Abstract
N-halamine derivatives are efficient antibacterial agents and have been widely used in different kinds of surfaces due to their biocidal functions against a broad range of microorganisms, long term stabilities and regenerable properties. In this study, 3-(3′-acrylicacidpropylester)-5,5-dimethylhydantoin was synthesized and bonded onto cotton fabric by an electron beam irradiation process. Upon exposure to household bleach, the coated cotton sample could be rendered antibacterial. SEM, FTIR and EDX were used to characterize the surface of modified cotton which confirmed that the N-halamine precursor was coated on the cotton successfully. The chlorinated cotton samples were challenged with Staphylococcus aureus and Escherichia coli O157:H7 and showed excellent biocidal efficacy by inactivating 100 % of the bacteria with the contact times of 10 and 5 min, respectively. Standard washing and UV irradiation tests demonstrated that the coated cotton presented remarkable regenerable properties. The tensile loss was about 20 %, which is in an acceptable range in antimicrobial finishing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile materials, especially cotton, insure a good condition for micro-organisms to grow and breed. The pathogens have a tendency to colonize on the cotton surface and can cause the spread of infectious diseases. It is reported that about sixty percent of investigated health care workers’ uniforms and gowns have been contaminated by pathogens in hospitals (Sun et al. 2001), and the microbes are very stubborn; some infectious bacteria even can survive on the surface of materials for more than 90 days (Sun et al. 2001). To prevent cross-infection of diseases, an antibacterial property is a necessary function which should be added to medical and healthcare used textiles. Numerous antibacterial agents have been reported, including quaternary ammoniums (Kenawy et al. 1998, 2006; Liang et al. 2006), metal ions (Du et al. 2009; Heidenau et al. 2005; Nagar 1989; Rai et al. 2009), chitosan (Alonso et al. 2009; Cheng et al. 2014; El-Tahlawy et al. 2005; Fu et al. 2011; Klaykruayat et al. 2010; Shin et al. 2013) and N-halamines (Akdag et al. 2007; Cerkez et al. 2012; Chen and Sun 2006; Liang et al. 2007a, b; Worley et al. 1988). Among these biocidal agents, N-halamines are the most promising candidates. They have been used in antibacterial finishing and gained more and more attention for their biocidal functions against a broad range of microorganisms. N-halamines have high biocidal efficacy and regenerable properties upon exposure to household bleach (Kocer et al. 2010a, b, 2011a, b, c). The disinfection mechanism is the direct transfer of oxidative halogen from N-halamine to the microbial cells, which will destroy or inhibit the enzymatic or metabolic processes inside the cells, leading to the expiration of the microorganisms (Kocer et al. 2011a, b, c, 2010a, b). Among different types of N-halamines, heterocyclic structures have been proved to be more stable than acyclic structures (Kocer et al. 2011a, b, c). The heterocyclic structures such as oxazolidinones, imidazolidinones, triazines and hydantoins are good representatives. Due to the relative inexpensive price and excellent antibacterial efficacy, hydantoins are widely used as the precursor of N-halamines (Barnes et al. 2006; Chen et al. 2003; Grunzinger et al. 2007; Kou et al. 2006). The coating procedure of producing antibacterial cotton has been usually carried out by a pad-dry-cure process and/or with the aid of cross-linking (Ren et al. 2009a, b) or an initiator (Liu et al. 2014).
In recent years, electron beam (EB) irradiation processes have been widely used in modifying different kinds of surfaces, including cotton (El-Naggar et al. 2005), polyacrylonitrile (Dietrich et al. 1996), ethylene vinyl acetate copolymer (Wang et al. 2011), microcrystalline cellulose (Driscoll et al. 2009), etc. Due to the uniform cross-linking, and energy-saving and less environmentally hazardous, EB has received much attention. Many functions can be added to the materials via the EB process which is able to produce free radicals on the materials within several minutes. Previous studies showed that the cotton from three types of carbon-centered radicals on the glucose ring result upon treatment of EB irradiation (Alberti et al. 2005). Some of these radicals may originate from β-fragmentation of oxygen-centered radicals which can lead to the cleavage of a glycosidic bond. The tensile strength of the coated cotton decreases in some degree with irradiation ranging from 20 to 200 KGy, but the degradation of the materials is not significant. The monomers which can be used to modify the cellulose by the EB process usually contain unsaturated double bonds.
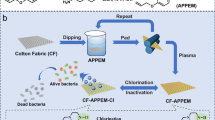
In this study, 5,5-dimethylhydantoin was chosen as a heterocyclic precursor to synthesize 3-(3′-acrylicacidpropylester)-5,5-dimethylhydantoin. The synthesis scheme is shown in Fig. 1. Electron beam irradiation was applied for the coating, followed by a chlorination process with diluted household bleach. There are three advantages of EB process for manufacturing antimicrobial cotton fabrics. First, the chemicals used in synthesizing monomer were not expensive. There is no need to use any initiator or cross-linker in EB process. Second, compared with traditional dipping process, EB irradiation is a continuous technology, which can be conducted efficiently in mass production. Third, the irradiation process is an energy-saving process and carried out at room temperature. The coated cotton after chlorination was evaluated for biocidal efficacy against 6-logs Staphylococcus aureus and Escherichia c oli O157:H7. The stability and recharge ability of the N–Cl bonds of the N-halamine coatings were also investigated by standard washing test and UV irradiation.
Experimental
Materials
1-Chloro-3-hydroxypropane and acryloyl chloride were provided by J&K Chemical Co., Ltd., Shanghai. 5,5-Dimethylhydantoin was purchased from Hebei Yaguang Fine Chemical Co., Ltd., China. Other chemicals used in this study were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai. All reagents were used as received without further purification. Fabric of 100 % bleached cotton was purchased from Zhejiang Guandong Printing and Dyeing Company, China. The bacteria employed in the research were S. aureus (ATCC 6538) and E. coli O157:H7 (ATCC 43895) (American Type Culture Collection, Rockville, MD). The Trypticase soy agar used was from Difco Laboratories, Detroit, MI.
Instruments
The EB150/20-250S1 electron beam accelerator (Hubei Eray Nuclear Technology Co., Ltd., China) was used for the irradiation treatment. A SU-1510 field-emission scanning electron microscope (Hitachi, Tokyo, Japan) was used to characterize the surface morphology of control and the treated cotton fibers. Fourier transform infrared (FTIR) spectra of cotton, coated cotton, and chlorinated coated cotton were obtained with a Nicolet NEXUS 470 spectrometer (Nicolet Instrument Corporation, Madison, WI). Nuclear magnetic resonance (NMR) spectra of the synthesized compound were recorded on an AVANCEIII 400 MHz Digital NMR spectrometer (Bruker AXS GmbH, Karlsruhe, Germany). EDX was conducted by a S5500 FESEM (Hitachi, Tokyo, Japan). UV light stabilities of the chlorinated cotton fabrics were measured using an Accelerated Weathering Tester (The Q-panel Company, USA). Tensile strength was recorded with YG (B) 026D-250 Electronic Fabric Strength Tester.
Synthesis of 3-(3′-hydroxypropyl)-5,5-dimethylhydantoin (HPDMH)
3-(3′-hydroxypropyl)-5,5-dimethylhydantoin was synthesized by the following procedure. The 5,5-dimethylhydantoin (0.1 mol) was mixed with NaOH (0.1 mol) in 100 ml distilled water and refluxed for 15 min to obtain the sodium salt of the 5,5-dimethylhydantoin. An equimolar quantity of 1-chloro-3-hydroxypropane was added to the above solution, and the mixture was stirred at 100 °C for 10 h. After the reaction was completed, solvent water was removed under reduced pressure. The solid product was recrystallized in acetone with a yield of 89 %. 1H NMR (D2O): δ 1.68–1.82 (6H), 2.09–2.22 (2H), 3.84–3.98 (2H), 3.98–4.06 (2H).
Synthesis of 3-(3′-acrylicacidpropylester)-5,5-dimethylhydantoin (APDMH)
0.1 Mol 3-(3′-hydroxypropyl)-5,5-dimethylhydantoin and an equimolar quantity of trimethylamine were added to 100 ml tetrahydrofuran (THF) and the mixture was stirred for 10 min to obtain a uniform solution. After cooling to 0 °C, 0.1 mol of acryloyl chloride was added drop wise to the solution and stirred for 1 h. Then the mixture was stirred for 20 h at ambient temperature. The product was collected via filtration and washed with THF and acetone (yield: 83 %). The product exhibited the following spectral data: 1H NMR (DMSO-d6): δ 1.26–1.33 (6H), 1.83–1.96 (2H), 3.42–3.52 (2H), 4.01–4.15 (2H), 5.90–6.00(1H), 6.10–6.22(1H), 6.28–6.40(1H), 8.23(1H).
Coating procedures
Electron beam (EB) irradiation was applied in this study. AHDMH was dissolved in distilled water at concentrations ranging from 3 to 8 %. Cotton swatches were impregnated in the water bath for 20 min with bath ratio of 50:1 (water to cotton by weight), and padded through a laboratory wringer. Two dips and two nips were used to get a wet pick-up of 100 wt%. Then the swatches were irradiated in air at room temperature by using an EB accelerator. Irradiation doses were set up in the range of 7–65 KGy. The energy of the EB accelerator was 130 KW. The average beam current was maintained at 1 mA. After irradiation, the swatches were dried at 100 °C for 3 min. The dried fabrics were soaked in 0.5 % detergent solution for 15 min, washed with distilled water to remove unbonded agent, and dried in air.
Chlorination and analytical titration
Cotton swatches coated with 3-(3′-acrylicacidpropylester)-5,5-dimethylhydantoin were soaked in a 10 % commercial aqueous sodium hypochlorite solution (0.5 % NaOCl) at pH 7 at room temperature for 60 min. Then the swatches were rinsed thoroughly with distilled water and dried at 45 °C for 60 min to remove the free chlorine absorbed on the surface of the cotton fabrics. The loaded chlorine concentration on the coated swatches was determined by the iodometric/thiosulfate titration method, and the weight percentage of chlorine was calculated according to the following equation:
where N and V are the normality (equiv/L) and volume (L) of sodium thiosulfate, respectively, and W is the weight (g) of the swatch.
Antimicrobial test
Both unchlorinated and chlorinated swatches were challenged with S. a ureus (ATCC 6538) and E. c oli O157:H7 (ATCC43895) using a modified AATCC Test Method 100-1999 to evaluate the biocidal efficacies of the coatings. Bacterial suspensions at designated populations were prepared in 100 mM phosphate buffer at pH 7. An aliquot of 25 µL bacterial suspension was taken and added to the center of a 1-inch square swatch. Then another 1-inch square swatch was put on it to insure that the suspension was sandwiched between two identical swatches which were held in place by sterile weights. After contact times of 5, 10, and 30 min, the samples were transferred to tubes contained 5 mL of sterile 0.02 N sodium thiosulfate solution and vortexed for 2 min to remove all oxidative chlorine residuals. The quenched solutions were diluted with 100 mM phosphate buffer (pH 7), and plated on Trypticase soy agar plates. After incubation at 37 °C for 24 h, the viable bacterial colonies were counted to determine antibacterial efficacy.
UV light stability testing
An Accelerated Weathering Tester was used to measure the UV light stabilities of coated cotton. The chlorinated swatches were exposed to the UV light (Type A, 315–400 nm, 0.89 W, 60 °C) chamber for contact times ranging from 1 to 24 h. After a specific time period of exposure to UV light, the cotton samples were removed from the UV chamber and titrated, or rechlorinated and titrated for the analysis of UV light stability.
Stability testing of chlorine on coated cotton
The washing stability of the treated cotton was recorded with a Launder-Ometer (Darong Textile Instrument Co., Ltd., Zhejiang, China) according to AATCC Test Method 61-1996 (Test 2A Procedure). The chlorinated cotton (1 in × 2 in), 50 stainless steel balls, and 150 mL of 0.15 % AATCC detergent water solution were added in stainless steel canisters which were fixed in a Launder-Ometer, and rotated at 42 rpm at 49 °C for 45 min for a washing cycle. Each cycle of washing in this method is equivalent to five machine washings. The cotton swatches were washed for the equivalents of 5, 10, 25, and 50 washing cycles. After the washings the swatches were rinsed thoroughly with distilled water and dried at room temperature. Then the chlorine loadings of washed swatches and rechlorinated swatches were determined by the titration procedure addressed above.
Tensile strength testing
The GB/T3923-1997 standard method was used to assess the tensile strength of the uncoated cotton and coated cotton before and after chlorination. Five replicates (25 cm × 5 cm) were tested for each sample in an electronic fabric strength tester, and their averages were reported. All the tests were performed at ambient temperature.
Result and discussion
Characterization of the coated cotton swatches
The scanning electron microscopy (SEM) morphologies of the uncoated and coated cotton with a magnification of 5000× are shown in Fig. 2. Compared with the uncoated cotton which has a very smooth surface, the coated cotton is uneven and rough. The differences between the two samples evidently demonstrated the presence of the copolymers on the surface of cotton fibers.
The FTIR spectra of cotton, unchlorinated APDMH-cotton and chlorinated APDMH-cotton are presented in Fig. 3. There are characteristic vibrational bands in the spectra of the coated cotton before and after chlorination at 1708 and 1729 cm−1, respectively, which are attributed to the vibration of C=O in the APDMH (Ren et al. 2008a, b). However, this band is not observed in the spectra of uncoated cotton. Due to the electron withdrawing effect of oxidative chlorine, the stretching vibrational band shifts from 1708 to 1729 cm−1 after chlorination. The shifts to higher wavenumber of the hydantoin carbonyl bands after chlorination have been also reported for other N-halamines (Ren et al. 2009a, b).
In order to confirm the elements on the surface of fibers, EDX was performed in cotton, treated cotton and treated cotton after chlorination. The three elements of C, O, Cl was tested in each sample. Figure 4 shows that both C and O can be seen on the three samples. The contents of elements in each sample are different due to the chemical reaction between APDMH and cotton fibers. It was observed that Cl (0.84 wt%) existed as a new peak in Fig. 4c which proved the presence of Cl on the sample. Compared with Fig. 4c, the content of Cl in Fig. 4b is only 0.04 % and could not be found in the image which might be due to the contamination. The new peak of Cl and the changes in contents (C, O, Cl) indicated that the surface of fiber has been modified successfully.
Biocidal efficacy
The antibacterial efficacy of modified cotton is shown in Table 1. Both unchlorinated and chlorinated samples were challenged with S. aureus and E. c oli O157:H7. Cotton coated with APDMH without chlorination was used as control sample. With contact time of 30 min, the control sample showed 0.585 log reduction for S. aureus and 0.338 log reduction for E. c oli O157:H7. The reduction of bacteria for the control samples was due to the adhesion of the bacteria to cotton instead of inactivation (Ren et al. 2008a, b). Meanwhile, the reduction of S. aureus is obviously higher than E. c oli O157:H7, which is due to the different cellular structures of bacteria. Compared with the control samples, the chlorinated samples showed excellent biocidal efficacy. With contact time of 5 min, the chlorinated samples could inactivate 99.99 % S. aureus and 100 % E. c oli O157:H7. When the contact time extended to 10 min, S. aureus was completely inactivated.
The UV light stability
The ultraviolet (UV) stability of the coated cotton upon chlorination is summarized in Table 2. With the increasing of UV irradiation time, the chlorine loading on the modified cotton decreased rapidly, especially in the first and second hours. After 4 h UV irradiation, 50 % of loaded chlorine was disappeared. With the irradiation time up to 24 h, most of the chlorines on the coated cotton were lost. The chlorine loss under UV irradiation is due to two reasons which are the dissociation of the N–Cl bond and the dissociation of the bond between the APDMH and cotton (Kocer et al. 2008). But about 86 % chlorine loading can be recovered upon exposure to an aqueous solution of sodium hypochlorite after 24 h UV irradiation, which is very remarkable compared with previous research (Kocer et al. 2010a, b). Considering the high recharge ability, the antibacterial cotton can be used for outdoor clothings.
Washing stability
The stability of the modified cotton towards washing was measured according to the method mentioned in the experimental section. The results are shown in Table 3. Two types of washing were performed to determine the stability of chlorine loading, prechlorinated samples upon certain machine washes (C), prechlorinated and rechlorinated samples after a given number of machine washes (R). Table 3 demonstrates that the chlorine loading on the samples decreased with the extension of washing cycles. After 5 washing cycles, the chlorine loading was decreased from 0.48 to 0.32 %. However, upon re-chlorination most of chlorine can be regained. After 50 washing cycles, 0.11 % chlorine was still left on the cotton which indicated that the N–Cl bonds on the APDMH coated cotton were more stable than on the hydantoin coated one reported previously (Kocer et al. 2010a, b; Kou et al. 2009; Ren et al. 2008a, b). After exposure to diluted sodium hypochlorite, the chlorine loading on the sample with 50 washing cycles could be recovered to 0.23 %, which is sufficient for rapid disinfection microorganisms according to the previous studies (Liang et al. 2007a, b).
Tensile strength test
Table 4 shows the results of tensile strength of cotton and coated cotton before and after chlorination. Both the warp and weft tensile strength of coated cotton showed some degree of decrease compared with uncoated cotton. The strength loss can be attributed to two reasons. The EB irradiation contained high energy which caused some breakage of bonds in the cotton fabric’s structure (Choi et al. 2008), and the grafting polymerization of APDMH on cotton could also cause the loss of strength. After chlorination, the sample could maintain 80 and 78 % of the original tensile strength in warp and weft directions, respectively. The small decrease in tensile strength demonstrates that the EB irradiation process has an advantage to produce antimicrobial N-halamine fabrics as compared to the traditional coating process. The reduction of breaking strength caused by the chlorination was due to oxidation of cotton fabric by household bleach, which has been reported in other studies (Ma et al. 2014).
Conclusion
A N-halamine precursor 3-(3′-acrylicacidpropylester)-5,5-dimethylhydantoin (APDMH) was successfully synthesized and confirmed by 1H NMR, and coated on cotton through an EB irradiation process. SEM, FTIR and EDX spectra showed that APDMH was successfully coated onto the surface of cotton. The coated cotton after chlorination presented outstanding antibacterial properties against S. a ureus and E. c oli O157:H7, which could inactivate both bacteria completely with 6.01 and 6.00 log reductions within the contact time of 10 and 5 min, respectively. The oxidative chlorine bonded to APDMH coated cotton is very stable and regenerable after standard washing and UVA irradiation. In addition, the tensile strength loss is in small degree, which shows a potential in applying the antibacterial cotton in the healthcare field.
References
Akdag A, Liang J, Worley SD (2007) Oxidation of organic sulfides by N-halamine compounds. Phosphorus Sulfur Silicon Relat Elem 182:1525–1533
Alberti A, Bertini S, Gastaldi G, Iannaccone N, Macciantelli D, Torri G, Vismara E (2005) Electron beam irradiated textile cellulose fibres: ESR studies and derivatisation with glycidyl methacrylate (GMA). Eur Polym J 41:1787–1797
Alonso D, Gimeno M, Olayo R, Vázquez-Torres H, Sepúlveda-Sánchez JD, Shirai K (2009) Cross-linking chitosan into UV-irradiated cellulose fibers for the preparation of antimicrobial-finished textiles. Carbohydr Polym 77:536–543
Barnes K, Liang J, Wu R, Worley SD, Lee J, Broughton RM, Huang TS (2006) Synthesis and antimicrobial applications of 5,5′-ethylenebis [5-methyl-3-(3-triethoxysilylpropyl) hydantoin]. Biomaterials 27:4825–4830
Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2012) N-halamine copolymers for biocidal coatings. React Funct Polym 72:673–679
Chen Z, Sun Y (2006) N-Halamine-based antimicrobial additives for polymers: preparation, characterization, and antimicrobial activity. Ind Eng Chem Res 45:2634–2640
Chen Y, Worley SD, Kim J et al (2003) Biocidal polystyrenehydantoin beads. 2. Control of chlorine loading. Ind Eng Chem Res 42:5715–5720
Cheng X, Ma K, Li R, Ren X, Huang TS (2014) Antimicrobial coating of modified chitosan onto cotton fabrics. Appl Surf Sci 309:138–143
Choi HY, Han SO, Lee JS (2008) Surface morphological, mechanical and thermal characterization of electron beam irradiated fibers. Appl Surf Sci 255:2466–2473
Dietrich J, Hirt P, Herlinger H (1996) Electron-beam-induced cyclisation to obtain C-fibre precursors from polyacrylonitrile homopolymers. Eur Polym J 32:617–623
Driscoll M, Stipanovic A, Winter W et al (2009) Electron beam irradiation of cellulose. Radiat Phys Chem 78:539–542
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 75:385–389
El-Naggar AWM, Zohdy MH, Said HM, El-Din MS, Noval DM (2005) Pigment colors printing on cotton fabrics by surface coating induced by electron beam and thermal curing. Appl Surf Sci 241:420–430
El-Tahlawy KF, El-Bendary MA, Elhendawy AG, Hudson SM (2005) The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan. Carbohydr Polym 60:421–430
Fu X, Shen Y, Jiang X, Huang D, Yan Y (2011) Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr Polym 85:221–227
Grunzinger SJ, Kurt P, Brunson KM, Wood L, Ohman DE, Wynne KJ (2007) Biocidal activity of hydantoin-containing polyurethane polymeric surface modifiers. Polymer 48:4653–4662
Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, Ziegler G, Gollwitzer H (2005) A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med 16:883–888
Kenawy ER, Abdel-Hay FI, El-Magd AA, Mahmoud Y (2006) Biologically active polymers: VII. Synthesis and antimicrobial activity of some crosslinked copolymers with quaternary ammonium and phosphonium groups. React Funct Polym 66:419–429
Kenawy ER, Abdel-Hay FI, El-Shanshoury AERR, El-Newehy MH (1998) Biologically active polymers: synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphonium groups. J Controlled Release 50:145–152
Klaykruayat B, Siralertmukul K, Srikulkit K (2010) Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr Polym 80:197–207
Kocer HB, Akdag A, Ren X, Broughton RM, Worley SD, Huang TS (2008) Effect of alkyl derivatization on several properties of N-halamine antimicrobial siloxane coatings. Ind Eng Chem Res 47:7558–7563
Kocer HB, Akdag A, Worley SD, Acevedo O, Broughton RM, Wu Y (2010a) Mechanism of photolytic decomposition of N-halamine antimicrobial siloxane coatings. ACS Appl Mater Interfaces 2:2456–2464
Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS (2011a) Polymeric antimicrobial N-halamine epoxides. ACS Appl Mater Interfaces 3:2845–2850
Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS (2011b) N-halamine copolymers for use in antimicrobial paints. ACS Appl Mater Interfaces 3:3189–3194
Kocer HB, Worley SD, Broughton RM, Acevedo O, Huang TS (2010b) Effect of phenyl derivatization on the stabilities of antimicrobial N-chlorohydantoin derivatives. Ind Eng Chem Res 49:11188–11194
Kocer HB, Worley SD, Broughton RM, Huang TS (2011c) A novel N-halamine acrylamide monomer and its copolymers for antimicrobial coatings. React Funct Polym 71:561–568
Kou L, Liang J, Ren X, Kocer HB, Worley SD, Tzou YM, Huang TS (2009) Synthesis of a water-soluble siloxane copolymer and its application for antimicrobial coatings. Ind Eng Chem Res 48:6521–6526
Kou L, Liang J, Worley SD, Lee J, Broughton RM, Huang TS (2006) Antimicrobial cellulose: preparation and application of 5-methyl-5-aminomethylhydantoin. Res J Text Appar 10:44–53
Liang J, Chen Y, Barnes K, Wu R, Worley SD, Huang TS (2006) N-Halamine/quat siloxane copolymers for use in biocidal coatings. Biomaterials 27:2495–2501
Liang J, Chen Y, Ren X et al (2007a) Fabric treated with antimicrobial N-halamine epoxides. Ind Eng Chem Res 46:6425–6429
Liang J, Wu R, Wang JW, Barnes K, Worley SD, Cho U, Huang TS (2007b) N-halamine biocidal coatings. J Ind Microbiol Biotechnol 34:157–163
Liu Y, Liu Y, Ren X, Huang TS (2014) Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl Surf Sci 296:231–236
Ma K, Xie Z, Jiang Q et al (2014) Cytocompatible and regenerable antimicrobial cellulose modified by N-halamine triazine ring. J Appl Polym Sci. doi:10.1002/app.40627
Nagar R (1989) Structural and microbial studies of some transition metal com-plexes. J Inorg Biochem 37:193–200
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Ren X, Kocer HB, Kou L, Worley SD, Broughton RM, Tzou YM, Huang TS (2008a) Antimicrobial polyester. J Appl Polym Sci 109:2756–2761
Ren X, Kocer HB, Worley SD, Broughton RM, Huang TS (2009a) Rechargeable biocidal cellulose: Synthesis and application of 3-(2, 3-dihydroxypropyl)-5,5-dimethylimidazolidine-2, 4-dione. Carbohydr Polym 75:683–687
Ren X, Kou L, Kocer HB, Worley SD, Broughton RM, Tzou YM, Huang TS (2009b) Antimicrobial modification of polyester by admicellar polymerization. J Biomed Mater Res B Appl Biomater 89:475–480
Ren X, Kou L, Kocer HB, Zhu C, Worley SD, Broughton RM, Huang TS (2008b) Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloid Surf A Physicochem Eng Asp 317:711–716
Shin HK, Park M, Chung YS, Kim HY, Jin FL, Choi HS, Park SJ (2013) Preparation and characterization of chlorinated cross-linked chitosan/cotton knit for biomedical applications. Macromol Res 21:1241–1246
Sun Y, Chen TY, Worley SD, Sun G (2001) Novel refreshable N-halamine polymeric biocides containing imidazolidin-4-one derivatives. J Polym Sci, Part A: Polym Chem 39:3073–3084
Wang B, Tai Q, Nie S, Zhou K, Tang Q, Hu Y, Song L (2011) Electron beam irradiation cross linking of halogen-free flame-retardant ethylene vinyl acetate (EVA) copolymer by silica gel microencapsulated ammonium polyphosphate and char-forming agent. Ind Eng Chem Res 50:5596–5605
Worley SD, Williams DE, Crawford RA (1988) Halamine water disinfectants. Crit Rev Environ Sci Technol 18:133–175
Acknowledgments
The financial supports were provided by the Project for Jiangsu Scientific and Technological Innovation Team, the research fund from the Science and Technology Department of Jiangsu Province of China (BY2014023-09), and the Scientific Research Foundation for Returned Overseas Chinese Scholars, Ministry of Education, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Liu, Y., Jiang, Z. et al. Synthesis of an N-halamine monomer and its application in antimicrobial cellulose via an electron beam irradiation process. Cellulose 22, 3609–3617 (2015). https://doi.org/10.1007/s10570-015-0763-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0763-3