Abstract
Endogenous interferon (IFN)-γ negatively regulates experimental autoimmune uveoretinitis (EAU), a Th1-mediated disease. Although it is well known that IFN-γ exerts its effects by binding to the IFN-γ receptor (IFN-γR), the role that IFN-γR plays in the development of EAU has not been investigated. Fyn has been reported to inhibit Th2 differentiation. We aimed to investigate how endogenous IFN-γR and fyn, which influence Th1/Th2 differentiation, participate in the development of EAU. Sex-matched 6- to 10-week-old C57BL/6 wild-type (WT), IFN-γR knockout (GRKO) and fyn knockout (fyn KO) mice were compared. Mice were immunized subcutaneously with human interphotoreceptor retinoid-binding protein peptide 1–20 emulsified in Freund’s complete adjuvant together with an intraperitoneal injection of Bordetella pertussis toxin. Three weeks later, mice were sacrificed, and their eyes and spleens were harvested for histopathologic analyses and examination of cellular immune responses, respectively. Cellular immune responses were evaluated by measuring the proliferative responses and cytokine production [interleukin (IL)-4, IL-5, IL-6, IL-13, IFN-γ and tumor necrosis factor (TNF)-α] of splenocytes. The incidence of EAU was 40.0% in WT mice, 59.3% in GRKO mice and 78.6% in fyn KO mice. The average EAU score was 0.294 in WT mice, 0.917 in GRKO mice and 1.063 in fyn KO mice. Upon EAU induction, significant infiltration of eosinophils into the eyes was observed in GRKO and fyn KO mice compared to WT mice. Splenocytes from GRKO mice proliferated against the antigen and a mitogen more vigorously than those from WT and fyn KO mice. Stimulation of splenocytes with the antigen induced a higher production of IL-4, IL-6, IL-13 and IFN-γ in GRKO mice compared to WT and fyn KO mice. In contrast, IL-5 and TNF-α were most abundantly produced by splenocytes from fyn KO mice compared to WT and GRKO mice. The incidence and mean severity of EAU were significantly higher in GRKO and fyn KO mice than in WT mice, suggesting that endogenous IFN-γR and fyn negatively regulate the development of EAU. The different cytokine production patterns by the GRKO and fyn KO mice indicate that the negative regulatory mechanism mediated by IFN-γR and fyn may differ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T cells, in particular, interferon-γ (IFN-γ)-producing Th1 cells, are involved in the development of organ-specific autoimmune diseases such as experimental autoimmune uveoretinitis (EAU) (Caspi 2002). Since Th2 cells counteract Th1 cell activity, the possibility that Th2 cells could inhibit the development of EAU has been raised. Supporting this notion is that the activation of Th2 cells in vivo successfully suppresses Th1 immune responses and blocks the development of EAU (Saoudi et al. 1993). Thus, the interaction between Th1 and Th2 cells may play an important role in the development of EAU. However, the relationship between Th1 and Th2 cells in EAU is by no means clear. This is shown by studies examining the role IFN-γ plays in EAU.
IFN-γ is an important effector cytokine produced by Th1 cells (Mosmann and Coffman 1989), and it has been speculated that IFN-γ may play a positive role in the development of EAU, a Th1-mediated disease. However, treatment of mice with a neutralizing monoclonal antibody specific to IFN-γ revealed that endogenous IFN-γ actually inhibits the development of EAU in mice (Caspi et al. 1994). In addition, the severity of EAU in IFN-γ knockout (GKO) mice is higher than that in wild-type (WT) mice (Jones et al. 1997; Avichezer et al. 2000). Taken together, endogenous IFN-γ appears to inhibit, rather than augment, the development of EAU.
The differentiation of naive Th cells into Th2 cells is regulated by many factors, including cytokines (Seder and Paul 1994) and costimulatory molecules (Harris and Ronchese 1999). In addition, molecules participating in T cell receptor (TcR) signaling have recently been shown to be involved in this differentiation process (Badou et al. 2001; Brogdon et al. 2002). Of the many molecules involved in TcR signaling, the T-cell-expressed src-family kinases p56lck (Lck) and p59fyn (fyn) are believed to be the first molecules to mediate the downstream passage of the signals from TcR (Zamoyska et al. 2003). Moreover, fyn has been reported to be involved in the differentiation of naive Th cells into Th2 cells. For example, fyn expression in Th2 clones is one third to one fifth the level found in Th1 clones (Tamura et al. 1995). Furthermore, fyn−/− T cells differentiate into Th2 cells even in the absence of IL-4 (Tamura et al. 2001). Thus, fyn appears to negatively regulate the differentiation of naive Th cells into Th2 cells.
We have previously demonstrated that endogenous fyn negatively regulates the development of experimental immune-mediated blepharoconjunctivitis (EC) (Fukushima et al. 2005), a Th2-mediated disease. However, the role fyn plays in the development of Th1-mediated EAU has not been evaluated. Here, to investigate the involvement of endogenous fyn in the development of EAU, we induced EAU in fyn knockout (fyn KO) mice and compared it to the EAU induced in WT mice. Moreover, to further assess the role IFN-γ plays in the development of EAU, we induced EAU in IFN-γ receptor (IFN-γR) knockout (GRKO) mice. Since IFN-γ exerts its effects by binding to IFN-γR (Celada 1988), it is likely that the EAU phenotype in GRKO mice is similar to that in GKO mice. However, the EAU phenotype in GRKO mice has not been investigated previously.
Materials and methods
Mice
C57BL/6 WT mice were purchased from Japan SLC Inc., Hamamatsu, Shizuoka, Japan. Homozygous IFN-γR1-chain-disrupted mice (Swihart et al. 1995) on the C57BL/6 background were kindly provided by Dr. Tadatsugu Taniguchi (Tokyo University, Japan) with the permission of Dr. Michel Aguet (University of Zurich, Switzerland). Homozygous C57BL/6 background fyn KO mice (Yagi et al. 1993) were kindly provided by Dr. Takeshi Yagi (Osaka University, Japan). The mice were maintained at Kochi Medical School’s animal facility. Sex-matched 6- to 10-week-old mice were used for all experiments. All research adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents
Human interphotoreceptor retinoid-binding protein (hIRBP) peptide 1–20 (GPTHLFQSLVLDMAKVLLD, Sawady Technology, Tokyo, Japan) was synthesized using t-butyloxycarbonyl amino acid derivatives and purified by HPLC to at least 95% purity. Purified Bordetella pertussis toxin (PTX), concanavalin A (Con A) and Freund’s complete adjuvant (FCA) were purchased from Sigma Chemical Co., St. Louis, MO. Purified protein derivative (PPD) was obtained from Japan BCG Co., Tokyo, Japan. Mycobacterium tuberculosis strain H37RA was from Difco, Detroit, MI, USA.
Induction of EAU
Mice were immunized subcutaneously in two sites (neck and base of tail) with hIRBP peptide (75 μg/site) in an emulsion with FCA that had been supplemented with M. tuberculosis strain H37RA to 2.5 mg/ml (total volume per mouse, 200 μl). The mice were intraperitoneally injected with 1.5 μg of PTX at the same time. Twenty-one days later, the mice were sacrificed, and their eyes and spleens were harvested for histopathologic analyses and evaluation of cellular immune responses, respectively.
Evaluation of EAU
Enucleated eyes were fixed in 10% buffered formalin for 48 h and then embedded in paraffin, cut into 4-μm-thick sections and stained with Giemsa. The incidence and severity of EAU in each eye were examined by two of us (A.F. and W.I.) in a masked fashion and scored by the method described elsewhere (Chan et al. 1990). When the score of the two observers differed, the average was calculated. The data presented show the averages of both eyes per mouse. In addition, the number of intraocular (vitreous, retina and choroids) infiltrating eosinophils was counted per section by an observer (W.I.) in a masked fashion. Data are presented as the average±SEM of each group.
Cellular proliferation assay
The splenocytes from each group of mice were combined per group, and the experiments were repeated at least three times. As a control, the splenocytes from three different types of naive mice were used. RBC-depleted splenocytes (3×105 cells/well) were cultured in 96-well flat-bottom plates in a final volume of 0.2 ml RPMI 1640 medium supplemented with 5% fetal calf serum (FCS; ICN Biomedical Japan Co., Tokyo, Japan) and 2-mercaptoethanol (2-ME, 5×10−5 M). The cells were stimulated with hIRBP peptide at final concentrations of 0.1, 0.5, 1 and 10 μg/ml; PPD at 5 μg/ml; or Con A at 5 μg/ml. After an 80-h incubation at 37°C in a humidified atmosphere containing 5% CO2, the cultures were pulsed for 16 h with 0.5 μCi/well [3H]thymidine (Japan Atomic Energy Research Institute, Tokai, Japan). The cultures were then harvested, and the incorporated radioactivity was measured by standard techniques. The data were expressed as stimulation indices [mean counts per minute (cpm) of Ag-stimulated cultures/mean cpm of unstimulated control cultures].
Measurement of cytokines in the culture supernatants
RBC-depleted splenocytes (107 cells/ml) were cultured for 48 h with Con A (10 μg/ml), PPD (5 μg/ml) or hIRBP peptide (10 μg/ml) in 96-well flat-bottom plates in a final volume of 0.2 ml RPMI 1640 medium supplemented with 10% FCS and 2-ME. The culture supernatants of unstimulated splenocytes served as the negative controls. The levels of IL-4, IL-5, IL-6, IL-13, IFN-γ and tumor necrosis factor (TNF)-α in the culture supernatants were measured by using the commercially available cytokine kits (Duoset, R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendations. Data were normalized by the number of CD4+ cells at the beginning of the culture, confirmed by flow cytometric analysis (4×105 CD4+ cells per well).
Statistical analyses
Statistical analyses of parametric data (infiltrating eosinophil number into the eye, proliferation and cytokine assays) were performed using an independent Student’s t-test. The EAU scores were analyzed by Mann–Whitney U-test.
Results
Actively induced EAU is more severe in GRKO and fyn KO mice compared to WT mice
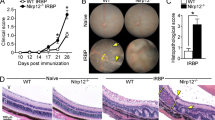
To compare the incidence and severity of EAU among WT (n=40), GRKO (n=27) and fyn KO (n=14) mice, the mice were actively immunized with hIRBP peptide. Figure 1 shows the distribution of EAU incidence and severity in each group and reveals that more GRKO and fyn KO mice developed EAU (16/27 and 11/14, respectively) than WT mice (16/40). In addition, the EAU was significantly more severe in GRKO (mean score 0.917, P=0.03) and fyn KO mice (mean score 1.063, P=0.002) than in WT mice (mean score 0.294). As shown in Fig. 2a–c, inflammatory cells consisting predominantly of mononuclear cells infiltrated the retina in WT mice with EAU. The retina in EAU-developing GRKO (Fig. 2d–f) and fyn KO mice (Fig. 2g–i) was also infiltrated by inflammatory cells, but these were composed not only of mononuclear cells but also eosinophils. The severity of cellular infiltration and granulomas was apparently more severe in GRKO and fyn KO mice than in WT mice (Fig. 2). As shown in Fig. 3, counting of eosinophils in the eye confirmed that significantly more eosinophis infiltrated into the eye in GRKO (average±SEM=123.15±44.73, P<0.01) and fyn KO mice (74.69±22.44, P<0.001) compared to WT mice (13.31±4.88).
Comparison of EAU grades in actively immunized WT, GRKO and fyn KO mice. C57BL/6 background WT, GRKO and fyn KO mice were immunized with hIRBP peptide (150 μg per mouse), and the histopathology of their enucleated eyes was analyzed for EAU grading 21 days later. Each point represents one mouse (the average of both eyes). The data shown are compilations of three to four sets of experiments. Horizontal bars show the average EAU scores. P values depicted are compared with WT mice
WT, GRKO and fyn KO mice with EAU show disparate immune responses
EAU is mediated by cellular, not humoral, immune responses. Therefore, it is possible that the variation in severity and histopathologic features of the EAU in the two kinds of KO mice relative to the EAU in the WT mice is due to different cellular immune responses. To investigate whether the cellular immune responses of WT, GRKO and fyn KO mice with EAU differ, we examined the proliferative ability of their splenocytes and the cytokine profile they produce when stimulated by hIRBP peptide, Con A or PPD. While the splenocytes from all three mouse types proliferated against hIRBP peptide in a concentration-dependent manner, the GRKO splenocytes proliferated most strongly (Fig. 4a). In contrast, the splenocytes from three different types of naive mice did not proliferate against hIRBP peptide (Fig. 4a). The proliferative responses by the GRKO mice were also higher when the stimulants were either Con A or PPD (Fig. 4b).
Proliferative responses by splenocytes from actively immunized WT, GRKO and fyn KO mice. Splenocytes were harvested 21 days after immunization and examined for their proliferative capabilities against hIRBP peptide (a) and PPD or Con A (b). As a control, the splenocytes from three different types of naive mice were used (a). Background (unstimulated) cpm±SEM of immunized mice was 388±15 in WT mice, 422±29 in GRKO mice and 1,099±54 in fyn KO mice (a, b), and the cpm±SEM of immunized mice at 10 μg/ml of hIRBP peptide was 3,057±186 in WT mice, 15,967±914 in GRKO mice and 5,949±436 in fyn KO mice (a). *P<0.05, **P<0.01 compared to WT mice
With regard to cytokine production, the profile induced by the three mouse types differed depending on the stimulant used (Fig. 5). When the stimulant was the specific Ag (hIRBP peptide), GRKO splenocytes produced higher amounts of IL-4, IL-6, IL-13 and IFN-γ than the splenocytes from WT or fyn KO mice, whereas fyn KO splenocytes produced more IL-5 and TNF-α than WT or GRKO splenocytes. In contrast, when the stimulant was a mitogen (Con A), GRKO splenocytes produced more IL-13 and IFN-γ than WT or fyn KO splenocytes, whereas fyn KO splenocytes produced more IL-4, IL-5, IL-6 and TNF-α than WT or GRKO splenocytes.
Cytokine production by splenocytes from actively immunized WT, GRKO and fyn KO mice. Splenocytes were stimulated with Con A, PPD or hIRBP peptide for 48 h, and the IL-4, IL-5, IL-6, IL-13, IFN-γ and TNF-α levels in the culture supernatants were measured. As a control, the culture supernatant of splenocytes without any stimulant (none) was used for measuring cytokines. Data (pg/ml) were normalized by the number of CD4+ cells at the beginning of culture (4×105 CD4+ cells per well). *P<0.05 compared to WT mice
Discussion
Here, we showed that the incidence and severity of EAU were higher in GRKO and fyn KO mice, which suggests that endogenous IFN-γR and fyn negatively regulate the development of EAU. Notably, the nature of the cells infiltrating the eye also differed depending on the mouse strain, as many eosinophils infiltrated the eye in GRKO and fyn KO mice, whereas eosinophils were much less in the WT infiltrates. Similar observations have been made using GKO mice (Jones et al. 1997; Avichezer et al. 2000). These changes are not only restricted to EAU, a Th1-driven ocular disease, but also observed in Th1-mediated diseases in other organs. For example, higher mortality was demonstrated in GKO and GRKO mice after EAE induction (Ferber et al. 1996; Willenborg et al. 1996). In addition, massive infiltration of granulocytes in the spinal cord was demonstrated. In these knockout mice, immune responses were dominantly Th2 phenotype. Although it has not been reported that Th1-mediated diseases are affected by the genetic ablation of fyn, it could be considered that upregulated Th2 immune responses in fyn KO mice as well as GKO and GRKO mice induce more severe EAU and eosinophil infiltration in the eye.
EAU has been reported to be inducible by transfer of Ag-primed lymphocytes, especially by T cells (Rizzo et al. 1996; Caspi 2002). In accord with this notion, in our hands, the transfer of Ag-primed splenocytes from WT and GRKO mice stimulated in vitro with hIRBP peptide was able to induce EAU in recipient WT mice (unpublished result). Furthermore, transfer of Ag-primed splenocytes from GRKO mice induced infiltration of eosinophils in recipient WT mice, whereas that from WT mice did not induce eosinophilic infiltration in recipient WT mice (unpublished results). Therefore, it could be considered that Ag-primed splenocytes play an important role in the initiation of EAU. Thus, a characterization of Ag-primed splenocytes would provide some clues to understand the different features of EAU among WT, GRKO and fyn KO mice. In addition, the different EAU features of GKO mice were attributed to the disparate cytokine profile of their immunocompetent cells (Jones et al. 1997; Avichezer et al. 2000). To test whether a similar phenomenon could explain our own observation, we compared the cellular immune responses of the splenocytes obtained from these three groups of mice after active immunization with hIRBP peptide.
First, we examined the proliferative responses of the splenocytes to the immunizing Ag or a mitogen and found that the GRKO splenocytes proliferated more profusely than the other splenocytes regardless of the stimulant used. This suggests that the interaction between IFN-γ and IFN-γR inhibits the proliferative responses of splenocytes. This is consistent with previous publications demonstrating that IFN-γ suppresses the expansion of T cells, possibly in an apoptosis-mediated fashion (Novelli et al. 1996). The proliferative responses of the splenocytes from fyn KO mice did not differ from those of WT mice regardless of the stimulant used. These observations together suggest that while endogenous IFN-γR and fyn are both involved in preventing the deterioration of EAU, they do so by different mechanisms.
Previous publications suggest that Th2 responses in GRKO (Shimizu et al. 2004) and fyn KO mice (Fukushima et al. 2005; Kudlacz et al. 2001) are generally upregulated relative to WT mice. To test this, we measured the concentrations of six cytokines released in the culture supernatants by stimulated splenocytes from GRKO and fyn KO mice with EAU. As indicated by previous studies, the Th2 cytokines were generally upregulated in both GRKO and fyn KO mice regardless of the stimulant used. However, the specific Th2 cytokines that were upregulated depended on the mouse strain. Comparing the GRKO and the fyn KO splenocyte responses, the recall Ag induced significantly higher IL-13 production by GRKO splenocytes but significantly higher IL-5 levels by fyn KO splenocytes. Thus, the involvement of endogenous IFN-γR and fyn in Ag-specific Th2 development appears to be different, which may explain why IFN-γR and fyn appear to be differently involved in the development of EAU.
Extremely high levels of IFN-γ were produced by GRKO splenocytes regardless of the stimulant used. This may reflect the possibility that the IFN-γ produced is not being consumed because of the absence of IFN-γR. While IFN-γ generally exerts its effects through IFN-γR (Celada 1988), it has been reported that IFN-γ has a direct cytotoxic effect that is not mediated by IFN-γR (Rodrigues and Travassos 2002). However, it is not yet known whether this direct effect is involved in the development of EAU. Therefore, it remains unclear whether the high IFN-γ production in GRKO mice is related to the severity of EAU. The precise role of IFN-γ during the effector phase of EAU remains to be clarified in the future.
TNF-α is known to augment the development of EAU by upregulating the expression of adhesion molecules, activating macrophages and driving into Th1 responses (Dick et al. 2004). In this study, TNF-α was most abundantly detected in the culture supernatant of splenocytes from fyn KO mice compared to the other two groups. This high production of TNF-α by fyn KO mice may be responsible, at least in part, for the greater severity of the EAU in these mice. Since it is not clear how fyn participates in TNF-α production by T cells, it may be of interest to investigate the involvement of fyn in the induction of TNF-α at the molecular level.
The involvement of IL-6 in the development of EAU has been less thoroughly investigated than other cytokines such as IFN-γ and TNF-α. However, it has been suggested that IL-6 counteracts TGF-β in the aqueous humor and inhibits the immunomodulation exerted by the aqueous humor during the development of EAU (Ohta et al. 2000). Although IL-6 was examined in our study by using culture supernatants only, the high production of IL-6 by the GRKO and fyn KO splenocytes suggests that this cytokine may be involved in inhibiting the immunomodulatory roles of the aqueous humor, thereby increasing the severity of the EAU in GRKO and fyn KO mice. Notably, a previous report showed that GKO and WT splenocytes produced equivalent IL-6 levels (Avichezer et al. 2000). This suggests that IFN-γ exerts its effects not only through IFN-γR but also through other pathways.
From the data of cytokine analyses using splenocytes, the strongest production of IFN-γ was noted in GRKO mice, whereas the strongest IL-4 production was detected in fyn KO mice. Because IL-4, which is a Th2 cytokine and is involved in the infiltration of eosinophils, was upregulated in the splenocytes of fyn KO mice, it is reasonable that eosinophils infiltrated in the eye of fyn KO mice. Although IFN-γ, which is a Th1 cytokine and inhibits Th2 responses, was upregulated in the splenocytes of GRKO mice, massive infiltration of eosinophils was observed in the eye of GRKO mice. This contradiction might be explained by the fact that the effects of IFN-γ are dominantly mediated through IFN-γR (Celada 1988), and therefore, the suppressive effects of IFN-γ on the infiltration of eosinophils were little, if any, in GRKO mice.
Taken altogether, our observations suggest that endogenous IFN-γR and fyn negatively regulate the development of EAU but via different mechanisms.
References
Avichezer D, Chan CC, Silver PB, Wiggert B, Caspi RR (2000) Residues 1–20 of IRBP and whole IRBP elicit different uveitogenic and immunological responses in interferon gamma deficient mice. Exp Eye Res 71:111–118
Badou A, Savignac M, Moreau M, Leclerc C, Foucras G, Cassar G, Paulet P, Lagrange D, Druet P, Guery JC, Pelletier L (2001) Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur J Immunol 31:2487–2496
Brogdon JL, Leitenberg D, Bottomly K (2002) The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J Immunol 168:3825–3832
Caspi RR (2002) Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol 21:197–208
Caspi RR, Chan CC, Grubbs BG, Silver PB, Wiggert B, Parsa CF, Bahmanyar S, Billiau A, Heremans H (1994) Endogenous systemic IFN-gamma has a protective role against ocular autoimmunity in mice. J Immunol 152:890–899
Celada A (1988) The interferon gamma receptor. Lymphokine Res 7:61–73
Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, Nussenblatt RB (1990) Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun 3:247–255
Dick AD, Forrester JV, Liversidge J, Cope AP (2004) The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res 23:617–637
Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG (1996) Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 156:5–7
Fukushima A, Yamaguchi T, Ozaki A, Taniguchi T, Udaka K, Ueno H (2005) Fyn regulates eosinophil infiltration into the conjunctiva by downregulating the Th2 response. Graefe Arch Clin Exp Ophthalmol (in press)
Harris NL, Ronchese F (1999) The role of B7 costimulation in T-cell immunity. Immunol Cell Biol 77:304–311
Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR (1997) IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol 158:5997–6005
Kudlacz EM, Andresen CJ, Salafia M, Whitney CA, Naclerio B, Changelian PS (2001) Genetic ablation of the src kinase p59fynT exacerbates pulmonary inflammation in an allergic mouse model. Am J Respir Cell Mol Biol 24:469–474
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173
Novelli F, Bernabei P, Ozmen L, Rigamonti L, Allione A, Pestka S, Garotta G, Forni G (1996) Switching on of the proliferation or apoptosis of activated human T lymphocytes by IFN-gamma is correlated with the differential expression of the alpha- and beta-chains of its receptor. J Immunol 157:1935–1943
Ohta K, Wiggert B, Yamagami S, Taylor AW, Streilein JW (2000) Analysis of immunomodulatory activities of aqueous humor from eyes of mice with experimental autoimmune uveitis. J Immunol 164:1185–1192
Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan CC, Caspi RR (1996) Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10. A mice. J Immunol 156:1654–1660
Rodrigues EG, Travassos LR (2002) Endogenous accumulation of IFN-gamma in IFN-gamma-R(−/−) mice increases resistance to B16F10-Nex2 murine melanoma: a model for direct IFN-gamma anti-tumor cytotoxicity in vitro and in vivo. Cytokines Cell Mol Ther 7:107–116
Saoudi A, Kuhn J, Huygen K, de Kozak Y, Velu T, Goldman M, Druet P, Bellon B (1993) TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol 23:3096–3103
Seder RA, Paul WE (1994) Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol 12:635–673
Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN (2004) Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest 114:300–308
Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Giudice G, Aguet M, Louis JA (1995) Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med 181:961–971
Tamura T, Nakano H, Nagase H, Morokata T, Igarashi O, Oshimi Y, Miyazaki S, Nariuchi H (1995) Early activation signal transduction pathways of Th1 and Th2 cell clones stimulated with anti-CD3. Roles of protein tyrosine kinases in the signal for IL-2 and IL-4 production. J Immunol 155:4692–4701
Tamura T, Igarashi O, Hino A, Yamane H, Aizawa S, Kato T, Nariuchi H (2001) Impairment in the expression and activity of fyn during differentiation of naive CD4+ T cells into the Th2 subset. J Immunol 167:1962–1969
Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA (1996) IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol 157:3223–3227
Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y (1993) A role for fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature 366:742–745
Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B (2003) The influence of the src-family kinases, lck and fyn, on T cell differentiation, survival and activation. Immunol Rev 191:107–118
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukushima, A., Yamaguchi, T., Ishida, W. et al. Mice lacking the IFN-γ receptor or fyn develop severe experimental autoimmune uveoretinitis characterized by different immune responses. Immunogenetics 57, 337–343 (2005). https://doi.org/10.1007/s00251-005-0805-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-005-0805-3