Abstract
Background
Recent data have demonstrated that long-lived memory T cells are present in the human lung and can play significant roles in the pathogenesis of specific allergic and autoimmune diseases. However, most evidence has been obtained from mouse studies, and the potential roles of memory T cells in human allergic diseases, such as asthma, remain largely unknown.
Methods
Thirty-three asthmatics, 26 chronic obstructive pulmonary disease (COPD) patients, and 22 healthy volunteers were enrolled in this study. Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood, and cell surface staining (CD4, CD45RO, CRTH2, CD62L, and CCR7) was performed for the detection of memory CD4+ T cells in blood. After stimulation with interleukin-27 (IL-27) or IL-4 for 15 min, the STAT1/STAT6 phosphorylation of memory CD4+ T cells was measured separately by flow cytometric techniques. The cytokine-releasing profiles after 6 days of culture under neutralization, TH2, TH2 + lipopolysaccharide (LPS), and TH2 + house dust mite (HDM) conditions were detected by intracellular protein (IL-5, IL-17, and interferon (IFN)-γ) staining. Correlation analyses between the profile of memory CD4+ T cells and clinical characteristics of asthma were performed.
Results
The number of circulating memory CD4+ T (CD4+ Tm) cells in asthmatics was increased compared with that in the healthy subjects (48 ± 5.7 % vs. 32 ± 4.1 %, p < 0.05). Compared with COPD and healthy subjects, the phosphorylation of signal transducer and activator of transcription 1 (STAT1-py) was impaired in asthmatics, whereas the phosphorylation of signal transducer and activator of transcription 6 (STAT6-py) was slightly enhanced. This imbalance of STAT1-py/STAT6-py was attributed to TH2 memory cells but not non-TH2 memory cells in blood. The cytokine-releasing profiles of asthmatics was unique, specifically IL-5high, IL-17high, and IFN-rlow, compared with those of COPD patients and healthy subjects. The IL-17 production levels in CD4+ Tm cells are associated with disease severity and positively correlated with medication consumption in asthma.
Conclusions
The long-lived, antigen-specific memory CD4+ T cells, rather than PBMCs or peripheral lymphocytes, might be the ideal T cell subset candidates for analyzing the endotype of asthma. Memory CD4+ T cells exhibiting a shift in STAT phosphorylation and specific cytokine-releasing profiles have the potential to facilitate the understanding of disease heterogeneity and severity, allowing the more personalized treatment of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytokines play a pivotal role in the development of asthma by regulating the expansion of TH2 cells and mediating the TH2-type responses that underlie the pathogenic events of an asthmatic response. It is known that cytokine-mediated signals are primarily transduced by the Jak-STAT signaling cascade. The STAT family has seven members, and STAT1 signaling regulates T helper type 1 (TH1) cell-specific cytokine production to alter both immune function and inflammatory responses by shifting the balance between TH1 and TH2 cells (Miklossy et al. 2013). STAT6 signaling is induced by IL-4 and IL-13 and regulates the balance between inflammatory and allergic immune responses. Recent studies revealed that the inhibition of activating cytokines (IL-4 and IL-13) and the associated transcription factors, such as JAKs and STATs, may be a potential therapeutic target for uncontrolled severe asthma(Chapoval et al. 2011).
However, numerous studies regarding the relationship of cytokines with the clinical properties of asthma as well as the classification and severity of asthma have not drawn consistent conclusions. One of the reasons for this difference may be the heterogeneity of peripheral blood mononuclear cells (PBMCs) or peripheral lymphocytes, which were widely used in cytokine-releasing experiments (Rudin et al. 2001). We hypothesized that long-lived, antigen-specific TH2 memory cells other than PBMCs may be ideal T cell subset candidates for analyzing the endotype of asthma. There are three types of memory T cells: effector memory T cells (Tem), central memory T cells (Tcm), and resident memory T cells (Trm) (MacLeod et al. 2010; MacLeod et al. 2009; Chen et al. 2006). Using a three-dimensional explant culture system, Purwar and colleagues demonstrated that more than ten billion memory T cells are present in the human lung. In an ovalbumin (OVA)-induced mouse asthma model, allergen-specific memory TH2 cells have also been identified in both the lung and spleen (Mojtabavi et al. 2002a). The intravenous transfer of memory TH2 cells into naive mice results in airway hyperresponsiveness and eosinophil infiltration in the recipient mice (Nakagome et al. 2005). Thus, memory T cells may play a harmful role in the pathogenesis of allergic and autoimmune diseases.
In the present study, we focused on circulating memory T cells and the relationship between the endotype of memory CD4+ T cells and the phenotype of asthma patients. The results revealed that the phosphorylation of signal transducer and activator of transcription 1 (STAT1) was impaired and the phosphorylation of signal transducer and activator of transcription 6 (STAT6) was slightly enhanced in asthmatics. The cytokine-releasing profiles of asthmatics were unique, specifically IL-5high, IL-17high, and IFN-rlow, and quite different from those of COPD patients and healthy subjects. The IL-17 production levels in CD4+ Tm cells are associated with disease severity and positively correlated with medication consumption in asthma.
Materials and methods
Human subjects
Patients with asthma (n = 32) and COPD (n = 26) and healthy donors (n = 22) were recruited from the outpatient clinic in the Respiratory Division at Zhongshan Hospital, Fudan University between December 2014 and August 2015. All asthma and COPD patients fulfilled the 2015 Global Initiative for Asthma (GINA) criteria and 2015 Global Initiative for Chronic Obstructive Lung Disease GOLD criteria, respectively. Healthy subjects with no prior history of allergic diseases or with negative allergy skin test results were enrolled. All subjects were 18 to 70 years of age. All included subjects had written informed consents (Table 1). The protocols (no. B2014-108) were approved by the institutional review board at Fudan University. Twenty milliliters of peripheral blood was drawn from each subject for CD4+ T cell isolation.
Reagents
A CD4+ T cell isolation kit was purchased from MACS (Bergisch, Germany). Surface and intracellular markers were determined using FACS antibodies purchased from BD Bioscience (USA). For surface staining, APC-Cy7-labeled anti-human CD4, FITC-labeled anti-human-CD45RO, Alexa 647-labeled anti-human-CD45RA, PE-labeled anti-human-CD3, APC-labeled anti-human CD62L, and PE-Cy7-labeled anti-human CCR7 were used. For intracellular staining, APC-labeled anti-human-IL-5, PerCP-Cy5.5-labeled anti-human-IL-17A, PE-Cy7-labeled anti-human IFN-γ, PE-labeled anti-STAT1 (pY-701), and PE-labeled anti-STAT6 (pY-641) were used. During cell culture and differentiation, the following reagents were used: human IL-2 (BD Bioscience, USA), human IL-4 (BD Bioscience, USA), human IL-27(BD Bioscience, USA), anti-human IL-4 antibody (BD Bioscience, USA), anti-human-IFN-γ antibody (BD Bioscience USA), anti-human CD3 (eBioscience), anti-human CD28 (eBioscience), LPS (L2630, Sigma-Aldrich,100 ng/mL), and house dust mite (HDM) (100 μg/mL) (a kind gift from Pro. Rui He from Immunology Department at Fudan University). A leukocyte activation cocktail with Golgi blocker (BD Bioscience, USA) was used to promote cytokine release.
Cell culture and polarization
CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) with the MACS CD4+ T cell isolation kit. Cells were cultured under four different conditions: (1) neutralization conditions, anti-human IL4 antibody and anti-human IFN-γ antibody were added on Day 0; (2) TH2 conditions, anti-human IFN-γ, and human IL-4 were added on Day 0; (3) TH2 + LPS conditions, anti-human IFN-γ, human IL-4, and lipopolysaccharide (LPS) were added on Day 0; and (4) TH2 + HDM conditions, anti-human IFN-γ, human IL-4, and HDM were added on Day 0. Cells were cultured in the respective medium for 6 days.
Stimulation of CD4+ T cells
-
1.
Stimulation of freshly isolated or cultured CD4+ T cells using the leukocyte activation cocktail: freshly isolated or cultured CD4+ T cells were collected and washed with phosphate-buffered saline (PBS) once. After centrifugation, the cells were resuspended with fresh culture medium. Leukocyte activation cocktail with Golgi blocker was added to each well and incubated overnight.
-
2.
Stimulation of freshly isolated CD4+ T cells by cytokines: to measure the phosphorylation of STAT1 and STAT6, freshly isolated CD4+ T cells were washed with PBS once and resuspended with 1640 complete culture medium. Next, the cells were treated with IL-27 (50 ng/mL) or IL-4 (15 ng/mL) for 15 min and rinsed with 10 vol of PBS to stop the reaction. After centrifugation, the cells were resuspended with FACS buffer for further staining.
Cell surface and intracellular staining of human CD4+ T cells
To determine the surface markers of CD4+ Tm cells, APC-Cy7-labeled anti-human CD4, FITC-labeled anti-human-CD45RO, Alexa 647-labeled anti-human-CD45RA, PE-labeled anti-human-CD3, APC-labeled anti-human CD62L, and PE-Cy7-labeled anti-human CCR7 were added, and the cells were incubated at 4 °C for 20 min in a dark environment (all FACS antibodies were used according to the product instructions). After washing with PBS once, the cells were ready for detection.
To detect intracellular cytokines, cell surface marker and intracellular cytokine staining was performed. The stimulated cells were harvested and washed once with PBS. APC-Cy7-labeled anti-human CD4 and FITC-labeled anti-human-CD45RO were added first at 4 °C for 20 min followed by 4 % paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co., Ltd., China) fixation for 7 min at room temperature. The cells were then permeabilized with prechilled Perm Buffer III (BD Bioscience) at 4 °C for 30 min. After washing once with PBS, the cells were stained with APC-labeled anti-human-IL-5, PerCP-Cy5.5-labeled anti-human-IL-17A, and PE-Cy7-labeled anti-human IFN-γ at 4 °C for 20 min. The samples were monitored using a FACS Aria II machine (BD Bioscience) and analyzed using FlowJo 10.0 software (Tree Star, Inc., Ashland, OR, USA). To determine STATs phosphorylation, CD4+ T cells were stained with FITC-anti-human CD45RO and APC-labeled anti-human CD294 (CRTH2). The stained cells were stimulated (or not stimulated) with hIL-27 (50 ng/mL) or hIL-4 (50 ng/mL) for 15 min. After fixation and permeabilization, the cells were stained with PE-labeled anti-STAT1 (pY-701) or PE-labeled anti-STAT6 (pY-641). The samples were monitored on FACS Aria II machine (BD Bioscience) and analyzed using FlowJo 10.0 software (Tree Star, Inc., Ashland, OR, USA).
Correlation analysis between IL-17+CD4+ Tm cells and clinical parameters of asthma
The frequencies of IL-17+CD4+Tm were calculated from different subgroups of asthma patients. Disease severities and medication consumption were also tested. The asthma medication consumption score was based on the GINA 2015 guideline. One to five represent the medication usage step one to five, respectively. For example, if a patient uses a medium dose of ICS + LABA, medication consumption score is 3. The correlation between the IL-17+CD4+ Tm cell frequency and these clinical parameters were analyzed through correlation analyses. The R and p values were calculated.
Statistical analysis
All data are presented as the means ± standard errors (SEs). The 95 % confidence interval (CI) was used to predict the total average. Pooled data are indicated in the figure legends. Differences between datasets were analyzed using one-way analysis of variance (ANOVA) with a Bonferroni posttest, which was used to determine the significance (Prism 5; GraphPad Software, San Diego, CA, USA). A p value <0.05 was considered statistically significant.
Results
Number of circulating memory CD4+ T cells in asthmatics was increased and present potent inflammatory cytokine-producing capacities compared with healthy subjects
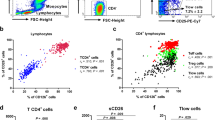
PBMCs were isolated from the peripheral blood of asthmatics and healthy volunteers. CD4+ T cells were purified using the MACS isolation kit with a purification rate of 93 to 95 %. CD4+ Tm cells were defined as CD4- and CD45RO-double-positive cells. On Day 0, CD4+ Tm cells increased in the peripheral blood of asthmatics compared with the healthy volunteers (48 ± 5.7 % vs. 32 ± 4.1 %, p < 0.05). Upon anti-CD3 plus anti-CD28 activation, CD4+ Tm cells also increased in the asthmatics, compared with the healthy subjects on Day 3 (63 ± 7.2 % vs. 51 ± 7.1 %, p < 0.05) (Fig. 1). In CD4+ Tm cells, CCR7- and CD62L-double-positive cells were central memory T cells (Tcm), whereas CCR7-negative and CD62L-positive cells represented effector memory T cells (Tem). Our data demonstrated that Tcm constituted the predominant memory T subset in the circulation in both asthmatic and healthy volunteers and that the percentage of Tcm in the asthmatics was greater than that in the healthy subjects. Although Tem was not a predominant subset, the percentage of Tem in the asthmatics was increased threefold compared with the healthy subjects (Fig. 1). The recall memory response can produce effective cytokines more rapidly and strongly. Our results indicate that CD4+ Tm cells have a more potent capacity of producing either TH1 (IFN-γ) or TH2 (IL-5) cytokines in both asthmatics and healthy subjects compared with non-memory CD4+ T cells (data not shown).
The number of circulating memory CD4+ T cells in the asthmatics increased compared with the healthy control. a Memory CD4+ T cells were stained as CD4+CD45RO+ cells using flow cytometry. b The percentage of memory CD4+ T cells increased in the asthma patients compared with the healthy controls. c Central memory CD4+ T cells (Tcm) were the predominant subgroup of memory CD4+ T cells in blood either in the asthma or the healthy subjects. The frequency of Tcm and Tem of CD4+ Tm cells was increased in the asthma patients compared with the healthy controls (data are representative of 12 independent experiments with similar results)
Imbalance of STAT1 and STAT6 phosphorylation in asthma patients
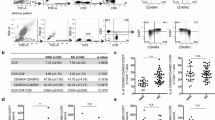
To assess the activation of STAT1 and STAT6 in memory CD4+ T cells, asthmatics, COPD patients, and healthy subjects were enrolled. Previous studies have demonstrated that STAT1-deficient mice exhibit a propensity toward mounting a TH2-type immune response (Hida et al. 2005; Fukushima et al. 2005) and that IL-4-induced STAT6 activation is crucial for the TH2 differentiation of naive T cells. However, the status of STAT1 and STAT6 in memory CD4+ T cells in asthma has not been reported. In the present study, we found that STAT1 phosphorylation (STAT1-Py) is relatively increased compared with STAT6-Py in memory CD4+ T cells of healthy patients. The ratio of STAT1-Py to STAT6-Py was greater than 1, similar to the pattern observed in COPD patients. In contrast, the ratio of STAT1-Py to STAT6-Py in memory CD4+ T cells of asthmatics was markedly less than 1 and thus significantly different from that observed in the COPD and healthy subjects (p < 0.01) (Fig. 2).
STAT1 phosphorylation is inhibited but STAT6 phosphorylation is enhanced in CD4+ Th2 memory cells in the asthmatics compared to the COPD and healthy subjects. a CD4+ T cells were stimulated with IL-27/IL-4 or PBS. STAT1-py/ STAT6-py was stained and monitored by flow cytometry. The numbers indicate the changes in fluorescence intensity (△MFI) after cytokine stimulation. b The ratio of △py-STAT1 to △py-STAT6 was reduced in asthmatics. Asthmatics (n = 32); COPD (n = 26); healthy volunteers (n = 22) (p < 0.05 statistical difference)
TH2 memory cells contribute to the imbalance of STAT1/STAT6 phosphorylation
To determine which CD4+ T cell subset is associated with the imbalance of STAT1/STAT6 phosphorylation, three populations were defined by staining isolated human CD4+ T cells with anti-human CRTH2, anti-human CD45RO, and anti-phosphorylated STAT (STAT-py) antibodies: naive CD4+ T cells (CD4+CD45RO−CRTH2−), memory TH2 cells (TH2m, CD4+CD45RO+CRTH2+), and memory non-TH2 cells (non-TH2m, CD4+CD45RO+CRTH2−). Non-TH2m cells consist of memory TH1 cells and memory TH17 cells. Our data indicate that the mean fluorescence intensity (MFI) of STAT1-py in both the naive CD4+ T-cell and non-TH2m populations in the asthmatic patients exhibited a decreasing tendency compared with that observed in the control subjects. However, these differences were not statistically significant (p > 0.05). CD4+ TH2m cells were the only subset that presented significantly reduced STAT1 phosphorylation in asthma patients compared with control subjects. Interestingly, a significant difference in the change in the mean fluorescence intensity (ΔMFI) of IL-4-induced STAT6-py between the asthma patients and the control subjects was observed in TH2m cells but not non-TH2m cells and naive CD4+ T cells. In addition, the ratio of STAT1-py to STAT6-py was less than 1 in the asthma patients, which is different from that observed in the COPD and healthy populations (Fig. 3).
STAT1 phosphorylation was inhibited in CD4+ TH2 memory cells in asthma patients. a Fluorescence-activated cell sorting analysis of STAT1 phosphorylation (STAT1-py) in various subsets of CD4+ T cells. Naive naïve CD4+ T cells, non-T H 2m non-TH2 memory CD4+ T cells, T H 2m TH2 memory CD4+ T cells. b Fluorescence-activated cell sorting analysis of STAT6-py. c △MFIs of STAT1-py and STAT6-py in gated cell populations ([P] patients, n = 6; [C] control subjects; n = 6; p < 0.05 statistical difference; ns, not statistical)
CD4+ Tm cells of asthma patients have a specific cytokine profile compared with those of COPD and healthy subjects
Do the cytokine-releasing profiles of memory CD4+ T cells differ among asthmatics, COPD patients, and healthy volunteers? CD4+ T cells purified from the enrolled subjects were cultured under neutralization, TH2, TH2 + LPS, and TH2 + HDM conditions for 6 days. After overnight activation with phorbol myristate acetate (PMA) and ionomycin, the cytokine protein levels (IL-5, IL-17, and IFN-γ) in CD4+ Tm cells were measured through intracellular staining. Figure 4a–c presents representative flow cytometry images. The CD4+ Tm cells of asthma patients produced more IL-5 under TH2 and TH2 + HDM conditions (4.4 ± 0.5 % and 6.1 ± 0.7 %) compared with those of the COPD patients (1.2 ± 0.09 % and 1.4 ± 0.08 %) and the healthy volunteers (1.0 ± 0.06 % and 1.0 ± 0.09 %). Similar results were observed for IL-17 secretion, which was increased in asthma patients under TH2 and TH2 + HDM conditions (4.7 ± 0.6 % and 5.3 ± 0.4 %). However, lower IFN-γ-producing capacities were observed in the CD4+ Tm cells of asthma patients under all culture conditions, particularly in the TH2 + LPS condition (asthma vs. COPD vs. healthy equal to 14.1 ± 0.15% vs. 24.2 ± 0.22 % vs. 22.4 ± 0.18 %). The histogram demonstrated the pooled data from all enrolled patients and volunteers, from which increased frequencies of IL-5- and IL-17-producing CD4+ Tm cells were noted in the circulation of asthma compared with COPD or healthy subjects. The capacity of producing-IFN-γ was reduced in asthma compared with COPD and healthy subjects (Fig. 4d).
Cytokine profiles of memory CD4+ T cells in the asthmatics compared with the COPD and healthy subjects. a–c CD4+ T cells were cultured under neutralization (Neu) and Th2, Th2 + LPS, or Th2 + HDM conditions for 6 days. After stimulation with Golgi blocker overnight, CD45RO and intracellular proteins (IL-5, IL-17, and IFN-γ) were stained and monitored using flow cytometry. The numbers indicate the percentage of double-positive cells. Similar results were obtained from subjects, including asthmatics (n = 32), COPD (n = 26), and healthy volunteers (n = 22). d The percentage of IL-15+CD4+ Tm, IL-17+CD4+ Tm, or IFN-γ+CD4+ Tm of CD4+ T cells in the asthmatics compared with the COPD and healthy subjects (p < 0.05 or p < 0.01 represented the statistical significance)
IL-17+CD4+ Tm cells may be a pathological cell type in asthma
CD4+ Tm cells have been proven to be flexible. For example, memory TH2 cells can develop into IL-17-producing memory TH2 cells with the capacity of releasing both IL-4 and IL-17 (13). Memory TH2 cells have the potential to express Foxp3 under a TGF-β and IL-6 microenvironment (14). Our experiment revealed that circulating CD4+ Tm cells in asthma, which present a shift in STAT1-py and STAT6-py, have a specific cytokine profile, specifically IL-5high, IL-17high, and IFN-γlow. However, whether the number of cell subsets could indicate the severity of the disease was still largely unknown. To answer this question, CD4+ Tm cells from healthy, mild asthma, and moderate to severe asthma patients were further analyzed. Our results indicate that only IL-17+CD4+ Tm cells were increased with an increase in the severity of asthma compared with IL-5+CD4+ and IFN-γ+CD4+ Tm cells (Fig. 5). The percentage of IL-17+CD4+ Tm was positively correlated with the asthma drug consumption score (R 2 = 0.8296, p < 0.05) (according to the 2015 GINA guidelines). However, the percentage of IL-5+CD4+Tm and IFN-γ+CD4+ Tm did not correlate with the drug consumption score (R 2 = 0.1408, p > 0.05; R 2 = 0.1934, p > 0.05) (Fig. 5).
IL-17+ CD4+ Tm cells might be a pathological T helper cell in asthma. a The percentage of IL-17+CD4+ Tm cells but not IL-5+CD4+ Tm cells or IFN-r+CD4+ Tm cells were associated with asthma severity. b The percentage of IL-17+CD4+ Tm cells was positively correlated with the asthma drug score (p < 0.05 statistical difference; ns not statistical )
Discussion
Although, the role of CD4+ memory T cells in the lung has not been fully elucidated (Seder et al. 2008; Wilkinson et al. 2012; Farber et al. 2014; Lumsden et al. 2011). Emerging evidence has suggested that circulating memory CD4+ T cells have the ability of recruiting to the lung quickly and initiate specific immune response against previously encountered pathogens (Turner and Farber 2014). In the present study, the number of circulating memory CD4+ T cells in asthmatics was increased compared with healthy control (49 vs. 30.3 %) on Day 0 when the cells were freshly isolated from the peripheral blood. The same trend was also observed when the cells were cultured for 3 to 6 days (data not shown). Our findings were consistent with those obtained in a previous study conducted by Abdulamir et al. (Abdulamir et al. 2008; Tang and Chen 2015), who showed that the percentage of CD45RO+CD4+ T cells was markedly different between severe asthmatics and healthy control subjects.
At birth, all T cells in the peripheral blood are naive. By the end of the second decade of human life, memory T cells consist of up to 35 % of circulating T cells (Sathaliyawala et al. 2013). More than ten billion resident T cells are present in the lung, and these are largely effector memory T cells (Tem), with a small number of central memory T cells (Tcm) and T regulatory cells (Treg). Our study revealed that Tcm is the predominant subgroup of memory T cells in the blood of asthma and healthy subjects and that the percentage of Tcm in asthma is greater than that in control subjects. Tem can also be detected in peripheral blood, with a proportion of 2.4 and 0.8 % noted in asthmatics and healthy subjects, respectively. Resident memory T cells (Trm) residing in tissues were more involved in the rapid control of infection and local pathogenesis of chronic disease than in circulating memory T cells, but it is hard to obtain from human tissues including the lung. Thus, the relationship between circulating memory CD4+ T cells (most of which are Tcm and Tem) and human asthma pathogenesis still requires further investigation.
STATs were originally described as latent cytoplasmic transcription factors. There are seven identified mammalian STAT family members, including STAT1, STAT2, STAT3, SATA4, STAT5 (STAT5A and STAT5B), and STAT6. The STAT1-T-bet pathway controls TH1 differentiation, whereas the STAT6-GATA3 pathway promotes TH2 differentiation. STAT1-deficient or interferon regulatory factor 1- and 2-deficient mice demonstrated a propensity toward mounting a TH2-type immune response against pathogens (Chen et al. 2013; Xiaoqiong et al. 2015; Kalliolias and Ivashkiv 2008; Ouaked et al. 2009). These results clearly demonstrated that TH1-promoting factors are critical in suppressing the differentiation of Th2 cells. In contrast, differentiated TH2 cells became insensitive to IFN-γ-mediated inhibition. Leung et al. (Leung et al. 2011) reported that atopic conditions suppressed the IFN-γ receptor expression on peripheral blood CD4+ T cell. We hypothesized that an imbalance of STAT1/STAT6, particularly their phosphorylation, is observed in the memory CD4+ T cells of asthmatics due to repeated exposure to allergen. First, we compared the change in the ∆MFI of STAT1-py/STAT6-py of asthma patients with that of COPD and healthy subjects. Interestingly, the ∆MFIs of STAT1-py/STAT6-py in asthma was much lower than 1, whereas the ratios in COPD and healthy subjects were closer to 1 or slightly higher than 1, thus indicating an impairment in the STAT1 phosphorylation of memory CD4+ T cells in asthma. Next, to determine whether STAT1 phosphorylation was inhibited in various CD4+ T cell subsets, the cells were divided into naïve CD4+ T cells, TH2 memory T cells, and non-TH2 memory T cells. Our finding demonstrated that the ∆MFI of STAT1-py/STAT6-py shift in asthma contributed to TH2 memory T cells rather than to non-TH2 memory or naive CD4+ T cells. Furthermore, whether the changed STAT1/STAT6 phosphorylation in memory CD4+ T cells affecting the cytokine-releasing capacities was performed.
Previous studies have analyzed the relationship between the cytokine profiles and the clinical characteristics of asthma, such as disease severity, exacerbation risk, and drug consumption, but no consistent results have been obtained (Rudin et al. 2001). We speculate that the use of peripheral PBMCs or CD4+ T cells as target cells may contribute to the inconsistent results obtained in these studies. Long-lived memory CD4+ T cells might be the ideal T cell subset candidates for analyzing the endotype of asthma. Data obtained from a long-term mouse asthma model showed persistent lymphocytic lung infiltration and proved that TH2 memory T cells play a crucial role in the pathogenesis of asthma (Mojtabavi et al. 2002b). We further studied the cytokine production profile of circulating CD4+ T memory cells in asthmatics, COPD patients, and healthy controls. To our excitement, the proportion of IL-5+CD4+ Tm cells and IL-17+CD4+ Tm cells under TH2 or TH2 + HDM culture conditions was considerably increased in the asthma patients compared with the COPD patients and the healthy controls. However, the proportion of IFN-γ+CD4+ Tm cells in asthmatics under either TH2 or TH2 + LPS culture conditions were significantly reduced compared with those of COPD patients and of healthy controls. So, impairment of the Jak/STAT1 pathway in memory CD4+ T cells of asthmatics may play a pivotal role in the development of TH2 cells and the production of TH2-like cytokines(Fig.6).
a During the primary immune response to allergens, disruption of the epithelial barrier ends to epithelial cell production of cytokines, which activate dendritic cell (DC) or macrophage in airway interface. Immune cells such as Th1, Th17, and Th2 cells recruit to the local inflammatory site. b DC maturation and trafficking to tissue draining lymph nodes (LN) where DC activate naive T (TN) cells to differentiate into effector (TEFF) cells (such as Th1, Th2, Th17, etc). TEFF cells reenter the systemic circulation and then the inflamed tissue through postcapillary venules. The majority of TEFF cells eventually die, but a minority differentiates into memory T (TM) cells several weeks after the resolution of inflammation. c Jak-STAT signaling pathway in memory CD4+ T cells. IL-27 induces STAT1 phosphorylation and promotes T-bet transcription and Th1 polarization. IL-4 induces STAT6 phosphorylation and promotes GATA3 transcription and Th2 polarization. Th2/Th1 unbalance was seen in memory CD4+ T cells
Numerous results have revealed the plasticity of long-lived TH2 memory cells. For example, TH2 memory cells redifferentiated into Foxp3+ T cells when stimulated in the presence of retinoic acid (Cosmi et al. 2010). TH17/TH2 memory cells were significantly increased in the circulation of patients with chronic asthma (Kim et al. 2010). In our study, the capacity of IL-17 production in CD4+ Tm cells differed among the three subgroups (healthy, mild asthma, and moderate-severe asthma). The more severe the disease, the more IL-17 was produced. Although the IL-5-producing capacities in CD4+ Tm cells were increased in asthma patients compared with healthy controls, it could not be used to distinguish between mild and severe patients. The percentage of memory CD4+ T cells was positively correlated with asthma medication consumption (R 2 = 0.8296, p < 0.01) rather than IL-5+CD4+ Tm and IFN-γ+CD4+ Tm cells (the asthma medication consumption score was based on the GINA2015 guideline). Our finding was consistent with previously published data in which the number of IL-17-TH2 cells in asthma was increased 5.8-fold compared with healthy donors (Wang et al. 2010). IL-17-producing TH2 memory cells might also represent the inflammatory TH2 cells in promoting the pathogenesis of asthma.
Taken together, the results show that CD4+ memory T cells in circulation were markedly increased in asthma patients. A shift in the balance of STAT1/STAT6 phosphorylation in memory CD4+ T cells contributes to the specific cytokine profiles in asthmatics compared with COPD patients and healthy subjects. Furthermore, one subset of memory CD4+ T cells (IL-17+CD4+ Tm cells) is positively correlated with asthma severity and medication consumption. This study sheds light on the potential of circulation memory CD4+ T cells for exploring asthma pathogenesis, precisely diagnosing asthma, and evaluating disease severity in the future.
Abbreviations
- STATs:
-
Signal transducer and activator of transcription
- CD4+Tm:
-
Memory CD4+ T Cells
- COPD:
-
Chronic obstructive pulmonary disease
- PBMCs:
-
Peripheral blood mononuclear cells
- STAT1-py:
-
STAT1 phosphorylation
- STAT6-py:
-
STAT6 phosphorylation
- LPS:
-
Lipopolysaccharide
- HDM:
-
house dust mite
- ILs:
-
interleukins
- Tem:
-
effector memory T cells
- Tcm:
-
central memory T cells
- Trm:
-
resident memory T cells
- Treg:
-
T regulatory cell
- MFIs:
-
Mean fluorescence intensities
- GINA:
-
Global Initiative for Asthma
References
Abdulamir AS, Hafidh RR, Abubakar F, Abbas KA. Changing survival, memory cell compartment, and T-helper balance of lymphocytes between severe and mild asthma. BMC Immunol. 2008;9:–73.
Chapoval SP, Dasgupta P, Smith EP, DeTolla LJ, Lipsky MM, Kelly-Welch AE, et al. STAT6 expression in multiple cell types mediates the cooperative development of allergic airway disease. J Immunol. 2011;186:2571–83.
Chen Z, Wang X, Gao L, Bai L, Zhu R, Bai C. Regulation of MUC5AC mucin secretion by depletion of AQP5 in SPC-A1 cells. Biochem Biophys Res Commun. 2006;342:775–81.
Chen Z, Wang S, Erekosima N, Li Y, Hong J, Qi X, et al. IL-4 confers resistance to IL-27-mediated suppression on CD4+ T cells by impairing signal transducer and activator of transcription 1 signaling. J Allergy Clin Immunol. 2013;132:912–21.
Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–30.
Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35.
Fukushima A, Yamaguchi T, Ishida W, Fukata K, Udaka K, Ueno H. Mice lacking the IFN-gamma receptor or fyn develop severe experimental autoimmune uveoretinitis characterized by different immune responses. Immunogenetics. 2005;57:337–43.
Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood. 2005;106:2011–7.
Kalliolias GD, Ivashkiv LBIL. 27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–33.
Kim BS, Kim IK, Park YJ, Kim YS, Kim YJ, Chang WS, et al. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc Natl Acad Sci U S A. 2010;107:8742–7.
Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73.
Lumsden JM, Schwenk RJ, Rein LE, Moris P, Janssens M, Ofori-Anyinam O, et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-alpha producing effector and central memory CD4 T cells. PLoS One. 2011;6:e20775.
MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: what are they and what can they do? Semin Immunol. 2009;21:53–61.
MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunol. 2010;130:10–5.
Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12:611–29.
Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002a;169:4788–96.
Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002b;169:4788–96.
Nakagome K, Dohi M, Okunishi K. To Y, Sato A, Komagata Y, et al. Antigen-sensitized CD4+CD62Llow memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res. 2005;6:46–52.
Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–9.
Rudin A, Macaubas C, Wee C, Holt BJ, Slya PD, Holt PG. Bystander" amplification of PBMC cytokine responses to seasonal allergen in polysensitized atopic children. Allergy. 2001;56:1042–8.
Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–97.
Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58.
Tang X, Chen XK. Z1. Memory T cells and asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38:69–71.
Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol. 2014;5:331.
Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–91.
Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–80.
Xiaoqiong S, Jue P, Jianjun J, et al. Intranasal administration of interleukin-27 alleviate the airway allergic inflammation of ovalbumin-induced mouse asthma model via the STAT1 signal pathway. Zhongguo Hu; Xi He Wei Zhong Jian Hu. 2015;14(5):425–31.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81270078 and 81470211 to ZHC) and the “Zhengyi” Scholar Program of Fudan University (JYH6273202/002/023/014 to GS and SXC).
Author contributions
ZHC, XDW, LZ, and JP conceived and designed the study. ZHC, DDL, ZHM, XQS, HLY, GS, and SXC performed the biological experiments. YJ was responsible for the statistical analysis. ZHC and JP wrote the paper. All the authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Zhihong Chen and Jue Pan contributed equally to this work.
Electronic supplementary material
Suppl Fig. 1
No liner correlations between IL-17+CD4+Tm cell number and several clinical characteristics (Eos, IgE and FEV1) (PDF 169 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., Pan, J., Jia, Y. et al. Effect of memory CD4+ T cells’ signal transducer and activator of transcription (STATs) functional shift on cytokine-releasing properties in asthma. Cell Biol Toxicol 33, 27–39 (2017). https://doi.org/10.1007/s10565-016-9357-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-016-9357-6