Abstract
The unique combinations of hard and soft components with core/shell structures were proposed to synthesize high strength nanocomposite hydrogels. The elastomeric hydrogels containing rod-like cellulose nanocrystals (CNCs) core and polyacrylamide shell were made from aqueous solutions via free radical polymerization in the absence of chemical cross-links. The obtained hydrogels possessed greater tensile strength and elongation ratio when compared with chemically cross-linked counterparts. Oscillatory shear experiments indicated that CNCs interacted with polymer matrix via both chemical and physical interactions and contributed to the rubbery elasticity of the hydrogels. The nanocomposite hydrogels were more viscous than the chemical hydrogels, suggesting the addition of CNC led to the increase of energy dissipating and viscoelastic properties. The network structure model was proposed and it suggested that the high extensibilities and fracture stresses were related to the well-defined network structures with low cross-linking density and lack of noncovalent interactions among polymer chains, which may promote the rearrangements of network structure at high deformations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are soft, wet and cross-linked three dimensional networks of hydrophilic polymeric materials that are capable of absorbing large amounts of water (Vermonden et al. 2012; Samchenko et al. 2011; Hu et al. 2012). They are highly swollen in aqueous media and the controlled changes in volume, mechanical or optical properties can be achieved by the tailored design of material structures and compositions (Yoon et al. 2011; Satarkar et al. 2010). Hydrogels have been studied for a wide range of biomedical, pharmaceutical and daily-care applications, such as contact lenses, tissue engineering, drug delivery, and superabsorbent agents (Vermonden et al. 2012). Due to the presence of solvent molecules during the gel formation process, most of polymeric hydrogels are brittle and prone to fracture when handled at swollen state, including low strain to break and poor extensibility (Cui et al. 2012; Isobe et al. 2011). Thus, the design of highly mechanical strength of hydrogels is critical to their performance where toughness is required. Recently, some progresses have resulted in these wet polymeric materials for creating highly stretchable hydrogels from different strategies, such as double-network gels (Gong et al. 2003), macromolecular microsphere composite gels (Huang et al. 2007), clay nanocomposite gels (Haraguchi and Takehisa 2002), polyrotaxane gels (Okumura and Ito 2001), and tetra-poly(ethylene glycol) gels (Akagi et al. 2011). Among them, nanocomposite gels have attracted many interests due to the simplicity of synthesis, multi-functionality of nanoparticles, and reversibility of self assembly (Simhadri et al. 2010; Wu et al. 2012).
Performance improvement by forming composite structures is not a new concept, while the capability of controlling the sizes, geometrical dimensions, and environmental response behavior of the composites is still challenging (Das et al. 2012; Zhang et al. 2012). In this scenario, there has been increasing precedence in literature where the gelator structure is not only predicted but also judiciously loaded with some specific moiety to impart desired functionality to the gel nanocomposites. With respect to the nanoparticles (NPs) embedded hydrophilic polymer matrix, the increasing control over surface characteristics of NP is especially important for the development of nanocomposite gels (Abbott and Bismarck 2010; Yang et al. 2012a). For example, some specific molecules are anchored on the surface of NP, and the presence of suitable functional groups in the gelator structures facilitates the subsequent in situ synthesis of the nanocomposite via intermolecular interactions (Zhang et al. 2012). This fact significantly simplifies the formulation of soft nanocomposites and eliminates the use of any hazardous exogenous reducing agents or harsh reaction conditions (Das et al. 2012). Thus, many successful technologies have been exploited to create an extensive variety of supermolecular architectures and offer desired physicochemical properties (Hu et al. 2010; Yang et al. 2012b).
Cellulose regarded as the most abundant polymer in nature and one of the oldest raw materials of chemical industry, has been attracting wide interests as advanced material due to its biocompatibility, renewability and multi-functionality (Moon et al. 2011). Cellulose nanocrystals (CNCs) are rod-like nanoparticles (average length 100–400 nm and diameter 10–25 nm depending on the cellulose source) and could be obtained by acid hydrolysis of cellulose (Siró and Plackett 2010; Nishio 2006). As derivatives from cellulose, CNCs offer many advantages such as high tensile strength (7.5–7.7 GPa), elastic modulus (110–220 and 10–50 GPa in axial direction and transverse direction, respectively), and high reactivity (esterification, etherification, oxidation, silylation, polymer grafting; Habibi et al. 2010). In this paper, a simple and versatile platform capable of synthesizing robust hydrogels is pursued that yields nanocomposite hydrogels with tunable mechanical properties, including tensile strength, elongation ratio at break, and Young’s modulus. The formulated networks herein are based on cellulose nanocrystals and acrylamide (AM) as starting materials, and the free radical polymerization occurs in an aqueous solution with propagating polyacrylamide (PAM) macroradical on the surface of modified CNCs. The resulting nanocomposite hydrogels possess excellent elastomeric properties that allow extensive stretching up to 1,000 %. The effects of CNC on the structure and mechanical properties of the nanocomposite hydrogels are evaluated and the results indicate that the hydrogels have great tensile properties with high elongation ratios and are more viscoelastic than the chemically cross-linked polymer counterparts. Overall, this study leads to a significant impact on the design and preparation of high-performance wet materials using the same synthetic platform, which further deepens the fundamental understanding of how hydrogels can be strengthened by cellulose in the absence of chemical cross-links.
Experimental
Materials
Pulp fibers were obtained from DongHua Pulp Factory, China. Acrylamide (AM, Sigma) was purified by recrystallization in a mixture of n-hexane/toluene (4:1, v/v) and dried under vacuum at 45 °C. Initiator potassium persulfate (KPS, Sigma) was recrystallized from water and dried under vacuum at 25 °C. The N,N′-methylene-bisacrylamide (BIS, Sigma) was used as chemical cross-link to prepared conventional hydrogels. The γ-methacryloxypropyl trimethoxy silane (A-174, Sigma) was used as silane coupling agent without further purification. Deionized water was used for all experiments and oxygen present in the water was removed by bubbling nitrogen gas for 30 min prior to use.
Preparation of cellulose nanocrystals
The fibers (5 g) were cut into small pieces and treated with a sodium hydroxide solution (4.5 %, w/v, 200 mL) at 80 °C for 2 h under mechanical stirring (200 rpm) to remove any residual noncellulosic contaminants, then washed and filtered with water until pH neutrality. The above purified fiber solution was subsequently hydrolyzed with a 60 % (w/w) sulfuric acid solution at 50 °C for 1.5 h under mechanical stirring (150 rpm), washed with water until neutrality. The obtained cellulose nanocrystal aqueous suspension was collected by centrifugation and stored at 4 °C as stock suspension (30 %, w/v).
Silane treatment of CNC
The CNCs in the aqueous suspension were solvent-exchanged with acetone and then with toluene by several successive centrifugation and redispersed operations. The sonication was performed after each solvent exchange step to avoid aggregation of the CNCs. The CNCs in toluene suspension (1.5 g in 100 mL) were introduced into a three-necked flask equipped with a mechanical stirring under nitrogen flow. The toluene distillation process was conducted to remove residual water in solvent before the reaction. The flask was then immersed into an oil bath and heated to 100 °C, then the required amount of silane agent A-174 (1.5 mL previously dissolved in 5 mL hot toluene) was added into the CNC suspension. The reaction was allowed to proceed for 6 h, filter and dried at 110 °C under vacuum for 2 h to ensure the formation of covalent bonds between CNC and A-174 (Abdelmouleh et al. 2002).
Preparation of CNCs based nanocomposite hydrogels
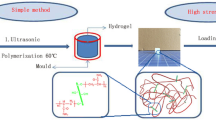
The CNCs based nanocomposite hydrogels were prepared by the free radical polymerization of AM in CNC suspensions. Typically, an aqueous solution of water (20 mL), CNC (0.3 mL, 10 %, w/v), and AM (6 g) was prepared, and the solvent was stirred vigorously for 1 h to produce a uniform suspension. Then the KPS (30 mg) was added and the solution was stirred for 15 min to dissolve the initiator, and the remaining bubbles were removed under vacuum. For the rheological experiments, a portion of this solution was transferred between the parallel plates of the rheometer. For swelling and mechanical measurements, and the remaining portion of solution was poured into a glass mold (10 mm height, 50 mm length, and 12 mm width). The free radical polymerization was allowed to proceed at 25 °C under nitrogen atmosphere over night. The specific compositions for nanocomposite hydrogels were shown in Table 1. Whereas the chemically cross-linked PAM gels for comparison to the nanocomposite hydrogels were prepared by the same procedures mentioned above, except an organic cross-links (BIS) was used instead of CNCs. The as-prepared hydrogels were purified by immersing into excessive water for 72 h with daily replacement of the water to remove any water-soluble fractions. For mechanical measurements, the purified hydrogels were dried under mild condition until their water content was equal to the initial gel compositions. The dried gels were prepared by drying purified hydrogels at 70 °C in an air-circulation oven for 48 h and subsequently further drying at a vacuum oven at 50 °C for 48 h (Fig. 1).
a Synthetic route for the CNC–PAM nanocomposite hydrogels with core/shell structures. The exposing vinyl groups on the surface of CNC act as initiator to anchor polymer chains. b Photographs of 0.5 % CNC hydrogels with the corresponding schematic illustrating the PAM chains (green curves) and CNCs (blue rod) that constitute the networks. (Color figure online)
Characterization
Swelling kinetics
The swelling behaviors of hydrogels were measured by immersing cylindrical samples (5 mm height × 15 mm diameter) into excessive water at 25 °C. The swollen samples were removed from water and weighted before blotting off the remaining water on the sample surface with a filter paper. The swelling ratio was defined as the weight ratio of the net liquid uptake to the dried hydrogel.
Rheological experiments
Gelation reactions were measured using a Physica MCR301 (Antor Paar Rheometer) equipped with a Peltier device for temperature control. The experiments were performed using a plate–plate geometry (diameter 25 mm) under N2 atmosphere at 25 °C, and the upper plate was set at a distance of 1 mm before the onset of the reactions. The silicon oil was applied on the edge of the plates to prevent water evaporation. The values of storage modulus (G′) and loss modulus (G″) were recorded at a frequency of 1 Hz with a strain of 0.01 (within the linear viscoelastic region which was determined by dynamic strain sweep experiments).
Mechanical test
The hydrogels tensile tests were performed at room temperature using a Zwick 005 (Germany). The tested samples had a uniform shape: 5 mm height × 40 mm length × 8 mm width. The crosshead speed was 30 mm/min and the gauge length was 20 mm. The initial cross section of 40 mm2 was used for calculating the tensile stress and the Young’s moduli were evaluated at the slope of between 20 and 120 % strain in the stress–strain curve. The tensile hysteresis was measured under the same conditions by the strain was limited to below 250 %.
Transmission electron microscopy (TEM)
The TEM observation of nanocomposite hydrogels was performed using a JEM-1010 (JEOL) at an acceleration voltage of 80 kV. The dilute suspension of the sample was firstly sonicated (100 W) for 15 min in an ice-water bath, then several drops of sonicated suspension were deposited onto a standard holey carbon-coated copper grid. The grid was then stained with uranyl acetate solution (2 wt%) for 30 s to enhance the microscopic resolution.
Results and discussion
The development of robust nanocomposite hydrogels for biomedical and industrial applications requires the control of the features and modes of interactions between nanoparticles and polymer matrix (Simhadri et al. 2010). By combining the polymer characteristics of PAM hydrogels with those rod-like CNCs, the elastomeric hydrogels can be prepared with excellent mechanical properties. They can endure high degrees of knotting, stretching without rapture, indicating the CNC based hydrogels possess high damage-tolerant ability and efficient energy dissipation mechanism. The elastomeric nature of the nanocomposite hydrogels is partially related to the long and flexible PAM chains that grafted on the surface of CNC via covalent bonds, but also to the unique core/shell network structures where the CNCs homogeneously disperse within polymer matrix and act as multifunctional cross-links. The specific evaluations of these properties which are critical to their performance as possible tissue engineering scaffolds would be discussed in the following sections.
Synthesis and characterization of nanocomposite hydrogels
The elastomeric and tough nanocomposite hydrogels were prepared by in situ free radical polymerization, involving only the mixing of all of the components before initiating network formation. By simply varying the content of CNC, the mechanical properties of the hydrogels were tunable, while maintaining high flexibility. Compared to other reported CNC based hydrogels (Zhou et al. 2011; Spagnol et al. 2012; Fajardo et al. 2012), there are two distinct differences comprised in this work: first of all there is no conventional chemical cross-link, which is generally applied to bind the polymer chains to each other covalently. The chemical cross-links (like bis-acrylamide) create a network which facilitate the absorption of solvent molecules but also pronouncedly quench the micro-Brownian motion along backbones and restrain the possible topological state of the chain molecules (Joly et al. 2002). The CNCs acting as multifunctional cross-links are able to induce PAM chains anchor to different CNCs as well as the same CNC, which accommodate the stability of network structures. Secondly, the polymerization reaction is performed with a higher monomer-to-CNC surface initiator ratio, which provides a much denser shell around the CNC-core than usually grown. Thus, the core component has a higher polymer density toward the interior and a more loosely interpenetrated cross-linked chains toward the periphery.
The Fig. 2 exhibited the TEM image of hydrogels showing morphologies of microstructures, one can note that the CNC–PAM hydrogels contained core–shell interconnected microscopic structures and led to porous structure with pore size in range of 50–200 nm. This observation verified that the uniform dispersion of CNC within the polymer matrix was achieved without any pronounced aggregation, even in a concentrated state. Thus it was concluded that the CNC–PAM core/shell structures were dispersed homogenously within the cross-linked networks, which definitely contributed to the excellent mechanical properties.
Swelling properties of CNC–PAM hydrogels
The swelling measurements of nanocomposite hydrogels were performed in water and NaCl aqueous solution. The hydrogels exhibited bulk swelling without any apparent dissolution or mass loss in the absence of chemical cross-links, suggesting the CNC act as cross-links within the polymer matrix (Fig. 3a). The swelling ratio of the hydrogels as a function of CNC content was exhibited in Fig. 3b. As the dried nanocompsite hydrogels were immersed into an aqueous media, such as water or NaCl solution, water molecules would enter the network where the swelling process occurred and the sample size increased. The equilibrium swelling ratio of the hydrogels strongly depended on the CNC content, and a decrease in the swelling ratio appeared with increasing content of CNC. This result was related to the CNC interaction with the polymer as well as role of filler that occupied void space within the polymer network, which in return led to a more compact gel and hindered the swelling process. To further demonstrate the effect of osmotic pressure on the hydrogels, the swelling measurements in aqueous NaCl solutions were performed as a function of NaCl concentration (Fig. 3c). It was noted that the swelling ratio decreased with increasing salt concentration in the external solution due to the decrease in the difference of counterions concentration between inside and outside of networks, which was usually observed in other hydrogels (Li et al. 2009; Nykänen et al. 2011).
Elastomeric and tough nanocomposite hydrogels
The mechanical properties of CNC–PAM nanocomposite hydrogels were evaluated by uniaxial tensile measurements (Fig. 4a) and the typical stress–strain curves were shown in Fig. 4b. It was impressive to find that the hydrogels with 0.5–3 wt% of CNC would lead to a 5–12-fold and 5–7-fold increase in toughness and in elongation ratio, respectively, when compared with the chemically cross-linked counterparts. In our CNC–PAM hydrogels, both covalent bonds and physical interactions between CNC and PAM chains affected the tensile strength, and this unique enhancement mechanism in mechanical behaviors would be related to the ability of CNC to change energy dissipation process. Besides, the addition of a small fraction of CNC led to a significant increase in Young’s modulus. The summary of mechanical properties of a series of hydrogels, including Young’s modulus, fracture strain, and elongation ratio at break, with different CNC contents were demonstrated in Fig. 4c. It was noted that the tensile strength and modulus increased pronouncedly as the content of CNC increased from 0.5 to 3 wt%, and this result was attributed to the presence of rigid CNC within the polymer matrix and role of cross-links. In contrast to the toughness of CNC–PAM hydrogels, PAM hydrogels that tested as controls were more brittle with elongation at break and tensile strength being less than 150 % and 18 KPa, respectively.
Mechanical properties of CNC–PAM nanocomposite hydrogels. a The rubbery-like CNC–PAM nanocomposite hydrogels exhibited excellent flexibility. b Typical stress–strain curves of hydrogels. The incorporation of CNC significantly reinforced the network and increased the tensile strength and modulus of hydrogels. c Summary of hydrogels mechanical parameters. The addition of CNC to polymer matrix significantly enhanced tensile strength and modulus without sacrifice much elongation ratio. d Loading and unloading cycle of hydrogels. They were highly elastic and recovered their original shape after the remove of stress (strain less than 250 %). e Energy lost during the cycle plotted against CNC content. The hydrogels had negligible hysteresis at low CNC contents. With increasing in CNC content from 0.5 to 3 wt%, there showed a nearly 12-fold increase in energy lost
Under the cyclic tensile measurements, an elastic network is fully recoverable to its original dimension, and a viscoelastic network may exhibit some certain of permanent deformation (Cui et al. 2012). The area between the loading and unloading curves was used to evaluate the energy lost during the deformation. The typical loading and unloading cycle of the hydrogel with 3 wt% CNC and the effect of CNC content on energy lost were plotted in Fig. 4d, e. There had not apparent hysteresis during consecutive loading and unloading cycles at low deformations, indicating that there was no permanent damage to the network and the hydrogels kept almost elastic. This result indicated that the addition of CNC to the polymer matrix induced viscoelastic properties to the network. Compared with other hydrogels where exhibited apparent hysteresis in the loading cycle and showed poor resilience, suggesting some permanent damage at strains due to the existence of covalent bonds in the randomly cross-linked network (Gaharwar et al. 2011; Webber et al. 2007). Whereas with an increase of CNC content from 0.5 to 3 wt%, it led to a pronounced increase in energy lost during the cycle. Wang et al. also reported a similar dependency of the elongation hysteresis where the hydrogels cannot recover their size simultaneously after the stress released at a high Laponite content (Wang et al. 2012).
Rheological measurements and viscoelastic properties
After formation of stable CNC–AM suspension, the initiator was added into the solution and the polymerization was started at 25 °C. The polymerization reactions of AM in the CNC aqueous suspension and the formation of nanocomposite hydrogels were monitored by rheometry. The elastic moduli (G′) and viscous moduli (G″) monitored at frequency of 1 Hz as a function of the reaction time were shown in Fig. 5a. It can be noted that both moduli increased rapidly in the initial 50 min and then approached plateau values after 200 min. The modulus rapid increase corresponds to the region of sol–gel transition and the gel growth, whereas the plateau regime corresponds to the post cross-linking reaction (Carlsson et al. 2010).
The gelation process is viewed to be complete when G′ and G″ approach a plateau value with time, and the estimated equilibrium modulus of the gels, \(G_{{\text{e}}}^{\prime } \), and loss factor tan δ (tan δ = G″/G′) at the reaction of 4 h are shown in Fig. 5b, c that plotted against the CNC content. Several interesting features can be seen from Fig. 5b, c. Firstly, the increase of CNC content from 0.2 to 3 wt% leads to a 2-order of magnitude increase in elastic modulus of the hydrogel. This pronounced increase in \(G_{{\text{e}}}^{\prime } \) with CNC content indicates that there exist strong interactions between PAM and CNC where the polymer chains are anchored on the surface of CNC and their free motion is restricted. Thus, the cross-linked CNC-PAM hydrogels with a Young’s modulus of 21.5 KPa is possible by using CNCs as multi-functional cross-links. Secondly, as mentioned above, the value of tan δ represents the ratio of dissipated energy to stored energy, the hydrogels show a gel-like behavior even at a low CNC content of 0.2 wt%. With increasing of CNC content, tan δ decreases and approaches 0.01 at high CNC contents (>3 wt%). Due to tan δ also represents the ratio of CNC–PAM chains adhesive bonds being broken down and reformed during dynamic strains to those remaining intact and unchanged, it suggests there has an efficient energy dissipation mechanism within the hydrogel networks (Wu et al. 2011).
At the beginning of reaction, the monomers were bound to the surface of CNC due to the hydrogen bonds, and the initiators were also linked to the surface of CNC by ionic interaction [CNC yielded a charged surface after acid hydrolysis (Habibi et al. 2010)]. After decomposition of the initiator and formation of primary macroradicals, the PAM chains began to grow on the surface of the CNC until they terminated by recombination with other radicals. The formed polymer chains led to the cross-linked network structures and bridged the neighboring CNCs as integrity. That is, the pendant vinyl groups with growing radicals reacted with monomers and led to the formation of CNC–PAM clusters, as basic unit of macroscopic network structures. One can speculate that if energetically favorable interactions exist between neighboring CNC–PAM particles, then the polymer chains can be bridged between the adjacent particles and further increase the cross-linking density, which lead to a stable network structure with a repeat distance of a few hundred nanometers (Joly et al. 2002). As seen in Fig. 4b, the elastic modulus of hydrogels is highly depended on the CNC content and hydrogels exhibit efficient energy dissipation process. This phenomenon is the result of orientation of the CNCs and relaxation of the grafted chains between different CNC–PAM clusters during the deformation process, where the reversible movement of polymer chains and cluster conformational rearrangements may be responsible for the rubbery elasticity and high toughness of the nanocomposite hydrogels.
CNC–PAM interactions and elastomeric network
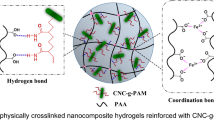
One can note from the results of strain–stress curves in Fig. 4b, the conventional PAM hydrogels can only be reversibly deformed within the constraints of the covalently cross-linked polymer junctions, and the higher extensions would lead to the fracture of covalent bonds. As a contrast, the CNC–PAM nanocomposite hydrogels exhibit mechanically robust behaviors, which are attributed to the combination of covalent bonds between CNCs and polymer chains via silane bridges (lead to elastic properties) and polymer chains relaxation and nanoparticle physical interactions (contribute to the viscoelasticity). Although the crystal underlying reasons for the extraordinary strength of these synthetic nanocomposite hydrogels are complicated (including polymer–polymer, hydrogen bonding, polymer-cellulose interactions) and will require further investigation, some answers could be found in the proposed network structures (Fig. 6). The current design strategy is based on the fundamental network elements of cellulose–polymer chain interactions that result in unique elastic properties. When dispersed into the host matrix at the nanoscale level (producing a very large polymer/filler interface), the CNC may exhibit pronounced enhancements in various properties even at a very low clay content (>0.5 wt%). In fact, the interactions between the nanoparticles and polymer chains always lead to unique mechanical properties as observed in a variety of hydrogel systems (Wu et al. 2011). The high concentration of AM in excess of what is required to encapsulate the surface of CNC would lead to a stable network if the polymer chains are long enough to bridge neighboring CNCs, even without chemical cross-links. As the reaction proceeds to a high conversion ratio, the initial isolated CNC–PAM clusters transfer to a continuous phase and interpenetrate into a cross-linked network. For the nanocomposite hydrogels in this study, although they do not possess a permanent network structure, they do exhibit elasticity if the relaxation time of the network is much longer than the application time of stress. Then, a physical gel may be indistinguishable from a covalent gel from the mechanical behaviors and appears to be permanent (Hao and Weiss 2011).
Schematic illustration of structural changes during network deformation in CNC–PAM hydrogels. Covalent bonds between CNC and PAM via silane bridges induce the formation of an elastic network, whereas polymer chains dynamic relaxation and CNC orientation process leads to viscoelastic properties. Before deformation, the PAM chains are attached to the surface of CNC preferentially via covalent bonds and hydrogen bonds. During deformation, the initial coiled chains present stretched and unfolded conformations, which are related to the unique energy release process
During the free radical polymerization, the reactive double bonding groups on the surface of CNC form highly branched polymer networks, which lead to CNCs act as multifunctional cross-links and bridge the adjacent CNCs. Under low deformations, the nanocomposite network can be viewed as near elastic one and this rubber elasticity originates from the entropy change of the polymer chains cross-linked by CNCs, whereas at high deformations, the network interconnects the physically entangled flexible chains and the viscoelastic properties exhibit. Except for the covalent bonds between CNC and PAM, there may also exist noncovalent interactions between CNC and PAM, which are ascribed to hydrogen bonds between amide side groups (–CONH) of PAM and the surface C–OH groups of the CNC. The factors that both hydrogen bonds rupture and reveal hidden lengths of highly coiled polymer chains contribute to the energy dissipative process. That is, the cross-linked polymer chains adopt a randomly coiled conformation at free-standing state, whereas they slide against each other during the dynamic deformation stage. Thus, the presence of covalent bonds and noncovalent interactions within networks jointly promote the enhancement of flexibility and toughness of the nanocomposite hydrogels. Besides, the dynamic polymer chains relaxation process within network is sensitive to applied stress, a partial of the energy accumulated in hydrogels would be efficiently released by this micro-Brownian motion. Thus, the above energy release mechanism during hydrogel deformation contributes to the excellent mechanical properties, especially when compare to the covalently cross-linked counterparts.
Conclusions
The core/shell nanocomposite hydrogels with unique network structures have been successfully fabricated by combination of a simple free radical polymerization and CNC-polymer particles self assemble. The resulting hydrogels had greater flexibility and toughness compared to their chemically cross-linked counterparts. The increase of CNC content from 0.2 to 3 wt% led to a 2-order of magnitude increase of the hydrogels elastic modulus, indicating that CNC mainly determined the rubbery elasticity of the systems. The covalent bonds at the interface between polymer chains and CNCs resulted in the formation of an elastic network, whereas physical interactions among PAM chains conformational rearrangements led to viscoelastic properties. The combined elastic and viscoelastic features contributed to strong mechanical properties, and it was believed that the low density of cross-links, homogeneous network structure, and multi-functionality of CNC within the primary chains promoted the remarkable strength of hydrogels. These findings provide new perspectives for understanding the relations between network structures and mechanical properties, and create another facile way for preparation of high-performance hydrogels, such as artificial muscles, sensors, and other environmentally friendly materials.
References
Abbott A, Bismarck A (2010) Self-reinforced cellulose nanocomposites. Cellulose 17:779–791
Abdelmouleh M, Boufi S, Salah A, Belgacem MN, Gandini A (2002) Interaction of silane coupling agents with cellulose. Langmuir 18:3203–3208
Akagi Y, Katashima T, Katsumoto Y, Fujii K, Matsunaga T, Chung UI, Shibayama M, Sakai T (2011) Examination of the theories of rubber elasticity using an ideal polymer network. Macromolecules 44:5817–5821
Carlsson L, Rose S, Hourdet D, Marcellan A (2010) Nano-hybrid self-crosslinked PDMA/silica hydrogels. Soft Matter 6:3619–3631
Cui J, Lackey MA, Madkour AE, Saffer EM, Griffin DM, Bhatia SR, Crosby AJ, Tew GN (2012) Synthetically simple, highly resilient hydrogels. Biomacromolecules 13:584–588
Das D, Kar T, Das PK (2012) Gel-nanocomposites: materials with promising applications. Soft Matter 8:2348–2365
Fajardo AR, Radovanovic E, Rubira AF, Muniz EC, Spagnol C, Rodrigues FHA, Neto AGVC, Pereira AGB (2012) Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur Polym J 48:454–463
Gaharwar AK, Dammu SA, Canter JM, Wu CJ, Schmidt G (2011) Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 12:1641–1650
Gong JP, Katsuyama Y, Kurokawa T, Osada Y (2003) Double-network hydrogels with extremely high mechanical strength. Adv Mater 15:1155–1158
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hao JK, Weiss RA (2011) Viscoelastic and mechanical behavior of hydrophobically modified hydrogels. Macromolecules 44:9390–9398
Haraguchi K, Takehisa T (2002) Nanocomposite hydrogels: a unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv Mater 14:1120–1124
Hu J, Chen M, Wu LM (2010) Organic-inorganic nanocomposites synthesized via miniemulsion polymerization. Polym Chem 2:760–772
Hu JM, Zhang GQ, Liu SY (2012) Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev. doi:https://doi.org/10.1039/c2cs35103j
Huang T, Xu HG, Jiao KX, Zhu LP, Brown HR, Wang HL (2007) A novel hydrogel with high mechanical strength: a macromolecular microsphere composite hydrogel. Adv Mater 19:1622–1626
Isobe N, Sekine M, Kimura S, Wada M, Kuga S (2011) Anomalous reinforcing effects in cellulose gel-based polymeric nanocomposites. Cellulose 18:327–333
Joly S, Garnaud G, Ollitrault R, Bokobza L (2002) Organically modified layered silicates as reinforcing fillers for natural rubber. Chem Mater 14:4202–4208
Li H, Lai FK, Luo RM (2009) Analysis of responsive characteristics of ionic-strength-sensitive hydrogel with consideration of effect of equilibrium constant by a chemo-electro-mechanical model. Langmuir 25:13142–13150
Moon RJ, Martini A, Nairn J, Simonsenf J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Nishio Y (2006) Material functionalization of cellulose and related polysaccharides via diverse microcompositions. Adv Polym Sci 205:97–151
Nykänen VPS, Nykänen A, Puska MA, Silva GG, Ruokolainen J (2011) Dual-responsive and super absorbing thermally cross-linked hydrogel based on methacrylate substituted polyphosphazene. Soft Matter 7:4414–4424
Okumura Y, Ito K (2001) The polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv Mater 13:485–487
Samchenko Y, Ulberg Z, Korotych O (2011) Multipurpose smart hydrogel systems. Adv Colloid Interface 168:247–262
Satarkar NS, Biswal D, Hilt JZ (2010) Hydrogel nanocomposites: a review of applications as remote controlled biomaterials. Soft Matter 6:2364–2371
Simhadri JJ, Stretz HA, Oyanader M, Arce PE (2010) Role of nanocomposite hydrogel morphology in the electrophoretic separation of biomolecules: a review. Ind Eng Chem Res 49:11866–11877
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Spagnol C, Rodrigues FHA, Pereira AGB, Fajardo AR, Rubira AF, Muniz EC (2012) Superabsorbent hydrogel nanocomposites based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 19:1225–1237
Vermonden T, Censi R, Hennink WE (2012) Hydrogels for protein delivery. Chem Rev 112:2853–2888
Wang T, Liu D, Lian CX, Zheng SD, Liu XX, Tong Z (2012) Large deformation behavior and effective network chain density of swollen poly(N-isopropylacrylamide)–laponite nanocomposite hydrogels. Soft Matter 8:774–783
Webber RE, Creton C, Brown HR, Gong JP (2007) Large strain hysteresis and mullins effect of tough double-network hydrogels. Macromolecules 40:2919–2927
Wu CL, Gaharwar AK, Chan BK, Schmidt G (2011) Mechanically tough iuronic F127/laponite nanocomposite hydrogels from covalently and physically cross-Linked networks. Macromolecules 44:8215–8224
Wu JJ, Zhao N, Zhang XL (2012) Cellulose/silver nanoparticles composite microspheres: eco-friendly synthesis and catalytic application. Cellulose 19:1239–1249
Yang J, Han CR, Duan JF, Ma MG, Zhang XM, Xu F, Sun RC, Xie XM (2012a) Studies on the properties and formation mechanism of flexible nanocomposite hydrogels from cellulose nanocrystals and poly(acrylic acid). J Mater Chem 22:22467–22480
Yang J, Gong C, Shi FK, Xie XM (2012b) High strength of physical hydrogels based on poly(acrylic acid)-g-poly(ethylene glycol) methyl ether: role of chain architecture on hydrogel properties. J Phys Chem B 116:12038–12047
Yoon J, Lee KJ, Lahann J (2011) Multifunctional polymer particles with distinct compartments. J Mater Chem 21:8502–8510
Zhang H, Liu Y, Yao D, Yang B (2012) Hybridization of inorganic nanoparticles and polymers to create regular and reversible self-assembly architectures. Chem Soc Rev. doi:https://doi.org/10.1039/c2cs35038f
Zhou CJ, Wu QL, Yue YY, Zhang QG (2011) Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. Colloid Interface Sci 353:116–123
Acknowledgments
This work was financially supported by Beijing Forestry University Young Scientist Fund (BLX2011010), Fundamental Research Funds for the Central Universities (124199, TD2011-10, YX2011-4, JD2011-2), National Natural Science Foundation of China (30901139).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Han, CR., Duan, JF. et al. Synthesis and characterization of mechanically flexible and tough cellulose nanocrystals–polyacrylamide nanocomposite hydrogels. Cellulose 20, 227–237 (2013). https://doi.org/10.1007/s10570-012-9841-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9841-y