Abstract
Superabsorbents hydrogel nanocomposites based on starch-g-poly(sodium acrylate) and cellulose nanowhiskers (CNWs) were synthesized. A set of experiments was performed to evaluate the influence of some factors such as NaAc/starch mass ratio, crosslinker, and nanowhiskers amount in the swelling capacity and swelling kinetics. Increasing the NaAc/starch mass ratio up to 7 leads to an increase in the water uptake at a maximum value, however, higher ratios decreased that value due to the increase of crosslinking points. Similarly, the incorporation of CNWs up to 10 wt% provided an improvement in the swelling due to the hydrophilic groups from cellobiose units. Further, the incorporation of CNWs diminishes the water uptake. Besides, the CNWs improved the mechanical properties. SEM images showed that CNWs increase the average porous size of composites. The composites presented good responsive behavior in relation to pH and salt presence allowing those materials suitable for many potential applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of novel materials composites filled with cellulose nanowhiskers (CNWs) is a well-established methodology capable of producing materials with excellent and desirable properties (Spagnol et al. 2012a, b). CNWs are formed when the cellulose is treated by an acid hydrolysis reaction (Beck-Candanedo et al. 2005). This reaction yields rod-shape structures with fibrous form, lateral dimensions ranging from 3–30 nm to 100–1,000 nm in length. These dimensions vary according to the cellulose source (Goetz et al. 2010; Sturcova et al. 2005). Cellulose, the most abundant natural polymer found in nature and considerable interest, has been recently focused on new material applications (Czaja et al. 2007). Plants, trees, bacteria and some animals (tunicates), via the condensation polymerization of glucose, produce cellulose. CNWs have been incorporated in a wide variety of matrices to improve strength properties (Siaueira et al. 2009; Pandey et al. 2010). Some works have reported that crosslinked composites filled with CNWs showed unique properties that were distinctly from the starting components and of special interest are the hydrogel (Barcus and Bjorkquist 1991; Goetz et al. 2009).
Hydrogels are three-dimensional networks of hydrophilic polymers that can swell to an equilibrium state that retains a significant amount of water and/or biological fluids. Among the class of hydrogels, the superabsorbent ones can exhibit fast swelling rate absorbing extraordinary amounts of water (Kuang et al. 2011; Omidian et al. 2005). These properties are observed because the hydrogel pores disposed in interconnected networks with diameters of several hundred microns create open channels that allow water movement inward the matrix for capillarity. A good strategy applied to produce superabsorbent materials relies on polysaccharides-based ones, which could increase their biocompatibility, biodegradability, water uptake capacity as well as decrease the toxicity broadening their potential of application (Salimi et al. 2010; Li et al. 2011).
Starch, which is a natural carbohydrate biopolymer, has been the subject of academic and industrial studies for many decades due to its low cost, biodegradability and versatility of use (Karlsson et al. 2007). In addition, the starch backbone can be grafted by several monomers to increase hydrophilicity, hydrophobicity, or polyelectrolyte nature of starch depending on the reagent and conditions used (Pereira et al. 2011; Sandhu and Singh 2007). In general, starch graft copolymers are synthesized by the free radical reaction with acrylic monomers. High viscosity, thermal stability, biodegradability, good film forming properties and water absorption capacity are some of the properties shown by the starch graft copolymers. Graft copolymers find various applications in industry as flocculants, wastewater treatment, and heavy metal ion removal, for sizing cotton, as mulch films, in oil drilling, as biodegradable polymers and in superabsorbents (Zhou et al. 2011; Saboktakin et al. 2009).
This work reports on the synthesis and characterization of CNWs from cotton cellulose and their application as filler in starch-g-poly(sodium acrylate) matrix to improve or even create new properties to this interesting material. The effects caused by the incorporation of CNWs into the hydrogel matrix on the water uptake capacity were evaluated. In addition, the effects of several experimental factors (such as starch/sodium acrylate mass ratio, crosslinker, and CNWs contents) and the swelling behavior at various pHs and effect of salt solution on water absorbency were tested. These data and the swelling kinetics parameters give lots of information for this promising material and allow its application in a satisfactory way.
Experimental
Materials
INPAL S.A. (Brazil) kindly supplied the cassava starch (Mw 5,400 kg mol−1). Sodium acrylate (NaAc) and the potassium persulfate (K2S2O8) were purchased from Sigma-Aldrich (USA). N,N-methylenebisacrylamide (MBA) was purchased from Pharmacia Biotech (USA). All reactants possess analytical grade and were used without further purification.
Cellulose nanowhiskers (CNWs)
Cotton fibers were purchased from Cocamar, a local agribusiness cooperative (Maringá, Paraná, Brazil). The fibers were firstly washed in a NaOH solution (2 wt%), under mechanic stirring for 1 h at room temperature, to remove some impurities (waxy, for instance). After this, the fibers were soaked in deionized water for 1 h at 80 °C and then oven dried for 5 h at 50 °C. The cellulose nanowhiskers were obtained through acid hydrolysis reaction using concentrate HCl at 45 °C for 1 h under vigorous magnetic stirring at cellulose/HCl ratio equal to 1/20 (grams/milliliters). After this step, the resulting solution was centrifuged at 10,000 rpm for 5 min and washed using deionized water up to pH 7. The resultant material was lyophilized (at −57 °C for 48 h). Then, CNWs were characterized by Fourier Transformed Infra-Red spectroscopy technique (FTIR), X-ray diffraction (XRD), and transmission electronic microscopy (TEM).
Synthesis of starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposites
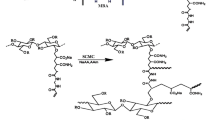
A set of hydrogels nanocomposites based on starch, sodium acrylate, and cellulose nanowhiskers was synthesized according to the following procedure. An appropriate amount of starch was solubilized in 30 mL of distilled water at 85 °C for 30 min using a 250 mL four-neck flask equipped with a reflux condenser, a funnel, and a N2(g) line. After, the temperature was cooled down to 60 °C, while 1 wt% of potassium persulfate was added to the system. After 15 min, the mixed solution of 7.2 g sodium acrylate (acrylic acid partially neutralized with 70 mol% NaOH solution), specific amounts of MBA (1–3 wt%) and CNWs (5–20 wt%) were added. The oil bath was heated slowly to 70 °C and kept at that temperature for 3 h to complete the polymerization reaction. In the whole process, the system was kept under mechanic stirring (1,000 rpm). The obtained material was washed several times with portions of distilled water to remove residual reactants. The hydrogels were oven-dried at 70 °C. The particles size distribution fall within 9–24 mesh range (2.00–0.71 mm). In addition, a blank sample, without CNWs, was synthesized according to procedures described above. This sample was labeled as starch-g-poly(sodium acrylate). A proposed mechanistic pathway for the synthesis of the starch-g-poly(sodium acrylate) is presented in Fig. 1. All the samples were characterized by the techniques described below.
Characterization techniques
FTIR spectroscopy
All the dried samples were characterized by infrared spectroscopy technique using a transform infrared spectrophotometer (Shimadzu Scientific Instruments, Model 8300), operating in the region from 4,000 to 500 cm−1, resolution of 4 cm−1. The dried material was blended with KBr powder and pressed into tablets before spectrum acquisition.
Wide angle X-ray scattering (WAXS)
The WAXS patterns were obtained in a diffractometer (DMAXB, Model Rigaku) equipped with a Cu-Kα radiation source (30 kV and 20 mA) in a scattering angle (2θ) from 5° to 70°, with resolution of 0.02°, at a scanning speed of 2° min−1.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
The hydrogel nanocomposites morphologies were evaluated through SEM images obtained from scanning electron microscope (Shimadzu, Model SS550 Superscan). Prior to analysis, the samples were immersed in distilled water at room temperature until the equilibrium swelling has been reached (approximately 24 h), frozen by immersion in liquid nitrogen and then lyophilized on a freeze dryer (Christ, Alpha 1–2 LD Plus) at −55 °C for 24 h. The dried-lyophilized hydrogels were gold-coated by sputtering before SEM visualization. The images were taken from fracture. TEM images of the as-obtained CNWs and starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposite were taken in a transmission electron microscopy TOPCON 002B. For the CNWs, a suspension in water containing the cellulose nanowhiskers was deposited on the grid (carbon-Formvar-coated copper) and left to dry at room temperature. For the starch-g-poly(sodium acrylate)/CNWs, a small droplet containing the hydrogel nanocomposite fraction was placed on the grid and the solvent was evaporated at room temperature. The images were taken at 200 kV acceleration voltage (point resolution 0.18 nm).
Swelling assays
Swelling assays were performed to evaluate the water uptake capacity of the starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels. For the assays, 15 mg of each sample were placed in 30 mL filter crucibles (porosity no. 0) pre-moistened and with a dry outer wall. This set was inserted in water in such a way that the gel was completely submerged. The crucible/composite hydrogel samples sets were removed at various time intervals, with the external wall of the set dried and the system weighed. For each sample 3 assays were performed (n = 3). The swelling capacity of the starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels was determined according to the Eq. (1):
where W is the gained water mass (in grams) per gram of hydrogel (absorbent), m is the mass of the swollen absorbent and m0 is the mass of the dry material (Cândido et al. 2012). It was possible to follow the kinetics of swelling in each studied medium. The size distribution of the hydrogel nanocomposites remained in the 9–24 mesh range.
Effect of salt solution on the water uptake capacity
The starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels were immersed in distinct salt aqueous solutions (concentration: 0.15 mol L−1) and their swelling capabilities were determined according to the procedures described previously. Aqueous solutions of NaCl, CaCl2, and AlCl3, at 25.0 °C, were used as swelling fluid. All solutions presented constant ionic strength (I = 0.1 M). All the procedures were done in triplicate (n = 3).
Effect of pH on the water uptake capacity
The effect of pH on the water uptake capacity was also evaluated using buffer solutions (pHs 2–12) with constant ionic strength (I = 0.1 M). The experimental procedures were the same describe in the previous subsections. All the procedures were done in triplicate (n = 3).
Mechanical properties
The mechanical properties of the starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposites, with different contents of CNWs, were determined through compressive tests in a Texturometer equipment (Stable Microsystems, Model TA.XT2). All samples were previously swelled in distilled water overnight; the superficial water excess was removed and then they were cut in small samples (10 × 10 × 10 mm) before the compression tests. The tests were performed at controlled temperature (25 °C) and relative humidity (50 %). The Young’s moduli were calculated according to the following equation:
where F is the force necessary to compress the sample (N), A is the area (m2) of the transversal section of the samples, L1 are the samples initial length (mm) and L2 is the samples length (mm) before the rupture point, respectively.
Results and discussion
FTIR and WAXS techniques
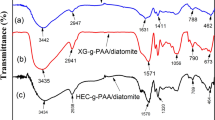
Figure 2 shows the FTIR spectra of the raw cotton fibers, CNWs, the synthesized starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels with 10 wt% of CNWs. Figure 2a, b shows the FTIR spectra of raw cotton fibers and CNWs, respectively, which are quite similar. Both presented bands at 3,344, 2,900, and 1,646 cm−1, which are assigned to stretching of –OH groups, C–H stretching, and –OH bending of the adsorbed water. Therefore, the bands of H–C–H and O–C–H in-plane bending vibrations appeared at 1,432 cm−1 and the C–H deformation vibration appeared at 1,367 cm−1. The C–O–C, C–C–O, and C–C–H deformation modes and stretching vibrations of the C5 and C6 atoms were observed at 898 cm−1, and the band referent to the C–OH out-of-plane bending at 670 cm−1 (Oh et al. 2005; Satyamurthy et al. 2011; Schwanninger et al. 2004; Sun et al. 2004). From these FTIR spectra, it was found that the acid hydrolysis reaction performed for obtaining the cellulose nanowhiskers did not affect the chemical structure of the cellulosic fragments, but rather their morphologies. The FTIR spectrum of starch (Fig. 2c) shows a broad band at 3,365 cm−1 assigned to –OH and bands at 1,158, 1,081, and 1,014 cm−1 assigned to C–O vibrational stretching. Although the FTIR spectra of starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels (Fig. 2d, e) presented the bands proceeding from pure starch they are weaker (Table 1).
The absorption bands at 1,647, 1,421 and 1,368 cm−1, attributed to C–OH bending from starch disappeared after the reaction with sodium acrylate. The above information indicates that the sodium acrylate has been grafted onto the cassava starch. Similar results have also been reported by Kiatkamjornwong et al. (2000) and by Zhang et al. (2006). The absorption bands at 1,723, 1,572 and 1,406 cm−1 (Fig. 2d) are assigned to –COOH stretching, COO− asymmetric stretching and COO− symmetric stretching, respectively. It confirms that a part of acrylic acid was neutralized by the NaOH solution. This phenomenon reveals that the grafting of sodium acrylate onto the starch backbone has some influence by the chemical environment around the −COOH and −COO− groups, and then on the water absorbency of the corresponding superabsorbent hydrogels. The FTIR spectrum of starch-g-poly(sodium acrylate)/CNWs (Fig. 2e) exhibited a similarity with the FTIR spectrum of starch-g-poly(sodium acrylate) as can been seen. Bands at 1,109 and 1,061 cm−1 are assigned to C–O–C asymmetric valence vibration, vibrational stretching C–C and C–O asymmetric pyran ring and C–O deformation in secondary alcohols and aliphatic ethers, regarding to cellulose nanofibrils, which confirms the hydrogel nanocomposite formation (Oh et al. 2005; Satyamurthy et al. 2011; Schwanninger et al. 2004; Sun et al. 2004).
Figure 3a, b shows diffraction profile of raw cotton fiber and CNWs, respectively. The diffraction patterns showed that both of them have cellulose nanocrystals within their structure and these nanocrystals present diffraction peaks at 2θ = 14.5°, 16.3°, 22.4°, and 34.1° that are characteristic of 101, 101′, 002 and 040 plane positions. These plane positions are typical of crystalline form from cellulose. The crystallinity index of cellulose nanowhiskers (Icr) was determined by the empirical method described by Segal et al. (1959), according to Eq. (3):
where Icr is the crystalline index in percentage, I002 is the maximum intensity of diffraction corresponding to 002 plan of cellulose crystals (reflection attributed to crystalline areas, detected at angle 2θ = 22.5°) and IAM is the diffraction intensity reported at angle 2θ = 18° (reflection attributed to amorphous areas).
The hydrolysis reaction, performed to obtain the cellulose nanowhiskers, increases the crystallinity index (Icr 90.3 %) of the fibers compared to cotton (Icr 80 %) due to the preference acidic attack in the amorphous regions in cellulose, which increases the level of the crystalline domains in obtained cellulose nanocrystals. The relative crystallinity index is about 90.3 %, which is in agreement with other literature values (Elazzouzi-Hafraoui et al. 2008).
The diffraction pattern of starch-g-poly(sodium acrylate) (Fig. 3c) did not exhibit diffraction peaks, which indicates the hydrogel presents a predominant amorphous structure. In spite of the addition of CNWs, a crystalline material, into the starch-g-poly(sodium acrylate) matrix, the amorphous diffraction pattern of starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposite (Fig. 3d) remained although being present the CNWs characteristic peak at 2θ = 22.8°. Therefore, it is suggestive that CNWs were incorporated during the gelling process confirming the formation of a nanocomposite and corroborating with the FTIR data.
SEM and TEM images
The changes on the starch-g-poly(sodium acrylate) morphology due to addition of CNWs into the hydrogel matrix were evaluated through SEM images (Fig. 4). Figure 4a shows the SEM image of the starch-g-poly(sodium acrylate) hydrogel and as can be seem its morphology is quite compact without porous with intercalated aspect. The incorporation of CNWs into the hydrogel matrix promoted the formation of a porous and irregular morphology. The pores are heterogeneous in size and distribution on the hydrogel composite network. The presence of CNWs into the hydrogel matrix can increase the amount of hydrophilic groups, which make the diffusion of liquids inward the hydrogel matrix easier and faster (Spagnol et al. 2012a, b). According to these SEM images is evidenced that the incorporation of CNWs into the starch-g-poly(sodium acrylate) hydrogel matrix changes its morphology as affects other properties.
In view of the data previously mentioned as well as the analysis of TEM images, the starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposite structure is sketched in Fig. 5. It is important to notice that the bars represent 100 nm and the TEM image at left is a TEM image of pure CNWs and at right a TEM image of the CNWs dispersed within the hydrogel matrix.
Evaluation of water uptake capacity
Effects of NaAc/starch mass ratio
The effect of NaAc/starch mass ratio on the water uptake capacity of starch-g-poly(sodium acrylate)/CNWs hydrogels nanocomposites, with 10 wt% CNWs, is shown in Fig. 6. In the synthesis of starch-g-poly(sodium acrylate)/CNWs hydrogels the amount of potassium persulfate was kept constant in all the experiments, for this reason is possible to infer that the number of free radicals generated by the decomposition of potassium persulfate did not vary in the reaction systems. Thus, the concentration of monomers may directly affect the reaction rate, grafting efficiency, and the amount of hydrophilic groups in polymer network (Wang et al. 2008, 2011 Wang and Wang 2010). It can be seen from Fig. 6 the water absorbency increased with increasing the amount of NaAc and the maximum water absorbency was achieved when the NaAc/starch mass ratio was equal to 7. When the NaAc/starch mass ratio changes from 3 to 7, both grafting efficiency and the molecular weight of the grafted poly(sodium acrylate) chain increased, which promoted an enhancement of the water uptake of the superabsorbent nanocomposite. As a result, the hydrophilicity of the superabsorbent material was improved due to increase of the polymerization of NaAc monomers. Moreover, the osmotic pressure is directly affected by the neutralization of acid groups from NaAc and the repulsive interaction among the negatively charged groups increased systematically with the increasing of the amount of NaAc, thus the water uptake is enhanced (Tokuyama et al. 2007). However, a high amount of NaAc in the reaction system will lead to a decrease in water absorbency. A higher monomer concentration, in this case NaAc, will increase the number of free radicals generated during the chain transfer process, resulting in an increasing of crosslinking density, which directly leads to a decrease in water absorbency (Spagnol et al. 2012a, b).
Effects of crosslinker content
The starch-g-poly(sodium acrylate)/CNWs hydrogels nanocomposites were synthesized using different concentrations of N,N-methylenebisacrylamide (MBA), which presents an important role in the swelling characteristics of the hydrogel (Hazer et al. 2008; Cuggino et al. 2008). Crosslinkers are indispensable to form a superabsorbent hydrogel in order to prevent dissolution of the hydrophilic polymer chains in aqueous solution. According to Flory’s network theory (Flory 1953), the increase of MBA concentration may cause the generation of more crosslinking points during radical polymerization and the increase of crosslinking density of polymer network, so the water absorption of the hydrogel was decreased with increasing the concentration of crosslinker (Dai and Kadla 2009). The MBA concentration range, handled in this work, was from 1.0 to 3.0 wt%. The dependence of the amount of absorbed water at the equilibrium (Weq) over to the MBA concentration is shown in Fig. 7. All the other parameters in these series of reactions were held constant. It is obvious that water absorbency, in distilled water, decreased with increasing of crosslinker content from 481 ± 14 to 245 ± 11 gwater/gabsorbent. Polymeric networks formed with high content of MBA are strongly crosslinked, this fact reflects a smaller expansion of matrix, and consequently smaller volume of water diffuses inward the matrix (Flory 1953).
Effect of content of CNWs
Figure 8 shows the dependence between the content of CNWs filled into the starch-g-poly(sodium acrylate) hydrogel matrix and Weq. According to results, when 10 wt% of CNWs was filling the hydrogel matrix the water uptake capacity achieved the highest value. This behavior can be attributed to the presence of hydroxyl groups form cellobiose, which provides greater hydrophilic character. The increasing in the interactions of the hydrogel surface with the water molecules allows a higher absorption capacity. On the other hand, as can been seen in Fig. 8 the water uptake capacity decreased when the content of CNWs was over than 10 wt%. The excess of CNWs into the hydrogel network avoids that a great amount of water could be held. In this stage the CNWs shows a typical behavior of inert filler (Dai and Kadla 2009).
The surface of CNWs contains large amounts of active –OH groups, which can take part during the polymerization reaction as well as the construction of 3D polymer network. As a result, the intertwining of polymeric chains was prevented and the hydrogen-bonding interactions among the hydrophilic groups, such as –COOH, –COO− and –OH, among others, were weakened. Thus, the degree of physical crosslinking decreased and the network sites for holding water molecules were well formed, fact that is extremely favorable to the improvement of water absorption. However, greater amounts of CNWs enhanced the crosslinking density of nanocomposite and minimized the network voids, responsible for absorbing and holding water molecules. In addition, the excessive CNWs particles may physically stack (inert filler) inside the network voids. Besides, some voids were plugged up and the water-holding capability decreased. In addition, with excess of CNWs the ratio of hydrophilic groups per volume unit decreased leading to nanocomposite with lower hydrophilic character.
Effect of cellulose nanowhiskers on the swelling kinetics
It is well known that the swelling kinetics of a superabsorbent material is significantly influenced by various factors such as composition of the superabsorbent, particle size, and surface area (Elazzouzi-Hafraoui et al. 2008). Thus, the incorporation of CNWs into the hydrogel matrix certainly affects its swelling kinetics. Figure 9 shows the swelling kinetics in aqueous media for the hydrogel nanocomposites for the blank sample. The profiles of swelling kinetic for both hydrogels are quite similar. The degree of swelling increased quickly during the first 30 min after the immersion, reaching ca. 90 % of the equilibrium value in this time range. Then, the swelling degree increased slowly until the equilibrium was reached (Weq) around 60 min, depending on the hydrogel. The incorporation of CNWs within the starch-g-poly(sodium acrylate) network improved the water uptake capacity at equilibrium and decreased the necessary time to reach the equilibrium condition. The starch-g-poly(sodium acrylate) hydrogel presented water uptake capacity at equilibrium (Weq) ca. 305 gwater/gabsorbent while starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposite presented higher water absorption capability (Weq = 481 gwater/gabsrobent, respectively). This statement corroborates with our previous works (Spagnol et al. 2012a, b), where different hydrogel matrixes were filled with CNWs and the resultant composites showed a higher water absorption capability than the hydrogels. In addition, Dai and Kadla (2009) observed a similar profile, where hydrogel nanocomposites with cellulose nanocristals had similar or improved swelling properties that the hydrogels without filler.
Some of the characteristics collected from the swelling curves for evaluating the mechanism of the swelling phenomenon and the incorporation of CNWs on the swelling kinetics of hydrogels, the Schott’s pseudo second order swelling kinetics model (Schott 1992) was adopted to fit the experimental data. The pseudo second order swelling kinetic model can be expressed through the relations proposed below:
where:
and
The A parameter corresponds to an initial swelling rate [(dW/dt)0] of the hydrogel, ks is the constant rate for swelling, Wt is a theoretical swelling value at equilibrium and the initial swelling rate (kis = ksW 2eq ). Wt and ks were calculated by fitting experimental data shown in Fig. 9a, b to Eqs. (4), (5), and (6). Based on the experimental data, the plot of t/W versus t for the starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogels give perfect straight lines with good linear correlation coefficient (R2 > 0.99), indicating that the swelling process of the superabsorbents follows the Schott swelling kinetic model.
Swelling behavior at various pH and effect of salt solution on water absorbency
The swelling behavior of starch-g-poly(sodium acrylate) and starch-g-poly(sodium acrylate)/CNWs hydrogel nanocomposites at various pHs were observed with the use of buffer solutions at pHs 2–12, maintaining the ionic strength equal to 0.1. According to Fig. 10a, the absorbency of the optimized hydrogel increased as the pH increased from 2.0 to 8.0 and then decreased at pH higher than 8.0. The water absorption is low at acidic pH values (≤4), but dramatically increases until a plateau was reached when the pH > 4. As an anionic polymer, the –COO− and –COOH functional groups in its structure can convert to each other. In acidic medium, –COO− converted to –COOH groups. The hydrogen-bonding interaction among –OH and –COOH groups was strengthened and the degree of physical crosslinking was increased. As a result, the water absorption was reduced. As pH increased to the basic conditions, numerous –COOH groups transform into –COO− groups and the hydrogen-bonding interaction was broken. In addition, the electrostatic repulsion among polymer chains was increased due to the rapid increase in the number of negatively charged –COO− groups. This effect causes to the hydrogel matrix an expansion and the amount of water absorbed increases considerably as can be seen by the increase in the swelling curves (Karadag et al. 2005). The set of results published previously by Spagnol et al. (2012a, b) allows us to infer that despite of the incorporation of CNWs to changed the water uptake capacity, the responsible by the pH-sensitive characteristic is attributed only the hydrogel matrix, as observed here.
In this work, the influence of some ions (cations and anions) on swelling capability of hydrogels was tested by the addition of different saline solution, including monovalent (NaCl), divalent (CaCl2) and trivalent (AlCl3) ions, at 0.15 mol L−1 and at 25.0 °C, as the swelling fluid. To achieve a comparative measure of salt sensitivity of the hydrogels, a dimensionless salt sensitivity factor (f) (El-Hamshary 2007; Zohuriaan-Mehr and Pourjavadi 2003) is defined as follow:
where Wsaline and Wwater are the swelling capacity in saline solution and in deionized water, respectively. It is obvious that the swelling decreasing is strongly depended on the “type” of salt added to the swelling medium. The effect of cation type (cations with different radius and charge) and sensitivity factor (f) on swelling behavior is shown in Fig. 11. The higher cation charges the higher degree of crosslinking and the smaller swelling value. Therefore, the absorbency for the hydrogel in the studied salt solutions is in the order of monovalent > divalent > trivalent cations.
The f values (Fig. 11) indicate that the starch-g-poly(sodium acrylate)/CNWs hydrogels undergo less influence to the presence of salt than the starch-g-poly(sodium acrylate). The increase in the ionic strength reduces the difference in the concentration of movable ions between the polymer matrix and the external solution (osmotic swelling pressure) and leads to an immediate contraction of gel. The decreasing is more significant to Ca2+ and Al3+ ions, which can be additionally caused by the complex formation ability of carboxamide or carboxylate groups including intramolecular and intermolecular complex formations, or because one multivalent ion is able to neutralize several charges inside the gel. Consequently, the crosslinking density of the network increases while water absorption capacity decreases.
Mechanical Properties
Figure 12 shows the mechanical properties of the starch-g-poly(sodium acrylate) hydrogel nanocomposites as function of the content of CNWs incorporated into the hydrogel matrix. As can be seen the incorporation of CNWs into the hydrogel matrix decrease considerably the Young’s modulus. This happens because the CNWs contribute to increase the water uptake capacity (see Fig. 8), which consequently became the hydrogel softer than the hydrogel without CNWs. On the other hand, the increase in the content of CNWs into the hydrogel matrix increases the Young’s modulus; the material acquires hardness again. The CNWs acts as efficient filler and contribute to improve the mechanical resistance against the compressive stress. This behavior corroborates with the swelling profile of the starch-g-poly(sodium acrylate)/CNWs. The high content of CNWs avoids that large amounts of water be absorbed into the hydrogel matrix, which contributes to keep the rigidity of the material. The hydrogel nanocomposite with 20 wt% of CNWs showed the highest Young’s modulus value ca. 40 kPa and is worthy to notice that even with increasing of rigidity the hydrogel is still of ease handling. That results corroborate with Dai and Kadla (2009) that demonstrated that high amount of CNWs favors the formation of a more rigid polymer matrix. The evaluation of the mechanical properties, swelling capacity and SEM images allows inferring that the incorporation of CNWs has a clear influence on these properties and contributes to change the original properties of starch-g-poly(sodium acrylate) hydrogel. These results and discussions strength the description of the CNWs applied as filler in different hydrogels matrixes.
Conclusion
Cellulose nanowhiskers (CNWs) were obtained from cotton fibers presenting crystallinity index of 90 %. Hydrogel nanocomposites based on starch-g-poly(sodium) filled with CNWs were successfully synthesized as confirmed by FTIR and XDR data. The evaluation of some factors on the swelling capacity of the hydrogel showed that the crosslinker presented an inverse relation to the water uptake as it was already expected, since an increase in crosslinker amount provides higher density of crosslinking points avoiding great extension of water influx inward the matrix. The increasing in the NaAc/starch mass ratio up to 7 leads to an increase in the water uptake at a maximum value, however, higher ratios decreased that value due to the increase of crosslinking points. Similarly, the incorporation of CNWs up to 10 wt% provided an improvement in the swelling due to the extra hydrophilic groups from cellobiose units. Further incorporation of CNWs diminishes the water uptake because some hydroxyl groups took part in the grafting reaction, which increase the crosslinking density. Besides, the incorporation of CNWs improved the mechanical properties and hydrogel handling. In addition, SEM images showed the hydrogel morphology changed due to the incorporation of CWNs. The nanocomposites presented good responsive behavior in relation to pH and salt presence allowing those materials suitable for many potential applications. The results presented here are quite similar with others published previously by our research group. The increase of knowledge about the incorporation of CNWs in different hydrogel matrixes allows developing novel materials with desirable and tailored properties.
References
Barcus RL, Bjorkquist DW (1991) US Patent Office, Pat. No. 5,049,235
Beck-Candanedo S, Roman M, Gray DG (2005) Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 6:1048–1054
Cândido JDS, Leitão RCF, Ricardo NMSP, Feitosa JPA, Muniz EC, Rodrigues FHA (2012) Hydrogels composite of poly(acrylamide-co-acrylate) and rice husk ash. I. Synthesis and characterization. J Appl Polym Sci 123:879–887
Cuggino JC, Igarzabal CIA, Rueda JC, Quinzani LM, Komber H, Strumia MC (2008) Synthesis and characterization of new hydrogels through copolymerization of N-acryloyl-tris-(hydroxymethyl) aminomethane and different crosslinking agents. Eur Polym J 44:3548–3555
Czaja WK, Young DJ, Kawecki M, Brown RMJ (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8:1–12
Dai Q, Kadla JF (2009) Effect of nanofillers on carboxymethyl cellulose/hydroethyl cellulose hydrogels. J App Polym Sci 114:1664–1669
Elazzouzi-Hafraoui S, Nishiyama Y, Putaux JL, Heux L, Dubreuil F, Rochas C (2008) The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 9:57–65
El-Hamshary H (2007) Synthesis and water sorption studies of pH sensitive poly(acrylamide- co-itaconic acid) hydrogels. Eur Polym J 43:4830–4838
Flory JP (1953) Principles of polymer chemistry. Cornell University, Ithaca
Goetz L, Mathew A, Oksman K, Gatenholm P, Ragauskas AJ (2009) A novel nanocomposite film prepared from crosslinked cellulosic whiskers. Carbohyd Polym 75:85–89
Goetz L, Foston M, Mathew AP, Oksman K, Ragauskas AJ (2010) Poly(methyl vinyl ether-co-maleic acid)-polyethylene glycol nanocomposites cross-linked in situ with cellulose nanowhiskers. Biomacromolecules 11:2660–2666
Hazer O, Soykan C, Karatal S (2008) Synthesis and swelling behavior analysis of poly(acrylamidoxime-co-2-acrylamido-2-methylpropane sulfonic acid) hydrogels. J Macromol Sci A 45:45–51
Karadag E, Uzum OB, Saraydin D (2005) Water uptake in chemically crosslinked poly(acrylamide-co-crotonic acid) hydrogels. Mater Design 26:265–274
Karlsson ME, Leeman, Bjorck C, Inger ME, Eliasson AC (2007) Some physical and nutritional characteristics of genetically modified potatoes varying in amylose/amylopectin ratios. Food Chem 100:136–146
Kiatkamjornwong S, Chomsaksakulb W, Sonsuk M (2000) Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiation Phys Chem 59:413–427
Kuang J, Yuk KY, Huh KM (2011) Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohyd Polym 83:284–290
Li H, Yang J, Hu, Liang J, Fan Y, Zhang X (2011) Fabrication and properties of transparent polymethylmethacrylate/cellulose nanocrystals composites. J Biomed Mater Res A 98:31–39
Oh SY, Yoo DI, Shin Y, Seo G (2005) FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohyd Res 340:417–428
Omidian H, Rocca JG, Park K (2005) Advances in superporous hydrogels. J Control Rel 102:3–12
Pandey JK, Lee CS, Ahn SH (2010) Preparation and properties of bio-nanoreinforced composites from biodegradable polymer matrix and cellulose whiskers. J Appl Polym Sci 115:2493–2501
Pereira AGB, Paulino AT, Nakamura CV, Britta EA, Rubira AF, Muniz EC (2011) Effect of starch type on miscibility in poly(ethylene oxide) (PEO)/starch blends and cytotoxicity assays. Mater Sci Eng, C 31:443–451
Saboktakin MR, Maharramov A, Ramazanov MA (2009) pH-sensitive starch hydrogels via free radical graft copolymerization, synthesis and properties. Carbohyd Polym 77:634–638
Salimi H, Pourjavadi A, Seidi F, Jahromi PE, Soleyman R (2010) New smart carrageenan-based super absorbent hydrogel hybrid: investigation of swelling rate and environmental responsiveness. J Appl Polym Sci 117:3228–3238
Sandhu KS, Singh N (2007) Some properties of corn starches II: physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem 101:1499–1507
Satyamurthy P, Jain P, Balasubramanya RH, Vigneshwaran N (2011) Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohyd Polym 83:122–129
Schott H (1992) Swelling kinetics of polymers. J Macromol Sci B 31:1–9
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spec 36:23–40
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Res J 29:786–794
Siaueira G, Bras J, Dufresne A (2009) Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 10:425–432
Spagnol C, Rodrigues FHA, Neto AGVC, Pereira AGB, Fajardo AR, Radovanovic E, Rubira AF, Muniz EC (2012a) Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur Polym J 48:454–463
Spagnol C, Rodrigues FHA, Pereira AGB, Fajardo AR, Rubira AF, Muniz EC (2012b) Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohyd Polym 87:2038–2045
Sturcova A, Davies RG, Eichhorn SJ (2005) Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 6:1055–1061
Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stabil 84:331–339
Tokuyama H, Ishihara N, Sakohara S (2007) Effects of synthesis-solvent on swelling and elastic properties of poly(Nisopropylacrylamide). hydrogels. Eur Polym J 43:4975–4982
Wang WB, Wang AQ (2010) Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: synthesis, characterization and properties. Carbohyd Polym 82:83–91
Wang WB, Zheng Y, Wang A (2008) Syntheses and properties of superabsorbent composites based on natural guar gum and attapulgite. Polym Adv Technol 19:1852–1859
Wang WB, Wang Q, Wang A (2011) PH-responsive carboxymethylcellulose-g-poly(sodium acrylate)/polyvinylpyrrolidone semi-IPN hydrogels with enhanced responsive and swelling properties. Macromol Res 19:57–65
Zhang J, Wang L, Wang A (2006) Preparation and swelling behavior of fast-swelling superabsorbent hydrogels based on starch-g-poly(acrylic acid-co-sodium acrylate). Macromol Mater Eng 291:612–620
Zhou M, Zhao J, Zhou L (2011) Utilization of starch and montmorrilonite for the preparation of superabsorbent nanocomposite. J Appl Polym Sci 121:2406–2412
Zohuriaan-Mehr MJ, Pourjavadi A (2003) Superabsorbent hydrogels from starch-g-PAN: Effect of some reaction variables on swelling behavior. J Polym Mater 20:113–120
Acknowledgments
The authors would like to thanks the financial support by FUNCAP (BPI 0280-106/08 and PIL-139.01.00/09), CNPq (Proc. 507308/2010-7), and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spagnol, C., Rodrigues, F.H.A., Pereira, A.G.B. et al. Superabsorbent hydrogel nanocomposites based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 19, 1225–1237 (2012). https://doi.org/10.1007/s10570-012-9711-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9711-7