Abstract
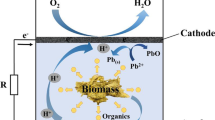
Herein, we report a new strategy for the simultaneous degradation of lignocellulosic biomass and bioelectricity generation using a novel three-chamber microbial fuel cell (MFC). Oscillatoria annae, a freshwater cyanobacterium, was used for the hydrolysis of cellulose to glucose. The electrocatalytic activity of the coculture of Acetobacter aceti and Gluconobacter roseus was used to oxidize the glucose for current generation in the MFC. Carbon felt was used as the anode and cathode material. Lignocellulosic materials such as sugarcane bagasse and corn cob were used as substrates. The performances of the MFC with two different substrates were analyzed by polarization studies, coulombic efficiency, percentage of COD removal and internal resistance. The three-chamber MFC produced a maximum power output of 8.78 W/m3 at 20.95 A/m3 and 6.73 W/m3 at 17.28 A/m3 with sugarcane bagasse and corn cob as substrates, respectively.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are electrochemical devices that make use of the complex enzymatic catalytic machinery of microorganisms to oxidize the substrates to generate bioelectricity (Schröder 2007). MFCs have several advantages over conventional energy sources because of their high conversion efficiency, wide range of operating conditions, simplicity as well as ecofriendly nature (Rabaey and Verstraete 2005). MFCs seems to be a boon to humankind because of their applications in sensors (Zhang et al. 2011), robotics (Ieropoulos et al. 2012), biodegradation (Bhuvaneswari et al. 2013), denitrification (Zhu et al. 2013), phenol removal (Yang et al. 2013), dye degradation (Niu et al. 2012), production of chemicals (Logan and Rabaey 2012), CO2 reduction (Chen et al. 2013) and bioelectricity generation. Scaling up of MFC is mainly hindered by the cost of the operation and installation of the MFC. The cost of the MFC operation can be decreased by using cheap and abundant materials such as lignocellulose as the fuel. Lignocellulosic wastes come from a wide range of sources including agricultural, domestic and industrial wastes (Elmekawy et al. 2013). Cellulose is a polysaccharide made up of 1,4-linked α-D-glucopyranose units with a polymerization degree up to 15,000 units. Cellulose is the major component of many natural lignocellulosic biomasses, and its concentration ranges from 40 to 50 % dry weight (Horvath 2005). The incineration technique is used to reduce the bulk amounts of lignocellulosic wastes generated globally. However, the incineration procedure produces gaseous pollutants such as carbon dioxide, dioxins and fly ash. Several approaches have been used for the disposal and recycling of the huge volumes of cellulosic waste being generated at increasing rates. Land filling is a simple and widely used strategy for the removal of cellulosic materials. However, it suffers from serious disadvantages such as leachate treatment and landfill gas (Maeda et al. 2009; Mahar et al. 2009; Cayuela et al. 2012). Other technologies such as microbial/vermicomposting of cellulose for fertilizer production (Trejo-Hernández et al. 2007; Singh and Sharma 2005), photocatalytic degradation of cellulose (Yeber et al. 2000) and pyrolysis of cellulosic wastes for charcoal production (Hesas et al. 2003), although seemingly attractive, have failed to treat the bulk volume of cellulosic wastes from a wide range of sources.
The use of the lignocellulosic biomass in any bioprocess operation is mainly hindered by the recalcitrant nature of the lignocellulose, which makes these materials a challenge to use as substrates directly in the MFC. Hence, pretreatment is necessary to break the structural and chemical complexity of the biomass (Mosier et al. 2005). Cellulose can be hydrolytically broken down into glucose by biological, physical or chemical methods (Yang and Wyman 2008). Among the different hydrolysis procedures, microbial hydrolysis confers immense benefits such as maximum rates of hydrolysis, simple operating conductions, ease of operation and an ecofriendly nature. The enzymatic hydrolysis procedures are not suitable for the treatment of lignocellulosic materials because of their low temperature requirements, need for narrow pH conditions, high cost and so on. Several bacteria, including Clostridium cellulolyticum sp. (Lee et al. 1985) and Pseudomonas (Cheng and Chang 2011), and several groups of fungi, including Ascomycota (Kudanga and Mwenje 2005), Trichoderma (Martinez et al. 2008) and Basidiomycota (Baldrian and Valaskova 2008), have good cellulolytic ability. Cyanobacteria exhibits good lignocellulolytic activity. Oscillatoria annae, a fresh water cyanobacterium, had higher hydrolysis rates when compared with other cyanobacterial strains and fungi such as Pichia stipitis (Viswajith 2008). Besides this, unlike other microorganisms, cyanobacteria require very simple growth conditions. They can thrive in a wide range of aquatic and terrestrial habitats in nature. They show high flexibility to varied environments and have a very fast growth rate (Singh et al. 2012). Cyanobacterium seems to be promising for pretreatment of lignocellulosic biomass in the MFC (Saha et al. 2010; Prabha et al. 2009; Krishnaraj et al. 2014).
Herein, we report a novel three-compartmental MFC by coupling the lignolytic and cellulolytic activities of O. annae and the electrogenic activity of the coculture of Acetobacter aceti and Gluconobacter roseus for bioelectricity generation.
Materials and methods
MFC construction
Two three-chamber MFCs were constructed with a 2-mm-thick Perspex sheet. The first chamber had dimensions of 5 cm × 3 cm × 4.5 cm. The second and third chambers had dimensions of 3 cm × 3 cm × 4.5 cm. The three chambers were interconnected with membranes of 2 cm × 2 cm dimensions. The first and second chambers were separated by a cellophane membrane. The second and third chambers were connected by a proton exchange membrane (Nafion 115) with 2 cm × 2 cm dimensions. The volume of the first compartment was 45 ml. The second and third chambers contained 15 ml of the anolyte and catholyte, respectively. Carbon felt (3.18 mm thick, procured from Alfa Aesar) was used as the anode and cathode materials. Two acetic acid bacteria, namely A. aceti (NCIM no. 2116) and G. roseus (NCIM no. 2049), were procured from NCL, Pune, India. A. aceti was subcultured in sterile broth containing glucose (1 g), yeast extract (1 g) and tryptone (1 g) in 100 ml of double-distilled water. G. roseus was subcultured in broth containing sorbitol (1 g) and yeast extract (1 g) in 100 ml of distilled water. Coculture of A. aceti and G. roseus was used for the formation of the biofilm on the carbon felt anode until it reached a stable negative potential. O. annae, a freshwater cyanobacterium, was used for the pretreatment of lignocellulose in the first compartment of the three-chamber MFC. O. annae was cultured in BG11 media in normal daylight conditions. The composition of BG-11 media is given in the supplementary information. The first chamber contained O. annae (1 g wet weight) in 45 ml of BG-11 media; 15 ml of phosphate buffer (pH 7) was used as the anolyte and 15 ml of potassium ferricyanide (3.3 g/100 ml) of phosphate buffer was used as the catholyte; 0.3 g of sugarcane bagasse and corn cob was used as the substrate in the first and second MFCs, respectively. The anode compartment was completely deaerated with nitrogen to maintain anaerobic conditions. The experiments were carried out with aseptic conditions at room temperature (37 ± 2 °C). A data logger (Agilent acquisition 34970A data acquisition/switch unit) was used to measure the voltage difference between the anode and cathode across the fixed external resistance for every 5-min interval. The data were collected automatically by a data acquisition program and personal computer.
Polarization studies
Polarization studies were carried out in both sugarcane bagasse and corn cob fed three-chamber MFCs by applying variable resistance from 10,000 to 100 Ω, and the final steady-state voltage was recorded for each resistance (Karthikeyan and Sheela 2012). Then current (I) was calculated using the formula I = V/R, where V is the voltage and R the applied resistance. Current density, i (A/m3), was calculated using the formula i = I/v. Power density, P (W/m3), was calculated using the formula P = IV/v, where v is the volume of the anolyte (15 ml). Ohmic resistance of the two MFCs was calculated from the slope of the linear region of the polarization curve (Fan et al. 2008).
Coulombic efficiency
Catalytic oxidation of fuel by the microorganisms was analyzed by measuring the levels of COD for every 24 h throughout the experiment. The MFCs with two different substrates were discharged under constant load, and the coulombic efficiencies were calculated using the formula (Heijne et al. 2006; You et al. 2006):
where Q obs = the current gained under constant load (C), and Q theor = the quantity of current expected from the glucose consumption under constant load (C). Q obs is calculated by integrating the current versus time. Q theor is calculated using the formula Q theor = bcvf/M where b is the number of moles of electrons produced per mole of substrate (4 mol e− mol−1 based on oxygen), C (mg/l) is the overall COD removal, V is the volume of the anolyte, F is Faraday’s constant [96,485 C (mol e−)−1], and M is the relative molecular mass (32 g/mol by oxygen). Catalytic oxidation of fuel by the microorganisms was analyzed by measuring the COD levels every 24 h throughout the experiment. O. annae was used for hydrolyzing the cellulose, and the kinetics of the hydrolysis of glucose in the three-chamber MFC was calculated by estimating the levels of reduced sugar (Miller 1959).
Electrochemical characterization of biofilms
Two small electrodes were prepared for the electrochemical studies by fixing carbon felt of 1 cm × 0.4 cm dimensions to a brass rod. The biofilm of the mixed culture (A. aceti and G. roseus) was allowed to form on both bare and modified felt in phosphate buffer solution containing glucose (0.2 g/30 ml of buffer) and the mixed culture (wet weight of 0.1 g A. aceti and 0.1 g G. roseus). After the biofilm formation, the electrode was gently rinsed with fresh phosphate buffer solution and scanned in the potential region from −1.2 to +1 V under 50 mV/s in the phosphate buffer using NCE (normal calomel electrode) and Pt as reference and counter electrode, respectively. Aseptic conditions were maintained throughout the procedure. Cyclic voltammograms were also recorded with the addition of O. annae hydrolyzed sugarcane bagasse and corn cob as substrates.
SEM analysis of microbial growth
The biofilm of the coculture of A. aceti and G. roseus formed over the anodes were characterized by SEM analysis. A piece of the felt with biofilm was carefully cut to 1 cm × 1 cm dimensions in aseptic conditions. The felt was gold sputter coated, and the biofilm analyses were carried out using the Hitachi model S-3000H SEM unit.
Results and discussion
SEM analysis
O. annae hydrolyzed lignocellulose to glucose in the first chamber of the three-chamber MFC. The hydrolyzed sugarcane bagasse and corn cob entered the second chamber, oxidized by the coculture of A. aceti and G. roseus. O. annae are trichomes, single or forming flat or spongy thalli, without a sheath. They are motile and show typical oscillatory movements. The terminal portion of the trichome cells is hooked or contains spindle-shaped hormogones. They contain chlorophyll-a as a primary photosynthetic pigment and phycobiliproteins as light-harvesting accessory pigments. They also contain carotenoids such as beta carotene and echinone (Varalakshmi 2007). An SEM image of the pure culture of O. annae is shown in Fig. 1A. SEM images of the sugarcane bagasse and corn cob along with O. annae are shown in Fig. 1B, C, respectively. The growth of O. annae over the surface of the lignocellulosic material indicates that these materials are promising substrate sources. Previously, Viswajith 2008 identified the presence of a few lignolytic enzymes, namely laccase, manganese-independent peroxidase and polyphenol oxidase, and cellulolytic enzymes, such as endogluconase and xylanase, in O. annae.
Polarisation studies and coulombic efficiency
The power outputs of the MFCs fed with two different substrates, namely sugarcane bagasse and corn cob, were analyzed according to their polarization curves. Figure 2A, B provides the polarization curves for the MFCs fed with sugarcane bagasse and corn cob as substrates, respectively. Polarization studies with sugarcane bagasse as substrate produced a maximum power density of 8.778 W/m3 at 20.95 A/m3. The power density of the MFC with corn cob was found to be 6.725 W/m3 at 17.28 A/m3. The power density and current density of the sugarcane bagasse-fed MFC was 30.52 and 21.53 % higher, respectively, when compared with the corn cob-fed MFC. Karthikeyan et al. (2009) reported a power output of 859.74 m W/m3 at 2.7644 A/m3 with the coculture of A. aceti and G. roseus as electro catalysts and glucose as substrate. Another report on the use of acetate, ethanol and bad wine as substrates in coculture of A. aceti and G. roseus based MFCs was reported in the literature (Karthikeyan and Sheela 2012). The use of a sodium lauryl sulfate (SLS)-MnO2 modified cathode and MnO2 cathode in A. aceti and G. roseus based MFCs resulted in a power output of 395 m W/m3 at 1.405 A/m3 and 693 m W/m3 at 3.04 A/m3, respectively, with glucose as substrate (Karthikeyan et al. 2012). Elmekawy et al. (2014) documented bioelectro-catalytic valorization of dark fermentation effluent in an MFC using farm manure-based microorganisms and produced a maximum power density of 12.5 W/m3, which is comparable to the power density obtained with the three-chamber MFC. The dark fermentation effluent in the MFC also aided in substrate degradation up to 72 % and yielded a coulombic efficiency of 48 % with reduced sugars, volatile fatty acids and alcohols as substrates.
The power density of the MFC was mainly affected by ohmic resistance. The ohmic resistance of the three-chamber MFC with different substrates was found from the slope of the linear region as the entire polarization curve was not strictly linear. In the MFC with sugarcane bagasse as substrate, the ohmic resistance was found to be 945 Ω between the potential regions from 0.162 to 0.419 V. The slope value in the linear region in the polarization curve of the MFC with corn cob as substrate was found to be 1,145 Ω between 0.182 and 0.359 V. The coulombic efficiency of the MFC fed with sugarcane bagasse and corn cob was found to be 68.2 and 42.3, respectively. Pant et al. (2013) reported the two-step continuous process with first volatile fatty acid and domestic wastewater for biohydrogen production and bioelectricity generation. The COD removal rate of the system was found to be nearly 90 % and had a coulombic efficiency of 46 %. The coulombic efficiencies of the three-chamber MFC with sugar bagasse and powdered corn cob were comparable to those reported in the literature.
Bioelectrocatalysis of biofilm
The microbial bioelectrocatalysis of the biofilm-formed anode with two different substrates such as O. annae hydrolyzed sugarcane bagasse and O. annae hydrolyzed corn cob was analyzed by cyclic voltammetry; the results are shown in Fig. 3A, B, respectively. Initially, the cyclic voltammogram of the anode with biofilm without the addition of substrate was recorded. The cyclic voltammogram confirmed the presence of electroactive redox species in the biofilm. Further, the electrocatalytic activity of the biofilm was evaluated by adding the reducing sugar formed during the hydrolysis of sugarcane bagasse by O. annae. In the sugarcane bagasse-fed anode, the current rose from 0.26 to 0.42 mA with the addition of 20 mM of the substrate. The potential also shifted from 0.068 to 0.080 V. On further addition of 20 mM of the substrate, the peak current and the peak potential increased to 0.062 mA at 0.096 V.
The electrocatalysis of the coculture biofilm was also analyzed with hydrolyzed corn cob as substrate. In the case of the hydrolyzed corn cob-fed anode biofilm, the current rose from 0.85 to 1.031 mA at the first addition of substrate. At the second addition, the current rose to 1.12 mA. The peak potential at 0.075 V in the biofilm shifted to 0.099 V on addition of the corncob hydrolyzed substrate. The bioelectrocatalysis experiments showed that the coculture biofilm had good electrocatalytic activity in relation to both the sugarcane bagasse and corn cob hydrolyzed substrates for MFC applications.
COD removal
The consumption of lignocellulosic feedstock in the MFC using the two different groups of microorganisms was analyzed by measuring the COD levels in both the first and the second chamber for every 24 h over a period of 20 days. The percentage of COD removal in the MFC fed with sugarcane bagasse was 78 %, whereas the corn cob-fed MFC had a 67 % percentage of COD removal. This confirms the good efficacy of the three-chamber MFC to significantly decrease the COD. This increased COD change is due to the utilization of fuel in the second chamber. Then, on the 8th day, 0.01 g of corresponding substrate was added to sugarcane bagasse and corn cob fed MFCs, respectively. The percentage COD removal in the second cycle was found to be 76.28 and 69.47 % in the first chamber of a MFC with sugarcane bagasse and corncob as substrates. On the 15th day, 0.01 g of lignocelluloses substrate was added again. The average COD removal rate in the first chamber of the MFC with sugarcane bagasse and corncob as substrates was found to be 73.78 and 63.27 %, respectively. Similar studies in the COD levels were performed for the anolyte in the second chamber and are shown in Fig. 4B. The COD levels were initially very low in the second chamber of both the bare and modified anode MFCs. They slowly increased and reached the maximum on the 3rd day after the addition of substrate in the first chamber. Then, the COD levels decreased on the 6th and 7th days. After the addition of substrate for the second cycle, the COD levels increased after 2 days and decreased on the 6th and 7th days. This trend continued in the third cycle. A similar pattern was observed in MFCs with both sugarcane bagasse and corn cob as substrates.
The COD levels in both the first and the second chamber during the operation of the sugarcane bagasse and corn cob fed three-compartment MFC are shown in Fig. 4A, B, respectively.
Conclusion
In conclusion, a novel three-compartment MFC was developed with the catalytic activities of two different microbial communities being utilized without any interactions between these two communities. Use of lignocellulose as a substrate in MFCs is a major challenge because of the recalcitrant nature of lignocellulose. This problem was resolved by using a combination of a cyanobacterium and acetic acid bacteria. This strategy can be applied for several bioprocess operations, and further studies will be made toward production of therapeutic biomolecules in MFCs.
References
Baldrian P, Valaskova V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32:501–521
Bhuvaneswari A, Krishnaraj RN, Berchmans S (2013) Metamorphosis of pathogen to electrigen at the electrode/electrolyte interface: direct electron transfer of Staphylococcus aureus leading to superior electrocatalytic activity. Electrochem Commun 34:25–28
Cayuela ML, Sánchez-Monedero MA, Roig A, Sinicco T, Mondini C (2012) Biochemical changes and GHG emissions during composting of lignocellulosic residues with different N-rich by-products. Chemosphere 88(2):196–203
Chen BY, Liu SQ, Hung JY, Shiau TJ, Wang JM (2013) Reduction of carbon dioxide emission by using microbial fuel cells during wastewater treatment. Aerosol Air Qual Res 13:266–274
Cheng CL, Chang JS (2011) Hydrolysis of lignocellulosic feedstock by novel cellulases originating from Pseudomonas sp. CL3 for fermentative hydrogen production. Bioresour Technol 102:8628–8634
Elmekawy A, Diels L, De Wever H, Pant D (2013) Valorization of cereal based biorefinery by-products: reality and expectations. Environ Sci Technol 47(16):9014–9027
Elmekawy A, Srikanth S, Vanbroekhoven K, De Wever H, Pant D (2014) Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J Power Sources 262:183–191
Fan Y, Sharbrough E, Liu H (2008) Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol 42:8101–8107
Heijne AT, Hamelers HVM, Wilde V, Rozendal RA, Buisman CJ (2006) A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ Sci Technol 40:5200–5205
Hesas RH, Ashri Wan Daud WM, Sahu JN, Arami-Niya A (2003) The effects of a microwave heating method on the production of activated carbon from agricultural waste: a review. J Anal Appl Pyrol 100:1–11
Horvath AL (2005) Solubility of structurally complicated materials: I. Wood 35:77–92
Ieropoulos IA, Greenman J, Melhuish C, Horsfield I (2012) Microbial fuel cells for robotics: energy autonomy through artificial symbiosis. ChemSusChem 5:1020–1026
Karthikeyan R, Sheela B (2012) Simultaneous degradation of bad wine and electricity generation with the aid of the coexisting biocatalysts Acetobacter aceti and Gluconobacter roseus. Bioresour Technol 104:388–393
Karthikeyan R, Sathishkumar K, Murugesan M, Sheela B, Yegnaraman V (2009) Bioelectrocatalysis of Acetobacter aceti and Gluconobacter roseus for current generation. Environ Sci Technol 43:8684–8689
Karthikeyan R, Uskaikar HP, Berchmans S (2012) Electrochemically prepared manganese oxide as a cathode material for a microbial fuel cell. Anal Lett 45:1645–1657
Krishnaraj RN, Berchmans S, Pal P (2014) Symbiosis of photosynthetic microorganisms with non photosynthetic ones for the conversion of cellulosic mass into electrical energy and pigments. Cellulose 21:2349–2355
Kudanga T, Mwenje E (2005) Extracellular cellulase production by tropical isolates of Aureobasidium pullulans. Can J Microbiol 51:773–776
Lee SF, Forsberg CW, Gibbins LW (1985) Cellulolytic activity of Clostridium acetobutylicum. Appl Environ microbiol 50:220–228
Logan BE, Rabaey K (2012) Converssion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337:686–690
Maeda K, Morioka R, Osada T (2009) Effect of covering composting piles with mature compost on ammonia emission and microbial community structure of composting process. J Environ Qual 38(2):598–606
Mahar RB, Liu J, Li H, Nie Y (2009) Bio-pretreatment of municipal solid waste prior to landfilling and its kinetics. Biodegradation 20:319–330
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn Hypocrea jecorina). Nat Biotechnol 26:553–560
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Niu CG, Wang Y, Zhang XG, Zeng GM, Huang DW, Ruan M, Li XW (2012) Decolorization of an azo dye Orange G in microbial fuel cells using Fe(II)-EDTA catalyzed persulfate. Bioresour Technol 126:101–106
Pant D, Arslan D, Van Bogaert G, Gallego YA, De Wever H, Diels L, Vanbroekhoven K (2013) Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environ Technol 34(13–16):1935–1945
Prabha DS, Karthikeyan K, Krishnaraj RN, Akila BM, Hemanth S, Harikrishnan R, Archunan G, Malliga P (2009) Effect of phenolic compounds released during degradation of Coir pith by Oscillatoria annae on Albino rat (Rattus norvegicus). J Appl Sci Environ Manage 13(4):87–90
Rabaey K, Verstraete W (2005) Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23:291–298
Saha SK, Swaminathan P, Raghavan C, Uma L, Subramanian G (2010) Ligninolytic and antioxidative enzymes of a marine cyanobacterium Oscillatoria willei BDU 130511 during Poly R-478 decolourization. Bioresour Technol 101(9):3076–3084
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9:2619–2629
Singh A, Sharma S (2005) Composting of a crop residue through treatment with microorganisms and subsequent vermicomposting. Bioresour Technol 85:107–111
Singh A, Pant D, Stig IO, Poonam SN (2012) Key issues to consider in microalgae based biodiesel production. Energy Educ Sci Technol Part A Energy Sci Res 29(1):563–576
Trejo-Hernández MR, Ortiz A, Okoh AI, Morales D, Quintero R (2007) Biodegradation of heavy crude oil Maya using spent compost and sugar cane bagasse wastes. Chemosphere 68(5):848–855
Varalakshmi P (2007) Plant growth regulators from cyanobacteria. PhD thesis, submitted to Department of Marine Biotechnology, NFMC, Bharathidasan University, Tiruchirappalli-24, Tamilnadu, India
Viswajith V (2008) Potentials of Oscillatoria annae in producing bioethanol and plant growth regulator by the degradation of selected lignocellulosic, PhD dissertation, Bharathidasan University, Tiruchirappalli-24, Tamilnadu, India
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Bioref 2:26–40
Yang J, Zhou M, Zhao Y, Zhang C, Hu Y (2013) Electrosorption driven by microbial fuel cells to remove phenol without external power supply. Bioresour Technol 150:271–277
Yeber MC, Rodríguez J, Freer J, Durán N, Mansilla HD (2000) Photocatalytic degradation of cellulose bleaching effluent by supported TiO2 and ZnO. Chemosphere 41(8):1193–1197
You S, Zhao O, Zhang J, Jiang J, Zhao S (2006) A microbial fuel cell using permanganate as the cathodic electron acceptor. J Power Sources 162:1409–1415
Zhang F, Tian L, He Z (2011) Powering a wireless temperature sensor using sediment microbial fuel cells with vertical arrangement of electrodes. J Power Sources 196:9568–9573
Zhu G, Onodera T, Tandukar M, Pavlostathis SG (2013) Simultaneous carbon removal, denitrification and power generation in a membrane-less microbial fuel cell. Bioresour Technol 146:1–6
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krishnaraj, R.N., Berchmans, S. & Pal, P. The three-compartment microbial fuel cell: a new sustainable approach to bioelectricity generation from lignocellulosic biomass. Cellulose 22, 655–662 (2015). https://doi.org/10.1007/s10570-014-0463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0463-4