Abstract
This article reports the effect of wet-pressing-induced fiber hornification on enzymatic saccharification of lignocelluloses. A wet cellulosic substrate of bleached kraft eucalyptus pulp and two wet sulfite-pretreated lignocellulosic substrates of aspen and lodgepole pine were pressed to various moisture (solids) contents by variation of pressing pressure and pressing duration. Wet pressing reduced substrate moisture content and produced irreversible reduction in fiber pore volume—fiber hornification—as reflected in reduced water retention values (WRVs), an easily measurable parameter, of the pressed substrates. Wet pressing resulted in a reduction in substrate enzymatic digestibility (SED) by approximately 20% for the two sulfite-pretreated substrates when moisture content was reduced from approximately 75% to 35%. The reduction in SED for the cellulosic substrate was less than 10% when its moisture content was reduced from approximately 65% to 35%. The results indicated that reduction in SED is negligible when samples were pressed to solids content of 40% but observable when pressed to solids content of 50%. It was also found that WRV can correlate to SED of hornified substrates resulting from the same never-dried or pressed sample independent of the hornification process (e.g., pressing or drying). This correlation can be fitted using a Boltzmann function. Cellulase adsorption measurements indicated that wet-pressing-induced fiber hornification reduced cellulose accessibility to cellulase. The results obtained in this study provide guidelines to high-solids enzymatic saccharification of pretreated biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzymatic hydrolysis of cellulose at high solids loadings of lignocellulosic substrates is necessary to produce high sugar concentrations in the hydrolysate. This ensures high biofuel (ethanol) titer through fermentation of the hydrolysate, reducing distillation (separation) costs downstream. It can also reduce process volume and therefore the capital cost in a biorefinery (Hodge et al. 2009; Wingren et al. 2003). To conduct high-solids hydrolysis using substrates from aqueous pretreatments, substantial dewatering of the pretreated substrate by means of wet pressing is required. It is well known that wet pressing of fibrous materials can induce fiber hornification (Carlsson and Lindstrom 1984; Häggkvist et al. 1998; Maloney et al. 1997; Robertson 1964), a term referring to the irreversible loss of water binding ability upon drying of cellulose (Jayme 1944; Laivins and Scallan 1993). It is a consequence of the irreversible change of cell wall structure (e.g., shrinkage and loss of pores) through the drying/dewatering–rewetting process (Scallan 1974). It was demonstrated that drying-induced fiber hornification significantly reduces enzymatic cellulose saccharification efficiency in our previous study (Luo and Zhu 2011). The shrinkage and loss of pores during wet pressing was verified by the measured volume distribution of pores upon fiber pressing (Häggkvist et al. 1998; Maloney et al. 1997), which can significantly reduce cellulose accessibility to cellulase. Therefore, it is expected that wet pressing can reduce enzymatic cellulose saccharification efficiency. However, this has never been verified and quantified.
Wet-pressing-induced fiber hornification can occur at a lower solids level than that for the drying-induced fiber hornification (Carlsson and Lindstrom 1984). Furthermore, wet-pressing-induced fiber hornification can occur at a pulp solids as low as 20% (Weise et al. 1996). High-solids enzymatic hydrolysis experiments of lignocellulosic substrate are often carried out at about 20% solids or higher (Hodge et al. 2009). Because most lignocellulosic substrates experience a certain degree of delignification during pretreatment, we hypothesize that wet pressing to dewater lignocellulosic substrates for high-solids enzymatic hydrolysis can induce hornification of the substrate, causing reductions in saccharification efficiency. It was found that nondelignified fibers were not hornified when subjected to wet pressing (Carlsson and Lindstrom 1984). The objective of this study is to quantify the effect of wet-pressing-induced fiber hornification on enzymatic cellulose saccharification efficiency. This study builds upon our previous study on the effect of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses (Luo and Zhu 2011). Water retention value (WRV), an easily measurable parameter, will be again used to characterize the degree of fiber hornification. The goal of this study is to provide quantitative guidelines from estimating the impact of wet-pressing lignocellulosic substrate on high-solids enzymatic saccharification.
Experimental
Materials
Three different pulps were used as “never pressed sample” in this study. The two lignocellulosic samples, pretreated lodgepole pine (PLPS) and pretreated aspen solid substrate (PASS), were produced in our previous study (Luo and Zhu 2011) by disk-milling wood chips at 10% solids content after sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL) (Wang et al. 2009; Zhu et al. 2009). The lodgepole pine log (BL-1), harvested from the Canyon Lakes Ranger District of the U.S. Arapaho–Roosevelt National Forest, Colorado, was chipped at the USDA Forest Service, Forest Products Laboratory, Madison, Wisconsin. Aspen wood chips were generously provided by the Wisconsin Rapids pulp mill of New Page Corporation (Wisconsin Rapids, Wisconsin). The third samples was a commercial wet-lap bleached eucalyptus pulp (BEP) provided by Kimberly Clark Corp. (Appleton, Wisconsin). The chemical compositions of the three samples are reported in our previous study (Luo and Zhu 2011).
Celluclast 1.5 L (cellulase) and Novozym 188 (β-glucosidase) were purchased from Sigma–Aldrich (St. Louis, Missouri). All chemicals used in this study were as received from Sigma–Aldrich.
Substrate mat preparation and wet pressing
A sample mat was first prepared for wet-pressing. Wet samples as received (for BEP at approximately 35% solids content) or produced (for PASS and PLPS at 10% solids content and then lightly pressed to approximately 25% solids) were used as “never pressed” sample. A 15-g (oven dry (od) weight) sample was suspended in 1000 mL deionized water and stirred by a magnetic stirrer for 30 min. The suspension was used to form a fiber mat on a 15-cm-diameter WhatmanTM #1 filter paper in a Büchner funnel by vacuum filtration. The collected filtrate was recycled three times through the sample mat on the filter paper to recover as much fines as possible. The filtration process resulted in a fiber mat with relatively uniform thickness. This mat was sandwiched between three layers of 18- by 18-cm blotter paper on each side. The mat sandwich was pressed by a hydraulic cylinder press (Model 2518, Fred S. Carver, Inc., Menomonee Falls, Wisconsin). Ranges of pressure and pressing duration were applied to obtain pressed mats (substrates) of different solids (moisture) contents. The pressed substrate was rapidly removed from the hydraulic press and placed into an airtight plastic bag overnight for moisture equilibrium. After equilibration, the solids (moisture) content of the pressed substrate was determined gravimetrically by oven drying a small portion of the sample at 105 °C overnight.
Measurements of substrate water retention value (WRV)
The degree of fiber hornification is defined in the literature as the percentage reduction in WRV (Jayme 1944). The WRVs of all substrates were measured following Scandinavian test method SCAN-C 62:00 (SCAN 2000). As described in our previous study (Luo and Zhu 2011), approximately 1 g (od weight) of substrate was suspended in 1 L deionized water and disintegrated for 10,000 revolutions at 312 rpm in a disintegrator (Model 73-06-01, TMI, Ronkonkoma, New York). The resultant suspension was carefully filtered using a nylon membrane (Sartonlon Polymid, pore size 0.45 μm, Sartorius Stedim Biotech GmbH, Gottingen, Germany). The filter cake was added to deionized water to make a suspension of about 10% solids consistency. After about 2 h, the suspension was wrapped by a nylon screen with mesh opening of 100 μm (Cole–Parmer, Vernon Hills, Illinois) and placed into a centrifuge tube with support to make space for water accumulation during centrifuge. The wrapped suspension was centrifuged at 3000×g for 15 min in a laboratory centrifuge (Thermo Fisher Scientific, Sorvall Legend 40/40R, Waltham, Massachusetts). WRV of the substrate is simply the amount of water retained after centrifuge as a percentage of the dry weight of the substrate. The averages of replicate measurements were reported. The standard deviations were used as error bars.
Enzymatic hydrolysis
The wet-pressed substrate was directly used for enzymatic hydrolysis at 2% substrate solids (w/v) (i.e., 2 g (od weight) substrate in a 100-mL solution). Sodium acetate solution of 50 mM concentration was used to buffer the hydrolysis suspension at pH 4.8. Hydrolysis was conducted at 50 °C in a flask on a shaker (Thermo Fisher Scientific, Model 4450, Waltham, Massachusetts) at 200 rpm using a commercial enzyme mixture of Celluclast 1.5 L and Novozyme 188 (β-glucosidase) (both Novozymes, Franklinton, North Carolina). Unless specified, the Celluclast 1.5 L loading was 7.5 FPU/g cellulose. The Novozym 188 loadings were 1.5 times the Celluclast 1.5 L amounts for all substrates. Five milliliters of 10 mg/mL tetracycline chloride was added to prevent the consumption of the released sugars by microorganisms. Hydrolysates were sampled periodically for measurement of glucose concentration by a commercial glucose analyzer (YSI 2700S, YSI Inc., Yellow Springs, Ohio). The substrate enzymatic digestibility (SED) values, defined as percentage of glucan in substrate converted to glucose, measured at 48 h were reported in this study. The averages of replicate experiments were reported. The standard deviations were used as error bars.
Measurements of cellulase adsorption
The amount of cellulase adsorption onto a lignocellulosic substrate was measured using a commercial endoglucanase, Novozym 476 (Novozymes, Franklinton, North Carolina) by an in situ and rapid UV–vis spectrophotometric method that directly measures protein absorption at 280 nm (Liu et al. 2011). The spectral interferences by light scattering from particulates and adsorption of lignin leached from lignocellulosic substrate were corrected using a dual wavelength technique (280 and 255 nm). Cellulase solution or cellulase and lignocellulosic substrate buffer suspension was circulated to a quartz flow cuvette (1.5 mL) by a peristaltic pump (Model M312, Gilson, Middleton, Wisconsin) using a flow loop consisting of a Erlenmeyer flask and a Teflon tube. A stainless steel tube screen of 400 mesh was wrapped at the inlet of the Teflon tube to filter fibers. Adsorption experiments were conducted at ambient temperature of 25 °C at pH 4.8. Absorption spectrum of free cellulase in the suspension was recorded every several minutes for 60 min using a commercial UV–vis spectrophotometer (model 8453, Agilent Technologies, Palo Alto, California). The amount of cellulase adsorbed onto lignocellulosic substrate is simply the difference between the amount measured and the amount of cellulase applied at the beginning of the adsorption experiment. The cellulase adsorption measured at 27 min was used to represent the total substrate-accessible area of cellulase. Our previous study indicated that 15 min is sufficient time to achieve endoglucanase adsorption equilibrium (Liu et al. 2011). Cellulase activities (hydrolysis) are negligible at 25 °C within 27 min at Novozyme 476 dosage of 15 IU/g substrate.
Results and discussion
Effects of wet pressing on substrate WRV
The effect of wet pressing on fiber hornification can be represented by the WRV because it mainly reflects the amount of water in the pores of a fibrous substrate. Wet pressing either by variation of pressure or duration reduced the moisture contents of all substrates as expected. The moisture content was reduced from approximately 75% to 35% for both PASS and PLPS, respectively, and from approximately 65% to 35% for BEP for the range of wet pressing experiments conducted. The wet-pressing-induced fiber hornification was apparent for all three samples as can be seen from the WRVs of the pressed substrates, which were significantly lower than those of the corresponding never-pressed substrate (Fig. 1). Although the substrate moisture content and WRV are correlated and the correlations are independent of the pressing methods (i.e., variation of pressures and duration), the correlations are nonlinear and dependent on the never-pressed sample (Fig. 1). Furthermore, the reductions in WRV were also different for the three samples examined. WRV was reduced from 145 to about 105 and 90, respectively, for the PASS and PLPS samples when their moisture content was reduced from approximately 75% to 35%. The WRV was reduced from 110 to about 90 for the BEP sample when moisture content was reduced from approximately 65% to 35%. This suggests variations in the substrate susceptibility to wet pressing. It should be pointed out that the BEP sample was pressed to solids content of about 35% by the supplier and may have already experienced some degree of hornification. It should also be pointed out that it is inaccurate to define the degree of fiber hornification as the relative reduction in WRV as widely used in the literature (Jayme 1944). This is because WRV also represents water associated with fibril surfaces; therefore, WRV of a completely hornified substrate is not zero. As a result, the degree of hornification determined from this definition is not 100% for a completely hornified sample. Therefore it is not obvious to determine the effects of pressing on the degree of hornification of different samples from the data shown in Fig. 1. The accurate definition of the degree of fiber hornification should be based on the reduction of fiber saturation point (FSP), because FSP represents only the water in the pores. Alternatively, one may also use the following expression,
where subscript NH represents never hornified and CH represents completely hornified.
Effects of wet pressing on substrate enzymatic digestibility (SED)
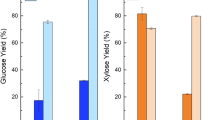
The reductions in WRV of pressed substrates suggest wet-pressing-induced fiber hornification, which should then be reflected in SED. SED decreased as moisture content was reduced (Fig. 2). The reduction is most significant for the two unbleached substrates, PASS and PLPS. SED reduction was over 20% when moisture content was reduced from approximately 75% to 35%. SED reduction was less than 10% for the BEP sample when moisture content was reduced from approximately 65% to 35%. The differences in wood species and chemical compositions made it difficult to compare the difference in the effect of wet pressing on reduction of cellulose accessibility for the substrate studied.
WRV is an easily measurable parameter and can reflect the change of pore size due to hornification. It was found that WRV can correlate well with pore radius (Andreasson et al. 2003) and pore volume (Jaturapiree et al. 2008), or the amount of water in cell wall pores (Hui et al. 2009; Weise et al. 1996). Furthermore, it was found that WRV can correlate well with SED as reported in our previous study (Luo and Zhu 2011). Quantitative determination of the change of porosity and pore size distribution using solute exclusion technique (Stone and Scallan 1967) or direct measurements of cellulose accessibility to cellulase (Hong et al. 2007) would be the most direct ways to study the effect of hornification on SED. However, these methods are laborious and time consuming. Moisture content, though, can correlate to WRV and SED for wet-pressed samples (Figs. 1, 2). However, it performs poorly in correlating with WRV and SED of hornified substrates when moisture content approaches zero through drying. To demonstrate the effectiveness of WRV for characterizing the effect of fiber hornification on SED, we plotted the WRVs and SEDs of hornified substrates produced through drying at 150 °C from our previous study (Luo and Zhu 2011) and produced through wet-pressing in the present study onto the same graph (Fig. 3). The results indicate that SEDs of hornified substrates produced from the same never-pressed and dried sample fall on the same curve that can be fitted by a Boltzmann function proposed in our previous study,
where δ SED–H can be considered as SED reduction due to current level of hornification as a fraction of the total SED reduction that would be caused by complete hornification of the never-dried sample. SED ND and SED CH are the SED of the never-dried and pressed and the completely hornified substrates, respectively; \( \overline{WRV} \) is a characteristic value that represents a hornification state at which rapid reduction in SED occurs due to a great number of large pores shrinking to a size equal to the effective size of the enzyme molecule. Δ should be the characteristics of the substrate cell wall structure, such as the shape of the pore size distribution curve of the never-dried sample. The parameters obtained from fitting the data of drying-induced hornified substrates and the data including both the dried and wet-pressed substrates were almost identical, with a maximal relative difference less than 4% (Table 1). This suggests that WRV can be used to characterize the effect of fiber hornification on substrate enzymatic digestibility independent of the process used to produce hornification. As explained in our previous study (Luo and Zhu 2011), the zero values of SEDCH for the PASS and PLPS samples suggest that cellulose accessible surface area becomes zero when a substrate is completely hornified (all pores are closed to cellulase). In other words, cellulases move mainly through the pores in the cell wall rather than to the external surface to access substrate cellulose, and substrate external surface does not contribute to cellulose accessibility for these two samples with high lignin content. SEDCH for BEP was about 20%, which suggests cellulose can be accessed by cellulases through the substrate external surface because BEP does not contain lignin.
Cellulase adsorption measurements to evaluate wet-pressing-induced fiber hornification and its effect on SED
The amount of cellulase adsorbed onto lignocellulosic substrates can be a direct indication of substrate accessibility to cellulase for a set of hornified substrates with identical chemical composition/structure produced from the same unhornified sample. The measured amounts of endoglucanase adsorbed onto wet-pressed substrates were plotted against the WRVs of the substrates (Fig. 4) to verify the suitability of using WRV to characterize cellulose accessibility to cellulase of hornified substrates. The results indicate that the measured amounts of cellulase adsorbed onto pressed substrates are nonlinearly correlated with substrate WRV for both samples examined. The nonlinearity is due to the fact that WRV is only a good representation of the total pore volume. Only those pores with diameter greater than the “effective diameter” of the cellulase contribute to measured cellulase adsorption. The slopes of the correlations are greater at low WRVs, suggesting that at higher degrees of hornification with the rapid shrinkages of many pores, the pores become inaccessible to the cellulase. The results also indicate that the substrates of the bleached kraft eucalyptus pulp (BEP) adsorbed less cellulase than did the sulfite-pretreated aspen substrate (PASS) (Fig. 4). SEM images show that the PASS sample has much shorter fiber length and is more swollen, perhaps due to sulfite pretreatment, than the BEP sample (Fig. 5a, b), which resulted in more cellulase adsorption.
The measured amounts of cellulase adsorbed onto the pressed substrates were found to be almost linearly proportional to SED (Fig. 6). The substrates derived from the same never-pressed sample are chemically identical, and the only difference among them is the accessible pore surface area due to pore shrinkage through fiber hornification. Therefore, cellulose accessibility to cellulase should be the only contributing factor to SED for the same never-pressed sample. One would expect a linear relation between SED and cellulose surface area accessible to cellulase. The linear relations shown in Fig. 6 suggest that the measured amount of cellulase adsorption can represent the cellulose accessible surface area or accessibility. As a matter of fact, cellulose accessibility to cellulase has been quantitatively characterized using protein adsorption, similar to that described in this study, with the addition of bovine serum albumin (BSA) to block nonproductive adsorption by lignin (Hong et al. 2007). The results in Fig. 6 also indicate that more cellulase is needed to achieve the same SED for the PASS than for BEP. This is because a lot of cellulase adsorbed onto PASS is associated with lignin—nonproductive adsorption.
Conclusions
We investigated the effect of wet-pressing-induced hornification on SED of lignocelluloses. Three different kinds of samples, namely PASS, PLPS, and BEP, were pressed under different pressures and durations to produce substrates with different moisture (solids) contents. For a given reduction of moisture content, PASS produced the most reduction in SED, followed by PLPS and then BEP. This may be caused by the different chemical compositions and cell wall structure (porosity or pore size distribution) among these substrates. For the three samples studied, wet pressing to solids content less 40% has negligible effects on SED. The reduction in SED due to wet pressing became obvious when solids content reached approximately 50%, and this is especially the case for the two pretreated samples, PASS and PLPS. It is expected that this critical solids content depends on the cellulose accessibility of the never-pressed sample. The results also indicate that WRV can represent the cellulose accessibility of hornified substrates produced from the same never-hornified sample, independent of the hornification process. Moreover, SEDs of the hornified substrates, from the same never-dried and pressed sample and produced by wet pressing and drying correlate very well with their WRVs. The correlation can be fitted using a Boltzmann function. Cellulase adsorption measurements indicate that hornification through wet pressing reduced cellulose accessibility to cellulase. SEDs of hornified substrates produced from the same never-pressed sample are almost linearly proportional to cellulase adsorption, suggesting hornification-induced pore shrinkage, reducing cellulose accessibility to cellulase, is the dominant and perhaps only factor to reduce substrate cellulose saccharification for a given never-hornified sample.
References
Andreasson B, Forsström J, Wågberg L (2003) The porous structure of pulp fibres with different yields and its influence on paper strength. Cellulose 10:111–123
Carlsson G, Lindstrom T (1984) Hornification of cellulosic fibers during wet pressing. Svensk Papperstidning 87(15):R119–R125
Häggkvist M, Li TG, Ödberg L (1998) Effects of drying and pressing on the pore structure in the cellulose fibre wall studied by 1H and 2H NMR relaxation. Cellulose 5:33–49
Hodge DB, Karim MN, Schell DJ, Mcmillan JD (2009) Model-based fed-batch for high-solids enzymatic cellulose hydrolysis. Appl Biochem Biotechnol 152:88–107
Hong J, Ye X, Zhang Y-HP (2007) Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications. Langmuir 23:12535–12540
Hui L, Liu Z, Ni Y (2009) Characterization of high-yield pulp (HYP) by the solute exclusion technique. Bioresour Technol 100(24):6630–6634
Jaturapiree A, Ehrhardt A, Groner S, Öztürk HB, Siroka B, Bechtold T (2008) Treatment in swelling solutions modifying cellulose fiber reactivity—Part 1: accessibility and sorption. Macromol Symposia 262(1):39–49
Jayme G (1944) Milkro-quellungsmessungen an Zellstoffenn. Papier-Fabr/Wochbl Papierfabr 6:187–194
Laivins GV, Scallan AM (1993) The mechanism of hornification of wood pulps. In: Baker CF (ed) Products of papermaking. Pulp and Paper Fundamental Research Society, Oxford, pp 1235–1260
Liu H, Zhu JY, Chai XS (2011) In situ, rapid, and temporally resolved measurements of cellulase adsorption onto lignocellulosic substrates by UV–vis spectrophotometry. Langmuir 27(1):272–278
Luo X, Zhu JY (2011) Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzyme Microb Technol 48(1):92–99
Maloney TC, Li TG, Weise U, Paulapuro H (1997) Intra- and inter-fibre pore closure in wet pressing. Appita J 50:301–306
Robertson AA (1964) Some observations on the effects of drying papermaking fibers. Pulp Paper Mag Can 65:T161–T168
Scallan AM (1974) The structure of the cell wall of wood—A consequence of anisotropic inter-microfibrillar bonding? Wood Sci 6:266–271
SCAN (2000) SCAN-C 62:00 water retention value of chemical pulps. Nordic Standardization Program
Stone JE, Scallan AM (1967) The effect of component removal upon the porous structure of the cell wall of wood. II. Swelling in water and the fiber saturation point. TAPPI J 50(10):496–501
Wang GS, Pan XJ, Zhu JY, Gleisner R (2009) Sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL) for robust enzymatic saccharification of hardwoods. Biotechnol Prog 25(4):1086–1093
Weise U, Maloney T, Paulapuro H (1996) Quantification of water in difference states of interaction with wood pulp fibres. Cellulose 3:189–202
Wingren A, Galbe M, Zacchi G (2003) Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog 19(4):1109–1117
Zhu JY, Pan XJ, Wang GS, Gleisner R (2009) Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour Technol 100(8):2411–2418
Acknowledgments
This work was sponsored by the USDA Forest Service through the Program of Woody Biomass, Bioenergy, and Bioproducts (WBBB, 2009) that provided financial support to Luo for his visiting appointment at the University of Wisconsin–Madison and USDA Forest Service, Forest Products Laboratory (FPL).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was conducted at the USDA Forest Service, Forest Products Laboratory (FPL) while Luo was a visiting student at the University of Wisconsin–Madison and FPL, and on official government time by Zhu and Gleisner. The work is in the public domain in the U.S.
Rights and permissions
About this article
Cite this article
Luo, X.L., Zhu, J.Y., Gleisner, R. et al. Effects of wet-pressing-induced fiber hornification on enzymatic saccharification of lignocelluloses. Cellulose 18, 1055–1062 (2011). https://doi.org/10.1007/s10570-011-9541-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9541-z