shaking bath for 1 h for equilibrium. Emission spectra [λ em = 350–500 nm, λ ex = 339 nm, E x (SBW) = 5.0 nm, E m (SBW) = 2.5 nm] of the above solutions were recorded.
The intensity ratio (I 1/I 3) was plotted against the logarithm of graft copolymer concentrations. The CMC values were obtained based on the point of two tangents to the curve at the inflection and low copolymer concentrations, respctively.
Characterization of HEC-based micelles
The average sizes of HEC-based micelles were acquired by the dynamic light scattering (DLS) method using a nanoparticle size analyzer (Malvern Instruments, Malvern, UK).
The morphology of HEC-based micelles was observed by a transmission electron microscopy (TEM, JEM-2100, Japan) at an acceleration voltage of 200 kV. Each sample solution was loaded on a copper grid coated with carbon film, negatively stained with 2 % (w/v) phosphotungstic acid for 3 min and then air-dried at room temperature.
Results and discussion
Synthesis of HEC-based amphiphiles
A series of amphiphilic HEC-based copolymers with different side chains (PLA, PCL and PPDO) was synthesized via homogeneous ROP in ionic liquid BmimCl. Figure 1 illustrates the routes of the preparation of HEC-based amphiphiles. BmimCl was employed to effectively dissolve HEC and the hydrophobic monomers and then provide a homogeneous environment for the reaction. DMAP was applied to catalyze the ROP of the monomers grafted onto HEC backbone because of its good availability and selectivity (Coulembier et al. 2006; Labet and Thielemans 2012). In addition, the WPG was calculated and is presented in Table 1. From PLA-1 to PLA-4, the WPG increases with the increasing weight ratio of l-LA to HEC, indicating increasing PLA content in the graft copolymer. The other samples of HEC-g-PCL and HEC-g-PDO present similar trends. Thus, the HEC-based graft copolymers with various side chain contents could be synthesized by adjusting the weight ratio of monomer to HEC. In addition, with the same conditions, PCL grafts had the lowest WPG of the three types of HEC-based copolymers, which may be due to the longer chain of PCL resulting in larger space steric hindrance than PCL and PDO and then the effect of the grafting reaction.
Characterization of HEC-based amphiphiles
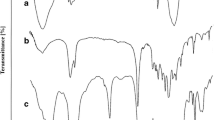
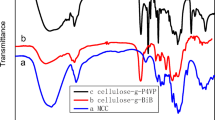
The chemical structures of HEC and its grafted copolymers were characterized by FT-IR and 1H NMR spectra. Figure 2 displays the FT-IR spectra of HEC, regenerated HEC, HEC-g-PLA, HEC-g-PCL and HEC-g-PPDO. In the spectra of HEC, the main adsorption bands at 3400 cm−1 (stretching vibration of the –OH group and a small amount of absorbed water), 2930 and 2874 cm−1 (C–H stretching vibration of –CH2) and 1417 and 1357 cm−1 (C–H bending vibration of –CH2 and –CH3) can be observed. Comparing the FT-IR spectra of HEC-grafted copolymers with unmodified HEC, new bands attributed to the carbonyl stretch at 1742 cm−1 and symmetric C–O–C at 1203 cm−1 indicated the side chains (PLA, PCL or PPDO) had been introduced into the HEC backbone. Moreover, in order to prove the new signals originate from the side chain grafted onto HEC but not from the monomer mixed with HEC, regenerated HEC was prepared by stirring a mixture of cellulose and monomer in BmimCl at 80 °C for 3 h and then removing the monomer by dichloromethane extraction (Guo et al. 2012). The spectra of regenerated HEC demonstrated that the unreacted monomer can be completely removed by extraction and confirmed a successful grafting copolymerization.
The 1H NMR spectra of HEC-based amphiphiles is shown in Fig. 3. In the 1H NMR spectra of HEC-g-PLA (Fig. 3a), the multiple peaks at δ = 3.0–5.0 ppm are attributed to the protons of glucose, while the single peak at δ = 3.49 ppm could be contributed by methylene protons (Ha) of HEC and a small amount of absorbed water. New peaks originating from the internal and terminal methyl protons (Hc and Hc′) of the PLA side chain appear at δ = 1.41 and 1.24 ppm, respectively, while the chemical shifts at δ = 5.02 and 4.14 ppm correspond to the internal and terminal methylene protons (Hb and Hb′) of PLA (Guo et al. 2013a). This result is in agreement with the results of the FT-IR spectrum. In the 1H NMR spectra of HEC-g-PCL (Fig. 3b), the methylene proton peaks of PCL could be observed at δ = 4.10 ppm (Hh), 3.42 ppm (Hh′), 2.29 ppm (Hd′), 1.51 ppm (Hd), 1.40 ppm (He and Hg) and 1.30 ppm (Hf), confirming the successful synthesis of HEC-g-PCL (Guo et al. 2013b). In the 1H NMR spectra of HEC-g-PDO (Fig. 3c), the signals from δ = 4.16 to 4.14 ppm assigned to the internal and terminal methylene protons of PPDO (Hi and Hi′) clearly demonstrated PDO was grafted onto the HEC backbone.
The thermal stabilities of HEC and hydrophobically modified HEC with different side chains in nitrogen were determined by TGA (Fig. 4). It can be seen that the TG curve of pure HEC shows a single decomposition stage with the onset of weight loss at about 255 °C, whereas the thermal stability of HEC-based graft copolymers is not as good as that of HEC. The graft copolymers show two decomposition stages and two maximum decomposition temperatures (T max). The T max1 is ascribed to the scission of the side chains (PLA, PCL or PDO) from the HEC backbone followed by the decomposition of the side chains, and T max2 corresponds to the decomposition of HEC residues. The lower T max2 of graft copolymers relative to HEC confirmed the fact that the ordered structure of HEC had been partially destroyed by the introduction of side chains; the modified HECs with looser and disordered structures were more easily decomposed by thermal treatment (Guo et al. 2013b).
GPC was employed to evaluate the M n , M w and M w /M n values of the HEC-based graft copolymers; the results are summarized in Table 1. The M w values of all copolymers are in the range of 50–280 kDa and increase with increasing side chain contents in copolymers. However, the M w /M n (polydispersity) is barely changed with the progressive increment of grafting chains. Taking HEC-g-PLA as an example, when the monomer dosage is increased from 1:1 to 6:1 with all of the other conditions kept constant, the Mw of HEC-g-PLA products increases from 144 to 275 kDa, whereas the M w /M n value has no obvious change. Moreover, it should be noted that graft copolymers with lower side chain contents (e.g., PLA-1, PCL-1) have a lower Mw than HEC. This may be attributed to the degradation of the HEC main chain or higher de-polymerization rate in the reaction process.
Self-assembly of HEC-based amphiphilic copolymers
The critical micelle concentration (CMC) is a vital parameter to exhibit the self-assembly ability of amphiphilic polymers. In this study, the self-assembly behavior of HEC-based graft copolymers was determined by a fluorescent probe method. It is well known that pyrene has much lower solubility in water than in hydrocarbon. Once the hydrophobic association occurs in aqueous solution, pyrene significantly transfers into hydrophobic regions, resulting in the change of its photophysical property (Liu et al. 2009). Accordingly, the intensity ratio (I 1/I 3) in the emission spectrum of pyrene is usually used to present the obvious change. Figure 5 shows the I 1/I 3 of the pyrene emission spectra versus the concentration of HEC-based graft copolymers. On the basis of the crossover point of the plot curve, the detailed CMC values are summarized in Table 1. The results show that the CMC decreases with increasing side chain contents in copolymers when other conditions are kept constant. This is reasonable because the hydrophobicity of the copolymer increased with increasing side chain content, so that the copolymers with higher side chain contents associated at lower concentrations (Jiang et al. 2011).
Characterization of HEC-based micelles
The sizes of HEC-based micelles were determined by DLS, as summarized in Table 1. The determined sizes of HEC-based micelles vary in the range of 40 to 150 nm and decrease with increasing side chain contents. Figure 6f–j displays the size histogram of HEC-based micelles determined by DLS, showing that the obtained nanomicelles have a narrow size distribution.
The morphology of the HEC-based nanomicelles was observed by TEM, as depicted in Fig. 6a–e. It can be seen that the micelles are uniformly distributed with spherical structures, and no obvious aggregation was observed. The particle sizes of micelles show a decreasing trend with increasing hydrophobic side chain content. Obviously, the size of PLA-4 micelles is larger than that of PLA-7, which is consistent with the result of DLS as shown in Table 1. A similar result was found for PCL-2 and PCL-6 micelles. Moreover, it is noteworthy that the size of the micelle observed by TEM is smaller than that determined by DLS, which is attributed to the micelle dehydration caused by solvent evaporation in the TEM experiment (Li et al. 2008).
Conclusions
In summary, amphiphilic HEC-based graft copolymers with different side chains, including PLA, PCL and PDO, were synthesized via homogeneous ROP in BmimCl. The obtained copolymers showed fine structures, significantly different thermal stabilities and molecular weights. Moreover, the amphiphiles were capable of self-assembling into core-shell micelles. The CMC values and sizes varied in the range of 0.03–0.24 mg/ml and 40–150 nm, respectively. They were both relevant to the molecular structure and hydrophobic side chain content of HEC-based copolymers. These HEC-based nanomicelles completely composed of biodegradable materials are potential vehicles and can be used in the form of liquid membranes for drug delivery (Chen et al. 2011a).
References
Chang C, Wei H, Quan CY, Li YY, Liu J, Wang ZC, Cheng SX, Zhang XZ, Zhuo RX (2008) Fabrication of thermosensitive PCL-PNIPAAm-PCL triblock copolymeric micelles for drug delivery. J Polym Sci Polym Chem 46:3048–3057
Chen C-H, Hsieh M-F, Ho Y-N, Huang C-M, Lee J-S, Yang C-Y, Chang Y (2011a) Enhancement of catechin skin permeation via a newly fabricated mPEG-PCL-graft-2-hydroxycellulose membrane. J Membr Sci 371:134–140
Chen CH, Cuong NV, Chen YT, So RC, Liau I, Hsieh MF (2011b) Overcoming multidrug resistance of breast cancer cells by the micellar doxorubicin nanoparticles of mPEG-PCL-graft-cellulose. J Nanosci Nanotechnol 11:53–60
Coulembier O, Degee P, Hedrick JL, Dubois P (2006) From controlled ring-opening polymerization to biodegradable aliphatic polyester: especially poly(beta-malic acid) derivatives. Prog Polym Sci 31:723–747
Gong P, Yang Y, Yi H, Fang S, Zhang P, Sheng Z, Gao G, Gao D, Cai L (2014) Polypeptide micelles with dual pH activatable dyes for sensing cells and cancer imaging. Nanoscale 6:5416–5424
Guo YZ, Wang XH, Shu XC, Shen ZG, Sun RC (2012) Self-assembly and paclitaxel loading capacity of cellulose-graft-poly(lactide) nanomicelles. J Agric Food Chem 60:3900–3908
Guo YZ, Liu Q, Chen H, Wang XH, Shen ZG, Shu XC, Sun RC (2013a) Direct grafting modification of pulp in ionic liquids and self-assembly behavior of the graft copolymers. Cellulose 20:873–884
Guo YZ, Wang XZ, Shen ZG, Shu XC, Sun RC (2013b) Preparation of cellulose-graft-poly(ε-caprolactone) nanomicelles by homogeneous ROP in ionic liquid. Carbohydr Polym 92:77–83
Hao Y, Peng J, Li J, Zhai M, Wei G (2009) An ionic liquid as reaction media for radiation-induced grafting of thermosensitive poly (N-isopropylacrylamide) onto microcrystalline cellulose. Carbohydr Polym 77:779–784
Hassani LN, Hendra F, Bouchemal K (2012) Auto-associative amphiphilic polysaccharides as drug delivery systems. Drug Discov Today 17:608–614
Jacobsen S, Degee PH, Fritz HG, Dubois PH, Jerome R (1999) Polylactide (PLA)—a new way of production. Polym Eng Sci 39:1311–1319
Jiang C, Wang X, Sun P, Yang C (2011) Synthesis and solution behavior of poly(ε-caprolactone) grafted hydroxyethyl cellulose copolymers. Int J Biol Macromol 48:210–214
Jones MC, Leroux JC (2010) Reverse micelles from amphiphilic branched polymers. Soft Matter 6:5850–5859
Kang H, Liu R, Huang Y (2013) Cellulose derivatives and graft copolymers as blocks for functional materials. Polym Int 62:338–344
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Edit 44:3358–3393
Labet M, Thielemans W (2011) Improving the reproducibility of chemical reactions on the surface of cellulose nanocrystals: ROP of ε-caprolactone as a case study. Cellulose 18:607–617
Labet M, Thielemans W (2012) Citric acid as a benign alternative to metal catalysts for the production of cellulose-grafted-polycaprolactone copolymers. Polym Chem 3:679–684
Li Y, Liu R, Liu W, Kang H, Wu M, Huang Y (2008) Synthesis, self-assembly, and thermosensitive properties of ethyl cellulose-g-P(PEGMA) amphiphilic copolymers. J Polym Sci Polym Chem 46:6907–6915
Lin C, Zhan H, Liu M, Habibi Y, Fu S, Lucia LA (2013) RAFT synthesis of cellulose-g-polymethylmethacrylate copolymer in an ionic liquid. J Appl Polym Sci 127:4840–4849
Liu Y, Cao X, Luo M, Le Z, Xu W (2009) Self-assembled micellar nanoparticles of a novel star copolymer for thermo and pH dual-responsive drug release. J Colloid Interface Sci 329:244–252
Liu XY, Chen J, Sun P, Liu ZW, Liu ZT (2010) Grafting modification of ramie fibers with poly(2,2,2-trifluoroethyl methacrylate) via reversible addition-fragmentation chain transfer (RAFT) polymerization in supercritical carbon dioxide. React Funct Polym 70:972–979
Lu H, Su F, Mei Q, Zhou X, Tian Y, Tian W, Johnson RH, Meldrum DR (2012) A series of poly N-(2-hydroxypropyl)methacrylamide copolymers with anthracene-derived fluorophores showing aggregation-induced emission properties for bioimaging. J Polym Sci Polym Chem 50:890–899
Miller T, Breyer S, van Colen G, Mier W, Haberkorn U, Geissler S, Voss S, Weigandt M, Goepferich A (2013) Premature drug release of polymeric micelles and its effects on tumor targeting. Int J Pharm 445:117–124
Moghaddam PN, Avval ME, Fareghi AR (2014) Modification of cellulose by graft polymerization for use in drug delivery systems. Colloid Polym Sci 292:77–84
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Quan SL, Kang SG, Chin IJ (2010) Characterization of cellulose fibers electrospun using ionic liquid. Cellulose 17:223–230
Sun N, Rodriguez H, Rahman M, Rogers RD (2011) Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem Commun 47:1405–1421
Wang XH, Guo YZ, Li D, Chen H, Sun RC (2012a) Fluorescent amphiphilic cellulose nanoaggregates for sensing trace explosives in aqueous solution. Chem Commun 48:5569–5571
Wang XL, Zhai YL, Tang DL, Liu GY, Wang YZ (2012b) Self-assembly, drug-delivery behavior, and cytotoxicity evaluation of amphiphilic chitosan-graft-poly (1,4-dioxan-2-one) copolymers. J Polym Res 19:1221–1227
Wang C, Yan D, Li Q, Sun W, Xing J (2014a) Ionic liquid pretreatment to increase succinic acid production from lignocellulosic biomass. Bioresour Technol 172:283–289
Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J (2014b) Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 35:7654–7665
Weerachanchai P, Kwak SK, Lee J-M (2014) Effects of solubility properties of solvents and biomass on biomass pretreatment. Bioresour Technol 170:160–166
Zhang Y, Wang M, Zheng Y, Tan H, Hsu BY, Yang ZC, Wong SY, Chang AY, Choolani M, Li X, Wang J (2013) PEOlated micelle/silica as dual-layer protection of quantum dots for stable and targeted bioimaging. Chem Mater 25:2976–2985
Acknowledgments
This work was financially supported by the Program for New Century Excellent Talents in University (grant no. NCET-13-0215), the Science and Technology Program of Guangzhou, China (grant no. 2014J4100039) and Open Foundation of State Key Laboratory of Pulp and Paper Engineering, South China University of Technology (grant no. 201312).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wenjiao Ge and Yanzhu Guo have contributed equally to this work and are considered first co-authors.
Rights and permissions
About this article
Cite this article
Ge, W., Guo, Y., Zhong, H. et al. Synthesis, characterization, and micellar behaviors of hydroxyethyl cellulose-graft-poly(lactide/ε-caprolactone/p-dioxanone). Cellulose 22, 2365–2374 (2015). https://doi.org/10.1007/s10570-015-0663-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0663-6