Abstract
Hydrogels with high water uptake were prepared by ionizing radiation induced crosslinking in aqueous solutions of four cellulose derivatives (carboxymethylcellulose sodium salt—CMC-Na, methylcellulose—MC, hydroxyethylcellulose—HEC and hydroxypropylcellulose—HPC). The gel fraction increased with absorbed dose, while water uptake decreased. At high polymer concentrations lower gel fractions were found due to the lower polymer chain mobility and inhomogeneity at low water content. The swelling rate gradually slowed down after 4–5 h. CMC and HEC gels reached equilibrium after 24 h, while HPC and MC gels required longer immersion times. Gels showed second-order swelling kinetics in water. The mechanism of the water diffusion proved to be anomalous. In pure water, CMC gels showed the highest, while HPC and MC gels the lowest water uptake. The derivatives had different sensitivities to ionic strength in the swelling solution. The salt type also proved to be a significant factor at uniform ionic strength. Thus different cellulose derivative based gels may be preferred at various applications depending on the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superabsorbents are three-dimensional polymer networks capable of absorbing large amounts of water: e.g. gels with carboxyl, hydroxyl or other hydrophilic functional groups. Due to their unique properties related to the very high water uptake and responsive behavior, they have a wide array of potential applications in various fields, such as in agriculture (Narjary et al. 2012), tissue engineering (Kim et al. 2008) and sensors (Richter et al. 2008).
Most of the practically applied superabsorbents (acrylate-based gels being the most widely used) are non-biodegradable. Therefore, producing gels with high water uptake using renewable and biodegradable materials is of great practical importance (Chang and Zhang 2011; Dastidar and Netravali 2012). Such renewable source is cellulose, the most abundant renewable material on earth, which can also be easily functionalized due to its hydroxyl groups (Mischnick and Momcilovic 2010).
Pure cellulose is also capable of forming gels, however, in order to produce hydrogels it requires the use of special solvents, such as alkali-urea (Cai and Zhang 2006; Qin et al. 2013), DMAc-LiAc (Ishii et al. 2006) or ionic liquid (Kadokawa et al. 2008) systems.
Difficulties of such solvents’ application suggest using a different way to get cellulose based hydrogels: proper gel stability and high water uptake can be achieved by developing inter- and intramolecular crosslinks in colloidal aqueous solution of cellulose derivatives. Substitution of the hydroxyl groups with carboxymethyl, hydroxyethyl, etc. groups on the cellulose backbone significantly changes the cellulose properties, including increased swelling in water up to solubility (BeMiller and Whistler 1993). Properties of cellulose derivatives depend on the type of substituent, molecular weight (Mw), degree of substitution (Ds), and distribution of substituents. Recently, several cellulose derivative-based hydrogels were synthesized using various methods (Chang and Zhang 2011; Saglam et al. 2002; Yang et al. 2011). While carboxymethyl cellulose is in the center of such research, various alkyl (Haque and Morris, 1993) and hydroxyalkyl (Rosén et al. 1998; Das et al. 2012; Uraki et al. 2006) derivatives may also be used for the purposes mentioned before. Both physical (Li et al. 2001; Saglam et al. 2002) and chemical gels were successfully synthesized, the latter by using various crosslinking agents (Anbergen and Oppermann 1990; Seki et al. 2014). Chemical crosslinking typically occurs by radical mechanism in the presence of initiator or induced by gamma, electron beam or UV irradiation (Clough 2001). A significant advantage of the latter methods is that gelation can be achieved without crosslinking agents. Radicals formed on the polymer chains during irradiation may initiate crosslinking or degradation (Charlesby 1955). Crosslinking is usually observed at higher solute concentrations (Fei et al. 2000; Yoshii et al. 2003), but recently successful gel formation in dilute solutions was also reported (Wach et al. 2014). Of the cellulose derivatives, most of the publications appear on the radiation induced synthesis of carboxymethylcellulose hydrogels (El-Din et al. 2010; Fei et al. 2000; Liu et al. 2002; Wach et al. 2003a), other derivatives getting significantly less attention (Furusawa et al. 2005; Pekel et al. 2004; Wach et al. 2003b). These cellulose based hydrogels are usually crosslinked by gamma or electron beam irradiation (Wach et al. 2002). Gelation proved to be dependent on the polymer properties: gels formed when the molecular weight was over a critical value (Wach et al. 2003b). Similar tendency was obtained for the degree of substitution (DS): CMC gels with higher DS had higher gel content (Fei et al. 2000). The atmosphere also had a significant effect on the gelling: irradiation in nitrogen atmosphere (Liu et al. 2002) or in vacuum (Fei et al. 2000) yielded higher gel content. In previous studies, the effect of absorbed dose (Furusawa et al. 2005) on gel properties was also thoroughly examined, but the effect of solution concentration was studied for a few derivatives only (Pekel et al. 2004, Yoshii et al. 2003). It is difficult to compare the results of gel fraction (GF) studies, due to the significantly differing methods of removing the sol, such as immersion in water for 2 days (Liu et al. 2005), for 2 weeks (Furusawa et al. 2005) or extraction in boiling water (Ibrahim et al. 2007). The time dependence of swelling was determined in numerous studies, but swelling kinetic models were proposed only for hydroxypropylcellulose gels (Wach et al. 2002), while the mechanism of water diffusion into the polymer network was not examined at all. As hydrogels are used in a number of applications in environment with high electrolyte concentration, the effect of ionic strength and pH on swelling properties was examined for various derivatives (Liu et al. 2005; Wach et al. 2003b), however, no studies were done on the ion type present at uniform ionic strength.
While the gelation of several derivatives was already reported, the comparison of various gel properties proves to be difficult due to the significantly differing synthesis parameters and characterization methods. The aim of this work is a comparative study of superabsorbent hydrogels prepared from various cellulose derivatives (CMC-Na, MC, HEC and HPC) by γ-irradiation initiated crosslinking. In a complex approach the effect of synthesis parameters on the gel properties including mechanism of water diffusion was determined and compared under the same conditions.
Experimental
Materials
CMC (Mw = 700,000 g mol−1, DS = 0.9; Mw = 100,000 g mol−1, DS = 1.0 was used as a reference only), HEC (Mw = 720,000 g mol−1, DS = 2.5) and MC (Mv = 88,000 g mol−1, DS = 1.5–1.9) were obtained from Sigma-Aldrich. HPC (Mw = 1,150,000 g mol−1) is a product of Ashland Specialty Ingredients. The molecular parameters were provided by the suppliers. For swelling studies, NaCl, KCl, BaCl2 and CaCl2 × 2H2O were purchased from Reanal (Budapest, Hungary), while Triton X-100 and Na-lactate are products of Sigma Aldrich. Chemical reagents were of analytical grade and used without further purification.
Synthesis
5–40 w/w% paste-like solutions of cellulose derivatives were prepared by dissolving their powder in deionized water. The solutions were stored for 24 h to achieve homogeneous structure. Spherical samples (~1 g) were prepared and closed into polyethylene bags. The irradiation was carried out in presence of air. 60Co γ-source (9 kGy h−1 dose rate) was used for irradiations, absorbed dose ranged from 1 to 200 kGy. Irradiated samples were dried at 60 °C to constant weight. All measurements (see Determination of gel fraction and Swelling studies) were performed on three samples.
Determination of gel fraction
Dried, weighted (w0) samples were immersed in deionized water for 48 h (liquid:gel (L:G) ratio of 1,000:1). Water was periodically changed in order to extract the sol fraction. The solutions were filtered by a sieve and the samples were dried at 60 °C to constant weight (w1). The GF was calculated by the following equation:
Swelling studies
After the removal of the sol fraction (see Determination of gel fraction), the dried, weighted gels (wd) were immersed again in deionized water (L:G = 1,000:1). Swollen gels were removed by using a sieve and weighted after blotting (ws). The degree of swelling (Q) was determined using the following formula:
Samples were characterized by the degree of swelling after 24 h.
The time dependence of the degree of swelling was measured by periodically removing and weighing the swollen gel. Samples were then again immersed in water. The change of Q was followed up to 48 h.
Swelling in salt solutions was also measured. Samples were immersed in NaCl solutions, salt concentrations were varied from 0.0025 to 0.5 mol dm−3. The degree of swelling after 24 h was determined and compared. The solvent uptake was also determined in three test solutions: two types of isotonic ones (Ringer’s solution: 8.60 g NaCl, 0.30 g KCl, 0.33 g CaCl2 × 2H2O/1,000 ml; Ringer’s lactate solution: 6.02 g NaCl, 0.41 g KCl, 0.26 g CaCl2 × 2H2O, 3.14 g Na-lactate/1,000 ml) and synthetic urine (10 g NaCl, 2.5 g Triton X-100/1,000 ml). These experiments were done to gain more information about certain potential applications (wound dressing and diapers).
Results and discussion
Synthesis parameters
Absorbed dose effect
The effect of dose on gel properties was determined using 20 w/w% solutions. Gel formation of CMC solutions (Mw = 700,000 g mol−1) required a dose over 5 kGy. GF increased with the dose up to 60 kGy as shown in Fig. 1a. Further irradiation led to a decrease of the gel fraction. The decrease may be due to chain degradation becoming more dominant. HEC gels showed similar behavior, though maximum gel ratio was achieved at a much lower dose (10 kGy). HPC and MC gels yielded significantly higher gel fractions, gel fractions decreased with the dose. The differences at lower doses can be attributed to the different polarities of the functional groups and to the differences in molecular weight. In case of HPC, the functional groups contain an additional methyl group compared to HEC and the molecular weight is 50 % higher than those of CMC and HEC derivatives. In solutions of low molecular weight CMC (Mw = 100,000 g mol−1) there is no gel formation during the irradiation (5–40 w/w% solution, 20 kGy). This can be explained by the shorter polymer chains not being able to form a continuous network due to the simultaneous degradation. However, in MC solutions gel formation was observed despite the low molecular weight (Mw = 88,000 g mol−1). In this case the substituent does not contain hydroxyl or carboxyl group, thus the water solubility is significantly lower.
The dose dependence of the degree of swelling was the opposite compared to the dose dependence of GF for CMC and HEC gels (Fig. 1b). The water uptake of CMC and HEC gels decreased with the dose up to 80 kGy, over which no changes were observed. This can be attributed to the increasing crosslinking density which hinders the water absorption. The degree of swelling of HPC and MC gels was similar and the swelling did not change much with the dose, only a very small increase was measured above 100 kGy, due to the degradation. CMC gels showed the largest water uptake, followed by HEC gels.
Effect of solute concentration
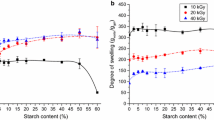
The concentration of the cellulose derivative in the solution basically influences the properties of the gels synthesized. The change of the gel concentration with the solute concentration (at 20 kGy absorbed dose) is shown in Fig. 2a. At very low solution concentrations (under 5–10 w/w% depending on the derivative) gels did not form. In dilute aqueous solutions the energy of irradiation is mainly absorbed by the water. The reactive intermediates produced during water radiolysis (OH radical, H atom and hydrated electron) attack the cellulose leading to formation of radicals on the cellulose chain. Due to the relatively large distance between the polymer chains in dilute solutions, these radicals cannot initiate crosslink formation between the chains; therefore, they initiate chain scission (degradation). Moreover, the crosslinking is also hindered by the electrostatic repulsion of the ionized carboxylic groups present in the slightly alkaline pH. However, higher concentrations lead to an increased chance of forming crosslinks (due to the lower distance between the chains), thus to an increased gel fraction. Over 15 w/w% cellulose derivative concentration no significant difference was observed up to 30 w/w%. Further increase led to a significant decrease in the gel fraction. This is the result of the low water content. Water enhances the mobility of the polymer chains (acting like a plasticizer) and at high concentrations (low water content) the polymer chains become less mobile, thus the crosslinking process is hindered. Over 40 w/w% no gel formation was observed. Similar tendency in the dose dependence of gel content was found by Yoshii et al. (2003). They came to the conclusion that crosslinking successfully competes with glycoside bond cleavage as the concentration of cellulose derivative in a solution exceeds some critical value. The lowest GF was obtained with CMC and the highest with HPC. HEC and MC showed similar behavior.
The dependence of the water uptake on the concentration is shown in Fig. 2b. The degree of swelling of all derivatives decreased with increasing concentration. CMC proved to be the most sensitive to the concentration changes. The decrease can be attributed to the increasing crosslinking density as indicated by the increasing gel fraction. At high concentrations the water uptake kept decreasing due to the hindered mobility of polymer chains as mentioned above.
Swelling studies
Swelling kinetics
Swelling rates of the cellulose derivative gels are shown in Fig. 3. The rate of water uptake is the largest in the first 5–6 h after which it gradually slows down. The equilibrium swelling ratio is reached after 24 h for CMC and HEC. The water uptake of HPC and MC gels was very low. The swelling rate is connected to the equilibrium swelling ratio—the higher the maximum water uptake, the faster is the swelling.
Swelling kinetics of the gels was described by supposing second order kinetics expressed by the following equation (Schott 1992):
where Qt and Q∞ are the degree of swelling at time t and equilibrium, respectively, while k is the swelling rate constant. By integrating the equation we have the linearized form:
where k∞ = kQ 2∞ . Second-order kinetics described well the swelling of all derivative gels (Fig. 4a). Table 1a contains the various kinetic parameters—the swelling rate constant increased with the equilibrium degree of swelling. The equilibrium degree of swelling is higher than the water uptake measured after 48 h, supposing further increase of water content at longer swelling times. The difference is not significant for CMC and HEC gels, while significant for the other two derivatives. This can be attributed to the much slower water uptake of the HPC and MC samples, thus a longer time is needed for reaching equilibrium.
The swelling kinetics was also described by applying the power-law to gain information about the mechanism of the water diffusion (Ganji et al. 2010):
where k and n are the power-law constants.
The value of n depends on the relation of solvent diffusion rate and polymer relaxation time. If n = 0.5, the polymer chain mobility is high, thus it does not hinder the diffusion of the solvent molecules and the process can be described as pure (Fickian) diffusion. Value of n = 1 indicates that solvent diffusion is significantly faster than the relaxation. This process can be described by zero order kinetics (Case II. diffusion). When 0.5 < n < 1 the diffusion is anomalous, the rates of the two processes are comparable. The linearized form of the equation was used:
The plots based on the equation are illustrated in Fig. 4b. Linear regression was applied only to values up to Q < 0.4Q∞, as the model is useable only at relatively low swelling degrees. The swelling parameters determined by the linear regression are shown in Table 1b. The value of n indicates anomalous diffusion mechanism for all samples. However, its value increases with decrease of equilibrium swelling ratio of various derivatives. This indicates that the swelling rate and equilibrium swelling are determined by the polymer chain relaxation: the slower the chain relaxation, the slower is the swelling, thus the mechanism is closer to Case II.
Salt effect
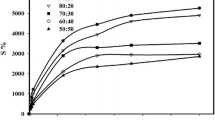
The effect of salt and salt concentration of the swelling solution varied depending on the cellulose derivative used (Fig. 5). The water uptake of CMC gels decreased significantly even at very low salt concentrations. This can be attributed to the carboxymethyl groups of CMC. The presence of the Na+ cations increase the water uptake through the Donnan osmotic pressure between the gel network and the outer solution (Rička and Tanaka 1984), while the repulsion of the charged groups on the polymer chain also helps the elongation of the network. However, with the increase in the ionic strength of the solvent the osmotic pressure between them decreases. HEC gels also showed a slight decrease in the water uptake with increasing ionic strength, while HPC and MC gels were unaffected by it. The type of salt also proved to be an important factor (Fig. 6). This is related to the ionic radius of the cations. The penetration of ions with large ionic radius to the gel network is more difficult than those with smaller ionic radius, thus the higher the hydrated ionic radius, the lower the swelling. The order of decreasing hydrated ionic radius is Ca2+ > Ba2+ > Na+ > K+ > NH4 + (Convay 1981), which is in good agreement with the changes in swelling. Only Ca2+ showed a slight difference, probably related to the larger standard error. The degree of swelling of CMC gels changed depending on the salt solution used, despite the uniform ionic strength. MC was not sensitive to the presence of salt as expected from the previous experiments. Thus due to the lower sensitivity to the salt solutions the use of gels other than CMC should be preferred in potential applications (agriculture, health) in environment with high electrolyte concentration liquids. The water uptake of HEC gels in various biologically relevant solutions is roughly the twice of those observed with CMC gels (Fig. 7).
Conclusions
Superabsorbent hydrogels based on aqueous solutions of cellulose derivatives (CMC, MC, HEC and HPC) were successfully prepared by γ-irradiation. In pure water second-order swelling kinetics described well the swelling behavior of all gels. The diffusion mechanism of the solvent into the polymer network proved to be anomalous.
In pure water the hydrophilic CMC-based hydrogel shows the highest swelling ability, while less hydrophilic derivatives, especially HPC and MC, can absorb much less water. However, the high water uptake is accompanied by high electrolyte sensitivity. In solutions, modeling the real environment of potential hydrogel applications, CMC loses its position among the derivatives, and HEC shows the best absorbing capacity. However, it should be noted that the molecular weight and the degree of substitution of various derivatives were not uniform, which should be taken into account when comparing them, as well.
References
Anbergen U, Oppermann W (1990) Elasticity and swelling behaviour of chemically crosslinked cellulose ethers in aqueous systems. Polymer 31(10):1854–1858. doi:10.1016/0032-3861(90)90006-K
BeMiller JN, Whistler RL (1993) Industrial gums: polysaccharides and their derivatives, 3rd edn. Academic Press, New York
Cai J, Zhang L (2006) Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules 7(1):183–189. doi:10.1021/bm0505585
Chang C, Zhang L (2011) Cellulose-based hydrogels: present status and application prospects. Carbohydr Polym 84:40–53. doi:10.1016/j.carbpol.2010.12.023
Charlesby A (1955) The degradation of cellulose by ionizing radiation. J Polym Sci 15(79):263–270. doi:10.1002/pol.1955.120157921
Clough RL (2001) High-energy radiation and polymers: a review of commercial processes and emerging applications. Nucl Instr Meth Phys Res B 185(1–4):8–33. doi:10.1016/S0168-583X(01)00966-1
Convay BE (1981) Ionic hydration in chemistry and biophysics. Elsevier, New York
Das R, Panda AB, Pal S (2012) Synthesis and characterization of a novel polymeric hydrogel based on hydroxypropyl methyl cellulose grafted with polyacrylamide. Cellulose 19:933–945. doi:10.1007/s10570-012-9692-6
Dastidar TG, Netravali AN (2012) ‘Green’ crosslinking of native starches with malonic acid and their properties. Carbohydr Polym 90(4):1620–1628. doi:10.1016/j.carbpol.2012.07.041
El-Din HMN, Alla SGA, El-Naggar AWM (2010) Swelling and drug release properties of acrylamide/carboxymethyl cellulose networks formed by gamma irradiation. Radiat Phys Chem 79(6):725–730. doi:10.1016/j.radphyschem.2010.01.011
Fei B,Wach RA, Mitomo H, Yoshii F, Kume T (2000) Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J Appl Polym Sci 78(2):278–283. doi:10.1002/1097-4628(20001010)78:2<278::AID-APP60>3.0.CO;2-9
Furusawa K, Dobashi T, Morishita S, Oyama M, Hashimoto T, Shinyashiki N, Yagihara S, Nagasawa N (2005) Structural and kinetic modification of aqueous hydroxypropylmethylcellulose (HPMC) induced by electron beam irradiation. Physica A: Stat Mech Applic 353:9–20. doi:10.1016/j.physa.2004.12.068
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E (2010) Theoretical description of hydrogel swelling: a review. Iranian Polym J 19(5):375–398
Haque A, Morris ER (1993) Thermogelation of methylcellulose. Part I: molecular structures and processes. Carbohydr Polym 22(3):161–173. doi:10.1016/0144-8617(93)90137-S
Ibrahim SM, Salmawi KME, Zahran AH (2007) Synthesis of crosslinked superabsorbent carboxymethylcellulose/acrylamide hydrogels through electron-beam irradiation. J Appl Polym Sci 104:2003–2008. doi:10.1002/app.25916
Ishii D, Tatsumi D, Matsumoto T, Murata K, Hayashi H, Yoshitani H (2006) Investigation of the structure of cellulose in LiCl/DMAc solution and its gelation behavior by small-angle X-ray scattering measurements. Macromol Biosci 6(4):293–300. doi:10.1002/mabi.200500231
Kadokawa J, Murakami M, Kaneko Y (2008) A facile preparation of gel materials from a solution of cellulose in ionic liquid. Carbohydr Res 343:769–772. doi:10.1016/j.carres.2008.01.017
Kim J, Lee KW, Hefferan TE, Currier BL, Yaszemski MJ, Lu L (2008) Synthesis and evaluation of novel biodegradable hydrogels based on poly(ethylene glycol) and sebacic acid as tissue engineering scaffolds. Biomacromolecules 9(1):149–157. doi:10.1021/bm700924n
Li L, Thangamathesvaran PM, Yue CY, Tam KC, Hu X, Lam YC (2001) Gel network structure of methylcellulose in water. Langmuir 17(26):8062–8068. doi:10.1021/la010917r
Liu P, Zhai M, Li J, Peng J, Wu J (2002) Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat Phys Chem 63:525–528. doi:10.1016/S0969-806X(01)00649-1
Liu P, Peng J, Li J, Wu J (2005) Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogel. Radiat Phys Chem 72:635–638. doi:10.1016/j.radphyschem.2004.03.090
Mischnick P, Momcilovic D (2010) Chemical structure analysis of starch and cellulose derivatives. Adv Carbohydr Chem Biochem 64:117–210. doi:10.1016/S0065-2318(10)64004-8
Narjary B, Aggarwal P, Singh A, Chakraborty D, Singh R (2012) Water availability in different soils in relation to hydrogel application. Geoderma 187–188:94–101. doi:10.1016/j.geoderma.2012.03.002
Pekel N, Yoshii F, Kume T, Güven O (2004) Radiation crosslinking of biodegradable hydroxypropylmethylcellulose. Carbohydr Polym 55:139–147. doi:10.1016/j.carbpol.2003.08.015
Qin X, Lu A, Zhang L (2013) Gelation behavior of cellulose in NaOH/urea aqueous system via crosslinking. Cellulose 20:1669–1677. doi:10.1007/s10570-013-9961-z
Richter A, Paschew G, Klatt S, Lienig J, Arndt KF, Adler HJP (2008) Review on hydrogel-based pH sensors and microsensors. Sensors 8(1):561–581. doi:10.3390/s8010561
Rička J, Tanaka T (1984) Swelling of ionic gels: quantitative performance of the Donnan theory. Marcomolecules 17:2916–2921. doi:10.1021/ma00142a081
Rosén O, Sjöström J, Piculell L (1998) Responsive polymer gels based on hydrophobically modified cellulose ethers and their interaction with ionic surfactants. Langmuir 14:5795–5801. doi:10.1021/la971168+
Saglam A, Yalçinkaya Y, Denizli A, Arica MY, Genç Ö, Bektas S (2002) Biosorption of mercury by carboxymethylcellulose and immobilized Phanerochaete chrysosporium. Microchem J 71(1):73–81. doi:10.1016/S0026-265X(01)00142-4
Schott H (1992) Kinetics of polymers and their gels. J Pharm Sci 81(5):467–470. doi:10.1002/jps.2600810516
Seki Y, Altinisik A, Demircioğlu B (2014) Carboxymethylcellulose (CMC)-hydroxyethylcellulose (HEC) based hydrogels: synthesis and characterization. Cellulose. doi:10.1007/s10570-014-0204-8
Tan R, She Z, Wang M, Fang Z, Liu Y, Feng Q (2012) Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohydr Polym 87(2):1515–1521. doi:10.1016/j.carbpol.2011.09.048
Uraki Y, Imura T, Kishimoto T, Ubukata M (2006) Body temperature-responsive gels derived from hydroxypropylcellulose bearing lignin II: adsorption and release behavior. Cellulose 13:225–234. doi:10.1007/s10570-005-9032-1
Wach RA, Mitomo H, Yoshii F, Kume T (2002) Hydrogel of radiation-induced crosslinked hydroxypropylcellulose. Macromol Mater Eng 287:285–295. doi:10.1002/1439-2054(20020401)287:4<285:AID-MAME285>3.0.CO;2-3
Wach RA, Mitomo H, Nagasawa N, Yoshii F (2003a) Radiation crosslinking of carboxymethylcellulose of various degree of substitution at high concentration in aqueous solution of natural pH. Radiat Phys Chem 68:771–779. doi:10.1016/S0969-806X(03)00403-1
Wach RA, Mitomo H, Nagasawa N, Yoshii F (2003b) Radiation crosslinking of methylcellulose and hydroxyethylcellulose in concentrated aqueous solutions. Nucl Instr Methods Phys Res B 211:533–544. doi:10.1016/S0168-583X(03)01513-1
Wach RA, Rokita B, Bartoszek N, Katsumura Y, Ulanski P, Rosiak JM (2014) Hydroxyl radical-induced crosslinking and radiation-initiatied hydrogel formation in dilute aqueous solutions of carboxymethylcellulose. Carbohydr Polym 112:412–415. doi:10.1016/j.carbpol.2014.06.007
Yang S, Fu S, Liu H, Zhou Y, Li X (2011) Hydrogel based on carboxymethyl cellulose for removal heavy metal ions. J Appl Polym Sci 119(2):1204–1210. doi:10.1002/app.32822
Yoshii F, Zhao L, Wach RA, Nagasawa N, Mitomo H, Kume T (2003) Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl Instrum Methods Phys Res B 208:320–324. doi:10.1016/S0168-583X(03)00624-4
Acknowledgments
The authors thank the Hungarian Science Foundation (NK 105802) for partial support, and Eva Horvathne Koczog for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fekete, T., Borsa, J., Takács, E. et al. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 21, 4157–4165 (2014). https://doi.org/10.1007/s10570-014-0445-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0445-6