Abstract
A pH-responsive cellulose-g-P4VP copolymer was synthesized by atom transfer radical polymerization (ATRP) in ionic liquid 1-allyl-3-methylimidazolium chloride [AMIM]Cl. The polymer structure was characterized by Fourier transform infrared (FT-IR), 1H nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC). The P4VP brushes that were covalently bonded to the cellulose backbone had a narrow molecular weight distribution, which was helpful for use in drug loading. The loading and controlled release of drug using aspirin as model drug in the micelles obtained the cellulose-g-P4VP copolymer was investigated. The structure and size of the copolymeric micelles were characterized by transmission electron microscopy (TEM), dynamic light scattering (DLS) and ultraviolet–visible (UV-Vis) spectroscopy, respectively. Blank micelles presented a stably spherical morphology with dimeter about 90 nm in aqueous solution. The resultant micelles had clear pH-sensitivity with a pH-dependent phase transition point at a pH of about 5.7. Drug-loaded micelles had a spherical, core–shell structure with dimeter about 150 nm. The polymeric micelles revealed an excellent controlled drug release at different pH values and the cumulative release of aspirin in phosphate buffer reached to 86.4% at pH 5.8, 60.9% at pH 7.4 and 42.2% at pH 8.0.at 50 h. The pH-sensitive cellulose-g-P4VP copolymer had an enormous potential as carriers for released drug delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a green biological resource, cellulose is widely used in the manufacture of textiles, foods, and pharmaceuticals because of its biocompatible, biodegradable and nontoxic properties [1,2,3,4]. Cellulose is a polymer with remarkable potential for functional application in controlled-release drugs. Cellulose polymers with environmental sensitivities such as pH, temperature, or ionic strength can be obtained by grafting functional groups onto the cellulose chain backbone [5, 6]. Cellulose polymeric micelles are used in drug encapsulation and sustained release formulations. Atom transfer radical polymerization (ATRP) can be used to synthesize cellulose copolymers, and is a popular method because it results in a uniform distribution of the grafted species along the molecular cellulose chain [7, 8]. However, microcrystalline cellulose is not easy to dissolve in common organic solvents. Ionic liquids, as green solvents, are favorable reaction media for the modification of cellulose [9,10,11]. Sui et al. prepared a cellulose graft poly (N,N-dimethylamino-2-ethyl methacrylate) (cellulose-g-PDMAEMA) by ATRP in 1-allyl-3-methylimidazolium chloride ([AMIM]Cl) and described its pH- and temperature-responsive properties [12]. Dong et al. synthesized cellulose graft-poly (l-lactide) in [AMIM] Cl, and found that the polymer could self-assemble spherical micelles with diameter of 30–80 nm and core–shell structure in an aqueous medium [13]. The micelle was an aggregate of hydrophobic cellulose, which can be used to load drugs [14, 15]. Guo et al. prepared a cellulose-g-poly (lactide) copolymer in ionic liquid that had good micelle-forming behavior and high encapsulation efficiency for paclitaxel [16]. Kang and colleagues used ATRP to graft poly(2-(diethylamino) ethyl methacrylate) onto a cellulose backbone and found that the polymeric micelles had an optimum pH-sensitivity for controlled release of rifampicin [17].
Aspirin is a widely used antipyretic and analgesic, and may have a cancer prevention function [18, 19]. However, it has low solubility, high chemical reactivity, low stability, and it irritates the gastric mucosa [20]. The disadvantages of aspirin have been overcome by encapsulation in pH-sensitive cellulose polymeric micelles, and the drug-loaded micelles were used for controlled-release drug delivery depending on the difference in pH of normal tissue and local lesions [21, 22]. The hydrophilic, pH-sensitive groups grafted onto the cellulose backbone formed the shell of the micelles via self-assembly, and the physicochemical properties of shell controlled the response to environmental stimuli [23]. The copolymer with poly(4-vinylpyridine) (P4VP) groups had good pH-sensitivity, biocompatibility and hydrophilicity [24], and its micelle released drugs locally in the acid environments of tumors (pH <7.2 and generally, 4.8–6.8), thus avoiding the side effects of drugs [25]. Most P4VP stimuli-responsive copolymer micelles used in pH-responsive drug carriers have been developed as block copolymers in organic solvents [26,27,28]. Grafting P4VP onto a cellulose chain backbone in ionic liquid is preferable for use as a stimuli-responsive drug-loading and controlled-release system. Cellulose-g-P4VP micelles as drug carrier to encapsulate ASP and study the pH-dependent release mechanism of aspirin may serve as a model for future study of the controlled release of hydrophobic anticancer drugs for cancer treatment.
In this study, P4VP molecular chains were evenly grafted onto a microcrystalline cellulose backbone using ATRP and [Amim] Cl ionic liquid. The pH-sensitivity and micellar size of the obtained copolymers were investigated by dynamic light scattering (DLS) and ultraviolet-visible spectrophotometry (UV-Vis). The drug loading property of cellulose-g-P4VP polymeric micelles was evaluated with aspirin as a model hydrophobic drug, and the release of aspirin was carried out in phosphate buffer solutions (PBS) at different pH value. Cellulose-g-P4VP had optimal use characteristics for sustained drug release.
Experimental
Materials
Microcrystalline cellulose (MCC) was obtained from Tianjin Fuguang Fine Chemical Research Institute, and 4-vinylpyridine (4-VP, 99%; Aldrich) was distilled under reduced pressure before use. Copper (I) bromide (CuBr, 98%; Aldrich) was stirred in glacial acetic acid to remove any soluble oxidized species, washed in ethyl alcohol, and then dried under vacuum at 25 °C. 2-Bromoisobutyryl bromide (BrBiB, 98%; Aladdin), N,N,N´,N´´,N´´-pentamethyldiethylenetriamine (PMDETA, 99%; Aladdin) ligand, ionic liquid 1-allyl-3-methylimidazolium chloride ([AMIM] Cl, >99%; Shanghai Cheng Jie Chemical Co.) and aspirin (ASP, Bomei, BioScience Co.) were used as purchased. Dialysis bags (MWCO = 3500) was purchased from Shanghai Yuanye BioScience Co. China. Sodium dihydrogen phosphate (Tianjin Bodi Chemical Co.) and disodium hydrogen phosphate (Tianjin Tianzheng Chemical Co.) were used to prepare PBS with different pH values.
Synthesis and characterization of cellulose-P4VP copolymers
The synthesis of cellulose-g-P4VP graft copolymers is shown in Scheme 1. First, 0.40 g MCC was dissolved in [Amim] Cl, and then a mixture of 0.02 g BrBiB and 5 ml toluene was added dropwise into the solution. The reaction mixture was kept at 25 °C for 24 h. An excess of deionized water was added to the solution, and the resulting cellulose-BiB flocculent precipitate was washed and freeze-dried. Thus, cellulose-BiB was generated with a degree of substitution (DS) of 1.05. Second, 0.040 g cellulose-BiB was dissolved in [Amim]Cl; CuBr, PMDETA, 4-VP were added to the solution, and the reaction mixture was held at 60 °C for 12 h under nitrogen atmosphere. An excess of deionized water was added to the solution, and obtained the cellulose-P4VP copolymer. The copolymer was diluted with distilled water and then transferred to a Soxhlet extraction reactor using acetone as the solvent. After 12 h, the sample was repeatedly washed with distilled water and freeze-dried at −50 °C for 24 h (Scientz-10 N Freeze dryer, NingBo scientz biological technology Co., Ltd.) obtain the graft copolymer. The grafting efficiency (GE) was calculated according to Eq. (1):
where W0 is the quantity of cellulose-BiB (g). W1 and W2 are the quantities of 4-VP (g) monomer and cellulose-g-P4VP(g), respectively.
Loading aspirin into the graft copolymer micelles and its release in vitro
The blank and drug-loaded cellulose-g-P4VP copolymer micelles were prepared by dialysis. Cellulose-g-P4VP 40 mg and aspirin 10 mg were dissolved in dimethylsulfoxide (DMSO) by stirring 12 h at room temperature. Then the solution was put into a dialysis bag and dialyzed against distilled water for 48 h, with the distilled water being changed every 6 h. The aspirin (ASP) loaded micelles were obtained by freeze-drying the micelle solution. The preparation of the blank micelles was same except that no aspirin was included in the DMSO solution. The drug loading capacity (LC) and entrapment efficiency (EE) were determined by Eqs. (2) and (3):
For the evaluation of drug release, 10 mg drug-loaded micelles were suspended in dialysis bags containing pH 5.8, pH 7.4, or pH 8.0 phosphate buffer. The dialysis bags were sealed and immersed in 90 mL of phosphate buffer with the corresponding pH value. The systems were shaken in a water bath at 37 °C, and 10 mL of phosphate buffer was removed and replaced by10 mL of fresh buffer. The aspirin released from the polymeric micelles was determined with a UV-Vis spectrophotometer (U-3900/3900H, Titachi Corp., Japan) at 208 nm, and the results were reported as mean of three independent experiments. Bulk drug release was determined by suspending equal amounts of aspirin-loaded micelles in pH 7.4 PBS phosphate buffer. Other experimental conditions were same as for experimental drug release. Cumulative drug release was calculated by Eq. (4).
W0 (mg) was the weight of drug loaded in the polymer micelles. C(n) (mg·L−1) was the drug concentration at nth measurement. ΣC(n-1) (mg·L−1) was the sum of the drug concentration of n-1 measurements.
Characterization
The Fourier transform infrared (FT-IR) spectra (thin casting films on KBr pallets) were recorded by a Nicolet 6700 spectrometer (Nicolet, USA) equipped with a diamond ATR device. 1H nuclear magnetic resonance (NMR) was used to determine the chemical composition of copolymers by a 500-MHz Bruker AVANCE NMR spectrometer (Bruker, Germany). The DS of BiB was also calculated from the 1H NMR spectrum. It was determined by comparing the integral area of the signal for the methyl hydrogen of 2-bromoisobutyryl (6H, 1.9 ppm and 1.7 ppm) to the integral area of the signal for the cellulose backbone protons(7H, 4.0 ppm–6.0 ppm). The molecular weight distribution of P4VP side chain hydrolysates was assayed by Waters 150 gel permeation chromatography (GPC). The P4VP side chains of the cellulose-P4VP copolymer were cleaved by acid solvolysis as previously described [29]. The surface tension value of micellar aqueous solution was determined by a DCAT21 tensiometer (Dataphysics Co., Germany). The size and polydispersity index (PDI) of micelles were determined by DLS on Nano-ZS90 zeta potential analyzer (Malvern, UK). The concentration of the micelle samples was 100 mg·L−1. Every test was repeated at least three times and the average value was calculated. Transmission electron microscopy (TEM) of micelles was carried out with a 2100HR electron microscope (JEOL, Japan). The sample of micelles solution was dropped on the formvar stabilized with carbon support films. The X-ray diffraction patterns of samples were collected on a D/MAX-2500 diffractometer (Rigaku, Japan; Cu Kα 0.1542 nm, ds1/2c, LS10mm, SS 1/2°, RS 0.15 mm, continuous 10°/min and step 0.02°).
Results and discussion
FT-IR spectroscopy of cellulose graft copolymers
The FT-IR spectra of MCC, cellulose-g-BiB and cellulose-g-P4VP are shown in Fig. 1. In the MCC spectra (Fig. 1a), the broad peak at 3200–3500 cm−1 corresponded to the stretching vibration of -OH. Compared with the spectrum of pure MCC, the cellulose-g-BiB copolymers (Fig. 1b) showed a new characteristic peak at around 1750 cm−1 that corresponded to the stretching vibration of carbonyl groups characteristic of cellulose-g-BiB copolymers. In the cellulose-g-P4VP spectra (Fig. 1c), new peaks at 1600 cm−1, 1558 cm−1 were observed, which belonged to the stretching vibration of C-N bonds of pyridine rings. The new peaks at 1452 cm−1, 1414 cm−1 belonged to the stretching vibration from C=C double bonds of pyridine rings. The peaks at 1070 cm−1 and 1000 cm−1 corresponded to the in-plane bending vibration of C–H bonds of pyridine rings. The peak at 825 cm−1 corresponded to out-of-plane bending vibration of C–H bonds of pyridine rings. These results confirmed that the P4VP side chain had been grafted onto the cellulose backbone.
1H NMR characterization of cellulose-BiB and cellulose-g-P4VP
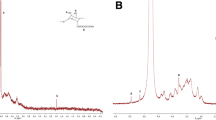
The chemical structure of cellulose-g-P4VP copolymers was characterized by 1H NMR, and the results are shown in Fig. 2. On 1H NMR spectra of the cellulose-g-BiB copolymer (Fig. 2a), new peaks at 1.9 ppm and 1.7 ppm were from the protons of 2-BIB. The resonance peaks at 4.0–6.0 ppm were from hydrogen in the sugar units of MCC. The 1HNMR spectra confirmed that the cellulose-g-BiB copolymer was obtained. On 1HNMR spectra of the cellulose-g-P4VP copolymer (Fig. 2b), the peaks at 1.5 ppm and 2.1 ppm were from the methylene (-CH2-) of P4VP. The peaks at 6.4 ppm (b) and 8.3 ppm (a) were from ortho-protons and meta-protons of pyridine ring. The peaks at 0.9 ppm were from the methylene (-CH2-) of MCC. The 1H NMR spectra confirmed that the cellulose-g-P4VP copolymer had been obtained.
Effect of the amount of 4-VP on cellulose-g-P4VP
The experimental details and the resultant cellulose-g-P4VP copolymers are summarized in Table 1. The graft efficiency and molecular weight of side chains increased with the amount of 4-VP monomer. When nCuBr: n4-VP molar ratio was 1:140, the polymer had a high 73.4% graft efficiency. The side chains of cellulose-g-P4VP synthesized by ATRP had a narrow molecular weight distribution. This suggested that ATRP had good control over the polymerization process, and that the P4VP side chains were evenly grafted on the cellulose chain backbone. As shown in Table 1, the Critical Micelle Concentration (CMC) content increased with enhanced graft efficiency, which occurred because increase in the number of hydrophilic groups led to a decrease of intermolecular and/or intramolecular hydrophobicity. The ability of polymers to form micelles was weakened and CMC value of polymer was increased. When nCuBr:n4-VP was 1:140, the polymer had a low CMC content of 0.068 mg/ml. As a drug loading and controlled-release system, the micelles can go through high power dilution and thus are more stable when the copolymer micelles had low CMC values. The optimum reaction conditions were attained at a CuBr/PMDETA/4-VP molar ratio of 1:20:140.
pH-dependent behavior of micelle formation
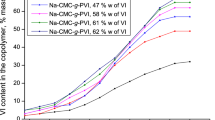
The UV-Vis transmittance of cellulose-g-P4VP micelles at different pH value is shown in Fig. 3. The transmittance of micelles solution declined with the increase of pH. At a pH above 4.3, the transmittance of the solution rapidly decreased. At the same time, the P4VP side chains began to deprotonate, and the electrostatic repulsion between the micelles decreased. As the micelles began to aggregate, the transmittance gradually decreased [30]. The transmittance sharply decrease at pH 5.7, which was very close to the pKb (about 5.4) of the P4VP side chains, and resulted from the collapse of deprotonated P4VP side chains in the shell of the micelles. Transmittance decreased as the micelles aggregated [31,32,33]. When the pH increased to 7.2, the transmittance of the micelle solution became constant.
As shown in Fig. 4, the pH response of the micelles included a change of diameter. The Dh of the micelles remained constant at a pH below 4.5, and the Dh of the micelles decreased sharply at around pH 5.5. This resulted from deprotonation of the P4VP side chains in the shell of the micelles and side-chain aggregation toward the core of micelles [34, 35]. When the pH increased to above 7.2, the dispersed micelles formed large micelle aggregates, and as the Dh of the micelles slightly increased, the pH-associated trend to increased diameter was detected by change in UV-Vis transmittance.
Morphology and diameter of blank and drug-load micelles
The particle size and size distribution of self-assembled micelles were evaluated by TEM and DLS, and the results are shown in Fig. 5. The particle size of blank micelles was 97.3 nm (PDI = 0.062). After drug loading with aspirin, the particle size increased to 156.3 nm (PDI = 0.153). Aspirin-loaded micelles had a uniform size distribution. The TEM images of blank micelles shown in Fig. 5a, reveal a regular spherical morphology with diameters of about 90 nm. Figure 5b shows TEM images of aspirin-loaded micelles. After aspiring loading, the diameters increased to about 150 nm, and the micelles had a regular spherical morphology with core-shell structure. The micelle particle size observed by TEM corresponded to the DLS results and demonstrated that the cellulose-g-P4VP could effectively entrap aspirin.
Analysis of the drug loading property of micelles
The XRD results for bulk drug, blank micelles and drug-loaded micelles are shown in Fig. 6. When the crystalline drug was encapsulated within polymeric micelles, its characteristic diffraction weakened or disappeared. The XRD diffraction pattern of aspirin (Fig. 6a) exhibited typical characteristic diffraction peaks at 5°-35° (2θ). Figure 6b shows the XRD diffraction pattern of cellulose-g-P4VP, with an amorphous scattering peak caused by its poorly crystalline properties. When aspirin was loaded into these poorly crystalline cellulose-g-P4VP micelles, the crystal diffraction peaks of the model drug nearly disappeared (Fig. 6c) because the drug lost its ordered molecular arrangement when present within the amorphous polymeric micelles [36].
Drug loading and loading efficiency obtained with different aspirin/micelle weight ratios are shown in Table 2. As the ratio of aspirin to micelle weight increased, the diameter and drug loading ratio of the micelle increased. However, when the ratio increased from 20/40 to 30/40, drug loading only increased by 34.38 to 35.01%. Even though the increase of drug loading capacity was small, the decrease of encapsulation efficiency was large. This reflected the limit of the encapsulation capacity of polymeric micelles for drugs. When the weight of the drug increased to a certain extent, the encapsulation capacity of micelles for aspirin reached a maximum and the loading efficiency declined with the increase of ASP and the amount of unbound drug increased. The optimum mass ratio of aspirin and micelles was 20/40 and demonstrated the optimum encapsulation capacity of micelles for aspirin.
pH-responsive release behavior of ASP in graft copolymer micelles
The cumulative release of aspirin by drug- loaded micelles at different pH values is shown in Fig. 7. In the initial stage of drug release, there was no obvious instant drugs release condition. At 50 h, the aspirin cumulative release in phosphate buffer was 86.4% at pH 5.8, 60.9% at pH 7.4, and 42.2% at pH 8.0. The cumulative release of drug was higher in an acidic than in a basic environment. A schematic diagram of drug release in low and high pH phosphate buffer solutions is shown in Fig. 8. In an acidic environment, nitrogen atoms of the P4VP side chains were protonated and contained many hydrogen ions. The increased electrostatic repulsion and enhanced solubility of these charged side chains resulted in micelle swelling or disaggregation [37]. These properties made for rapid drug release by loaded micelles at a pH characteristic of tumor tissue, i.e., pH < 7.2 and generally, 4.8–6.8. In a basic environment, drug-loaded micelles had a tight, spherical morphology because of the deprotonation of P4VP side chains, which favored the retention of drug within the micelles. Compared with acidic conditions, the cumulative release of aspirin was lower at the same time. Cellulose-g-P4VP has potential value in cancer therapy as a pH-responsive carrier with sustained drug release. The drug-loaded micelles had excellent pH-sensitivity and sustained drug release characteristics.
Conclusions
The pH-responsive drug carrier, cellulose-g-P4VP copolymers were synthesized by ATRP in [AMIM]Cl. The side chains of cellulose-g-P4VP had a narrow molecular weight distribution and were evenly grafted onto the cellulose chain backbone. Cellulose-g-P4VP copolymers could self-assemble into spherical nanoscale micelles with diameter of around 90 nm. The micelles showed an excellent pH responsiveness and appeared the phase transition at pH 5.7 value. After drug loading, the micelles presented a core–shell structure with a diameter of around 160 nm, and the polymeric micelles possessed an excellent aspirin encapsulation capacity. The hydrophobic aspirin release from the polymeric micelles displayed clearly pH sensitive, with cumulative release of aspirin in phosphate buffer of 86.4% at pH 5.8, 60.9% at pH 7.4 and 42.2% at pH 8.0.at 50 h, respectively. The cumulative release was higher in acidic than that of in basic environments. Collectively, the study results indicate that these pH-sensitive cellulose-g-P4VP polymeric micelles can be used as carriers of anticancer drugs and in other biomedical applications.
References
Marcus WO, Heike H, Michael G, Markus B (2016) Cellulose-graft-polystyrene bottle-brush copolymers by homogeneous RAFT polymerization of soluble cellulose macro-CTAs and“CTA-shuttled” R-group approach. Polymer 98:505–515
Murray BS, Durga K, De Groot PWN, Kakoulli A, Stoyanov SD (2011) Preparation and characterization of the foam-stabilizing properties of cellulose-ethyl cellulose complexes for use in foods. J Agric Food Chem 59:13277–13288
Chen NS, Tong ZH, Yang WH, Brennan AB (2015) Biocomposites with tunableproperties from poly (lactic acid)-based copolymersand carboxymethylcellulosevia ionic assembly. Carbohydr Polym 128:122–129
Massoumeh B, Shaghayegh S (2012) Thermosensitivenanosized micelles from cholesteryl-modifiedhydroxypropyl cellulose as a novel carrier of hydrophobic drugs. Iran Polym J 21:365–373
Wan S, Jiang M, Zhang GZ (2007) Dual temperature- and pH-dependent self-assembly of cellulose-based copolymer with a pair ofcomplementary grafts. Macromolecules 40:5552–5558
Li QM, Kang HL, Liu RG, Huang Y (2012) Block and hetero ethyl cellulose graft copolymers synthesized via sequent and one-pot ATRP and “click” reactions. Chin J Chem 30:2169–2175
Matyjaszewski K, Xia JH (2001) Atom transfer radical polymerization. Chem Rev 101:2921–2990
Lin CX, Zhan HY, Liu MH, Fu SY, Zhang JJ (2009) Preparation of cellulose graft poly (methyl methacrylate) copolymers by atom transfer radical polymerization in an ionic liquid. Carbohydr Polym 78:432–438
Zhang SJ, Lv XM (2006) The ionic liquids-from basic research to industrialapplication1th edn. Science Press, Beijing (Chapter 6)
Luan YH, Wu J, Zhan MS, Zhang JM, Zhang J, He JS (2013) “One pot” homogeneoussynthesis of thermoplastic cellulose acetate-graft-poly (L-lactide) copolymers from unmodified cellulose. Cellulose 20:327–337
Xu AR, Wang JJ, Wang HY (2010) Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem 12:268–275
Sui YF, Yuan JY, Zhou M, Zhang J, Yang HJ, Yuan WZ, Wei Y, Pan CY (2008) Synthesis of cellulose-graft-poly (N,N-dimethylamino-2-ethylmethacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 9:2615–2620
Dong HQ, Xu Q, Li YY, Mo SB, Cai SJ, Liu L (2008) The synthesis of biodegradable graft copolymer cellulose-graft-poly (l-lactide) and the study of its controlled drug release. Colloids Surf B: Biointerfaces 66:26–33
Yuan WZ, Zou H (2016) Amphiphilic graft copolymers with ethyl cellulose backbone: synthesis, self-assembly and tunable temperature-CO2 response. Carbohydr Polym 136:216–223
Yan Q, Yuan JY, Zhang FB, Sui XF, Xie XM, Yin YW, Wang SF, Wei Y (2009) Cellulose-based dual graft molecular brushes as potential drug nanocarriers: stimulus-responsive micelles, self-assembled phase transition behavior, and tunable crystalline morphologies. Biomacromolecules 10:2033–2042
Guo YZ, Wang XH, Shu XC, Shen ZG, Sun RC (2012) Self-assembly and paclitaxel loading capacity of cellulose-graft-poly (lactide) nanomicelles. J Agric Food Chem 60:3900–3908
Wang DQ, Tan JJ, Kang HL, Ma L, Jin X, Liu RG, Huang Y (2011) Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr Polym 84:195–202
Rodríguez LG, González-Pérez A (2004) Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer 91:525–529
Ratnasinghc LD, Graubard BL, Kaele L, Tangrea JA, Taylor PR, Hawk E (2004) Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res 24:3177–3184
Jung KH, Chu K, Lee ST, Yoon HJ, Chang JY, Nam WS, Yoon SH, Cho JY, Yu KS, Jang IJ, Kim M, Lee SK, Roh JK (2011) Prolonged use of aspirin alters human and rat intestinal cells and thereby limits the absorption of clopidogrel. Clin Pharmacol Ther 90:612–619
Sadhukhan S, Bakshi P, Datta R, Maiti S (2015) Poly (ethylene oxide)-g-gellan polysaccharide nanocarriers for controlled gastrointestinal delivery of simvastatin. J Appl Polym Sci 132:42399–42408
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–131
Luo YL, Yang XL, Xu F, Chen YS, Zhao X (2014) pH-triggered PMAA-b-HTPB-b-PMAA copolymer micelles: physicochemical characterization and camptothecin release. Colloid Polym Sci 292:1061–1072
Yang RM, Liu YH, Wang YM (2009) Hydroxyethylcellulose-graft-poly(4-vinylpyridine) as a novel, adsorbed coating for protein separation by CE. Electrophoresis 30:2321–2327
Ganta S, Devalapally H, Shahiwala A, Amiji M (2008) A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126:187–204
Sary N, Rubatat L, Brochon C, Hadziioannou G, Ruokolainen J, Mezzenga R (2007) Self-assembly of poly (diethylhexyloxy-p-phenylenevinylene)-b-poly(4-vinylpyridine) rod-coil block copolymer systems. Macromolecules 40:6990–6997
Peng HS, Chen DY, Jiang M (2003) Self-assembly of formic acid/polystyrene-block-poly(4-vinylpyridine) complexes into vesicles in a low-polar organic solvent chloroform. Langmuir 19:10989–10992
Liu H, Shi RH, Wan WM, Yang RM, Wang YM (2008) A well-defined diblock copolymer of poly (ethylene oxide)-block-poly(4-vinylpyridine) for separation of basic proteins by capillary zone electrophoresis. Electrophoresis 29:2812–2819
Tang EJ, Du KD, Feng XY, Yuan M, Liu SJ, Zhao DS (2015) Controlled synthesis of cellulose-graft-poly[2-(diethylamino)-ethyl methacrylate] by ATRP in ionic liquid [AMIM] cl and its pH-responsive property. Eur Polym J 66:228–235
Luo YL, Yu W, Xu F (2012) pH-responsive PMAA-b-PEG-b-PMAA triblockcopolymer micelles for prednisone drug release and release kinetics. Polym Bull 69:597–620
Dan N, Tirrell M (1993) Self-assembly of block copolymers with a strongly charged and a hydrophobic block in a selective, polar solvent, micelles and adsorbed layers. Macromolecules 26:4310–4315
Wittmer J, Joanny JF (1993) Charged diblock copolymers at interfaces. Macromolecules 26:2691–2697
Arimura H, Ohya Y, Ouchi T (2005) Formation of core-shell type biodegradable polymeric micelles from amphiphilic poly (aspartic acid)-block-polylactide diblock copolymer. Biomacromolecules 6:720–725
Chauhan GS, Singh B, Dhiman SK (2004) Functionalization of poly(4-vinyl pyridine) grafted cellulose by quaternization reactions and a study on the properties of postquaternized copolymers. J Appl Polym Sci 91:2454–2464
Sidorov SN, Bronstein LM, Kabachii YA, Valetsky PM, Soo PL, Maysinger D, Eisenberg A (2004) Influence of metalation on the morphologies of poly (ethylene oxide)-block-poly(4-vinylpyridine) block copolymer micelles. Langmuir 20:3543–3550
Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, Aquilano D, Trotta M, Zara GP, Cavalli R (2010) Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm 74:193–201
Ranjha NM, Ayub G, Naseem S, Ansari MT (2010) Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J Mater Sci Mater Med 21:2805–2816
Acknowledgements
The authors are grateful to the financial support by National Natural Science Foundation of China (No.21304030), Key basic research project in Hebei province, China (No.18961211D), Natural Science Foundation of Hebei Province, China (No.B2013208183) and Scientific Research Foundation for returned scholars preferred project of Hebei Province, China (No. C201400516).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, H., Han, R., Tang, E. et al. Synthesis of pH-responsive cellulose-g-P4VP by atom transfer radical polymerization in ionic liquid, loading, and controlled release of aspirin. J Polym Res 25, 205 (2018). https://doi.org/10.1007/s10965-018-1601-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1601-8