Abstract
Telocytes (TCs) are typically defined as cells with telopodes by their ultrastructural features. Their presence was reported in various organs, however little is known about their presence in human trigeminal ganglion. To address this issue, samples of trigeminal ganglia were tested by immunocytochemistry for CD34 and examined by transmission electron microscopy (TEM). We found that TCs are CD34 positive and form networks within the ganglion in close vicinity to microvessels and nerve fibers around the neuronal–glial units (NGUs). TEM examination confirmed the existence of spindle-shaped and bipolar TCs with one or two telopodes measuring between 15 to 53 μm. We propose that TCs are cells with stemness capacity which might contribute in regeneration and repair processes by: modulation of the stem cell activity or by acting as progenitors of other cells present in the normal tissue. In addition, further studies are needed to establish if they might influence the neuronal circuits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different morphological studies indicated the presence of cells with a peculiar morphology, the telocytes (TCs), in many organs and in various species (Cretoiu and Popescu 2014; Rusu et al. 2012c; Vannucchi et al. 2015; Yang et al. 2015; Zhang et al. 2015). Telocytes are a distinct type of interstitial cells characterized by a small cell body and extremely long and thin processes named telopodes (Tps), with uneven caliber (Bei et al. 2015a; Cretoiu and Cretoiu 2016; Popescu and Faussone-Pellegrini 2010; Rusu 2014; Rusu et al. 2014a; Rusu et al. 2014b). Additional information about Tps spatial configuration was brought recently by FIB-SEM tomography (Cretoiu et al. 2015a; Cretoiu et al. 2014).
Although in transmission electron microscopy TCs could be identified by their morphology, a distinctive marker for these cells has not been yet demonstrated. Several markers have been found to be expressed on TCs, such as CD34, CD117, vimentin, PDGFRα/β, and α-SMA (Campeanu et al. 2014; Diaz-Flores et al. 2013; Manole et al. 2015; Rusu et al. 2012d; Rusu et al. 2011). For example, Bei et al. showed that cardiac TCs are positive for CD34/c-kit, CD34/vimentin, and CD34/PDGFR-β (Bei et al. 2015c), while in other organ systems (i.e., human gastrointestinal tract), TCs express either CD34 or PDGFRα but do not express c-kit, which makes possible to distinguish gut telocytes, which are CD34-positive/PDGFRα-positive/c-kit-negative, and interstitial cells of Cajal, which instead are CD34-negative/PDGFRα-negative/c-kit-positive (Manetti et al. 2015; Milia et al. 2013; Vannucchi et al. 2013). In addition, various studies reported that the co-expression of CD34 and PDGFRα antigens can be used as the most reliable method for TCs identification, especially in cell cultures (Diaz-Flores et al. 2014; Vannucchi et al. 2013; Zhou et al. 2015).
Recently, there is growing evidence for a evident distinction between fibroblasts and TCs based on microRNA signatures of TCs and studies of gene-expression and proteomic profiles (Albulescu et al. 2015; Cismasiu and Popescu 2015; Cismasiu et al. 2011; Song et al. 2015; Sun et al. 2014; Wang et al. 2015; Zheng et al. 2014; Zhu et al. 2015). Various roles have been attributed to TCs, such as stromal signaling or participation in regenerative processes (Bei et al. 2015b; Roatesi et al. 2015; Rusu et al. 2014b).
Within stromal compartments TCs build three-dimensional networks which may belong to an integrated system which maintains tissue/organ function (Vannucchi et al. 2015). Indeed, TCs interrelations with each other as well as with other cells types or structure point out toward their affiliation to a intercellular signaling system (Cretoiu et al. 2012; Gherghiceanu and Popescu 2012; Popescu et al. 2015), hypothesis strengthened by the fact that they are also able to release extracellular vesicles (Cretoiu et al. 2015a; Cretoiu et al. 2013; Fertig et al. 2014). In adult, TCs could be considered mesenchymal stromal (stem) cells (MSCs) with multilineage potential (interstitial Cajal cells, fibroblasts, myofibroblasts) (Vannucchi et al. 2015). Indeed, it was discussed that CD34-expressing stromal fibroblastoid cells, which were applied confusing terms such as fibroblasts, fibrocytes, telocytes, gain MSCs potentiality after they lose the expression of CD34 (Diaz-Flores et al. 2015b; Diaz-Flores et al. 2014).
In view of these recent concept updates, this study aims to test the expression of CD34 within the human adult trigeminal ganglion, which was previously documented for housing c-kit + fibroblastoid interstitial cells (Rusu et al. 2011), as well as quiescent stem/progenitor cells included within the satellite glial cells sheaths (Rusu et al. 2013; Rusu et al. 2014c). Cells positive for CD34 were also examined by transmission electron microscopy (TEM) which revealed the ultrastructural peculiarities suggestive for TCs.
Materials and method
Tissue samples
For the present study, autopsy samples of trigeminal ganglia were dissected out from 15 adult cadavers (sudden cardiac or traumatic deaths), in the “Mina Minovici” Institute of Legal Medicine, Bucharest. Donor cases were not diagnosed nor died from neurodegenerative disorders; the mean age was 62 years and the sex ratio was 3:2. Approval for the present study was granted by the Bioethics Committee of the “Mina Minovici” Institute of Legal Medicine, Bucharest, according to the generally accepted international standards and national laws.
Immunohistochemistry
Tissue samples, fixed for 24 h in buffered formalin (8 %), were processed with an automatic histoprocessor (Diapath, Martinengo, BG, Italy) with paraffin embedding. Sections were cut manually at 3 μm and mounted on SuperFrost® electrostatic slides for immunohistochemistry (Thermo Scientific, Menzel-Gläser, Braunschweig, Germany). To assess an accurate embedding and tissue integrity the 3-μm thick sections were stained with hematoxylin and eosin (HE).
Samples of trigeminal ganglia were tested by immunocytochemistry using a primary antibody against CD34 (clone QBEnd 10, Dako, Glostrup Denmark, 1:50). Sections were deparaffinized, rehydrated, and rinsed in PBS buffer solution at pH 7.4. Retrieval by incubation in EDTA, pH 9 was completed. The standard ABC technique used a DAB protocol. Appropriate blocking of endogenous peroxidase was completed before immune labeling (Peroxidazed 1, Biocare Medical, Concord, CA, USA). Sections incubated with non-immune serum served as negative controls. The immune labeled sections were counterstained with hematoxylin.
The microscopic slides were analyzed and micrographs were acquired and scaled using a Zeiss working station which was described elsewhere (Rusu et al. 2012b).
Transmission electron microscopy
Small tissue fragments about 1–2 mm3 were prefixed in fresh ice-cold 4 % glutaraldehyde in sodium cacodylate buffer, pH 7.4 for 4 h at 4 °C. After fixation, the tissues were washed 6× in 0.05 M sodium cacodylate buffer (pH 7.4) at 4 °C, postfixed in 2 % osmium tetroxide in 0.1 M sodium cacodylate at room temperature for 2.5 h, stained en bloc with 0.5 % aqueous uranyl acetate overnight at 4 °C and washed with 0.05 M sodium cacodylate buffer. After dehydration in graded series of ethanol and infiltration with propylene oxide, specimens were embedded in Glycid ether (Epon 812-equivalent) and finally polymerized at 60 °C for 48 h. Semithin sections were stained with 1 % toluidine blue for light microscopy. Ultrathin sections (80–100 nm) were cut using a diamond knife and collected on 200-mesh copper grids, and double counterstained with uranyl acetate and subsequently lead citrate. The grids were examined in a Philips electron microscope EM 208S operated at an acceleration voltage of 80 kV. An image acquisition system consisting of a video camera Veleta and the iTEM Olympus Soft Imaging System was used.
Results
General histological structure of the trigeminal ganglion
The morphological features of the trigeminal ganglion (TG) were examined on histological slides stained with HE, as well as on semithin slides stained with toluidine blue. Different characteristic structures were identified: the neuronal–glial units (NGUs), intrinsic nerve bundles, and connective stroma (interstitial tissue) with microvessels, myelinated and unmyelinated nerve fibers, rare mast cells, and various other cells stromal cells difficult to identify precisely under light microscopy.
The NGUs of the TG, which showed a tendency to cluster, consisted of trigeminal neurons (usually single neurons, but also neuronal doublets and triplets were observed), surrounded by satellite glial cells (SGCs) forming a discontinuous neuronal envelope under light microscopy. Over the envelope built up by SGCs, the ganglionic interstitial tissue (connective stroma) appears to create distinctive layers of variable thickness. The interstitial tissue separating the NGUs was continuous with the connective sheaths of the intraganglionic nerve fascicles.
CD34-expressing cells of the trigeminal ganglion
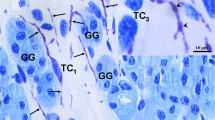
Immunohistochemistry for CD34 revealed positive interstitial cells with long and moniliform processes, chained in networks within the ganglion (Fig. 1). The networks were found in close vicinity to microvessels and nerve fibers and were also detected around NGUs. CD34 expression was also found, as expected, in vascular endothelial cells.
CD34 labeling of human adult trigeminal ganglion. a. CD34+ stromal cells (arrows) with long, slender, and moniliform processes surrounding the neuronal–glial units and the intraganglionic nerves (scale bar 50 μm). b, c Detailed telocytes positive for CD34 are indicated by white arrows (scale bar 20 μm). One can note their moniliform telopodes bordering the neuronal envelope. Blood vessels are indicated with arrowheads
Ultrastructural identification of telocytes within the trigeminal ganglion
Transmission electron microscopy (TEM) examination of human trigeminal ganglia showed NGUs as well as nerve fascicles and fibers, with the corresponding Schwann cells. In between NGUs, the interstitial tissue comprises microvessels of various calibers, having their walls built up from endothelial cells surrounded occasionally by discontinuous pericytes.
Telocytes, usually spindle-shaped and bipolar, with one or two telopodes visible on a single section (with lengths ranging from 15 to 53 μm) were identified at lower magnifications, and were either in neutral positions within the interstitial tissue, or topographically, closely related to microvessels, intraganglionic nerve fibers, and NGUs. These TCs were further evaluated at higher magnifications. Frequently, one can observe, around the cell body and along telopodes, a discontinuous basal lamina, interposed between the plasma membrane and pericellular fibrils of collagen. After the reconstruction of 28 micrographs and 39 micrographs respectively, we were able to observe the telopodes, as striking features of TCs (Figs. 2, 3, and 4). We identified the presence of elongated, spindle-shaped cell bodies with two telopodes emerging from the extremities, and we also observed triangular cell bodies for TCs presenting three telopodes. The nucleus was ovoid or triangular, with eccentric condensed chromatin and occasionally 1–2 nucleoli were observed. A thin rim of perinuclear cytoplasm was identified, its quantity increasing at the base of the primary processes, such as a “cone of insertion” of the respective process to accommodate mitochondria and endoplasmic reticulum. The presence of the Golgi apparatus and the lysosomes were inconspicuous. A primary cilium-like structure was also identified (Fig. 5). Telopodes were defined by their uneven caliber determined by intermittent dilations along their length, and which usually enclosed mitochondria and smooth and rough endoplasmic reticulum. At lower magnification, telopodes were observed to configure a veritable labyrinthine system. Longitudinal bundles of intermediate filaments were also a constant feature of these processes (Fig. 3). Lipid droplets and glycogen granules were occasionally seen within in telopodes, while an abundant vesicular content was constantly identified in the form of multivesicular bodies/endosomal carrier vesicles and endosomes (Figs. 3). The TCs we evaluated contained a high number of plasmalemmal caveolae, equally present in cell bodies and processes. Clathrin-coated pits and vesicles were also observed. Plasmalemmal adhesion plaques were constantly observed, seemingly configuring fibronexuses, as well as in regions of intercellular adherens-like junctions (Fig. 5).
Ultrastructural details (A–C) of the telocyte illustrated in the previous figure. a Several intercellular junctions (black arrows) and plasmalemmal adhesion plaques (black arrowheads) can be observed. b A telopode fragment with plasmalemmal caveolae (white arrows), an endosomal-rich zone (asterisk), and plasmalemmal adhesion plaques (black arrowheads) c an internal primary cilium (white arrowhead) can be observed. m mitochondrion
Discussion
Studies of the gasserian ganglia are in general addressed to the immunohistochemical and ultrastructural characterization of the different types of pseudounipolar neurons (Kai-Kai 1989; Maxwell 1967). Little attention was paid to the characterization of the interstitial space. The presence of c-kit positive neurons in sensory ganglia has been verified in various species. In one of our previous studies, we showed the presence of c-kit/CD117 receptors at the level of trigeminal neurons (TNs), mast cells and interstitial cells within the trigeminal ganglion (Rusu et al. 2011). Ultrastructurally, the interstitial cells were identified as telocytes and were described as completely different from the Schwann cells and the satellite glial cells (Rusu et al. 2011).
This study is the first report describing the presence of CD34 positive interstitial cells in the human trigeminal ganglion. The identified stromal cells are telocytes (TCs) meeting the key features for the ultrastructural identification: a small cell body (15–53 μm) with emerging long telopodes containing endoplasmic reticulum and mitochondria located in the podoms. Moreover, a primary cilium and attachment plaques configuring fibronexuses were also detected as previously reported by other studies (Cantarero et al. 2011; Rusu et al. 2012a). CD34 was detected on these cells with long processes interconnected in a network.
Lately, a significant body of in vitro and in vivo research confirmed that CD34+ stromal cells are TCs (Cretoiu et al. 2015b; Diaz-Flores et al. 2016). Recent studies underline the fact that TCs frequently express CD34 sometimes in association with other stem cell markers such as c-kit (Bei et al. 2015c; Zhou et al. 2015). CD34 is a glycosylated transmembrane protein and signifies a general marker for bone marrow-derived progenitor cells. It was suggested that CD34 is involved in maintenance of the progenitor cells in a phenotypically undifferentiated state (e.g., hematopoietic and endothelial progenitor cells) (Chotinantakul and Leeanansaksiri 2012). These stem cell properties of TCs along with the fact that they also express mesenchymal marker CD29 (Bei et al. 2015c) raise the possibility that they might be involved in regeneration and repair processes, either by modulating the stem cell activity (Albulescu et al. 2015; Diaz-Flores et al. 2016) or by acting as progenitors of other cells present in the normal tissue, e.g., mesenchymal cells or interstitial cells of Cajal or in affected tissue (granulation tissue) where it might participate as a source of α-smooth muscle actin positive stromal cells, with myofibroblastic characteristics (Diaz-Flores et al. 2015a; Diaz-Flores et al. 2015b; Diaz-Flores et al. 2014). In opposition to this proposed explanation, several studies provided convincing evidence that TCs are decreased and ultrastructurally damaged during tissue fibrosis of different etiologies, such as in scleroderma, failing human heart, and inflammatory bowel disease-related intestinal fibrosis (Ibba-Manneschi et al. 2016; Manetti et al. 2013; Manetti et al. 2014; Manetti et al. 2015; Milia et al. 2013; Richter and Kostin 2015). As an example, CD34/α-SMA combined immunostaining did not reveal the presence of double-positive transitioning stromal cells in the colonic wall of ulcerative colitis patients (Manetti et al. 2015). Furthermore, there is clear ultrastructural evidence of fibrosis-related TC degenerative processes rather than transdifferentiation into myofibroblasts, as shown in human heart failure and scleroderma skin (Ibba-Manneschi et al. 2016; Manetti et al. 2013; Richter and Kostin 2015).
The trigeminal TCs presence in the interstitium between NGUs having telopodes extended in a neutral position or in close proximity of microvessels seem to play an important role in the physiology of the gasserian ganglia. Besides the aforementioned plausible functions, TCs might represent key players in many events such as proliferation, differentiation, communication, and migration of the neurons and glial cells during development, processes regulated mainly by the cell secretome (Haslene-Hox et al. 2013). In a recent study, TCs secretome was analyzed, targeting the characterization of the several growth factors, cytokines, and other molecules secreted by cardiac TCs (Albulescu et al. 2015). Taken together, the findings suggested that TCs “could sense and re-direct the cellular microenvironment” to increase the renewal capacity of stem cells, phenomenon attributed to interleukin (IL)-6, VEGF, macrophage inflammatory protein 1α (MIP-1α), MIP-2, and MCP-1 (Albulescu et al. 2015).
Moreover, since TCs release extracellular vesicles some of their content might also influence the neuronal circuits, as we suggested before (Rusu et al. 2011). Future experiments will be designated to provide additional data about the relationships between the populations of c-kit positive and c-kit negative neurons existing in trigeminal ganglion and CD34+ TCs. It remains to be established if the TCs population is modified in different pathologies, e.g., trigeminal neuralgia. It is established that neuron–glia interactions are involved in all stages of inflammation and pain (Old et al. 2015); however, no data exists regarding the influence of the surrounding interstitial tissue. Thalakoti et al. demonstrated that activation of trigeminal neurons leads to changes in adjacent glia determined by nonsynaptic communication through gap junctions and paracrine signaling. They suggested that neuronal–glial cell signaling might play a key role in peripheral sensitization within the ganglion in migraine, rhinitis, and temporomandibular joint (TMJ) disorders that involve trigeminal nerve activation (Thalakoti et al. 2007). The primary cilium we have identified in trigeminal TCs is also an additional evidence for their role in signaling processes knowing that primary cilia are sensory organelles they play an essential role in the intracellular signal transduction pathways, including the platelet-derived growth factor (PDGF), Notch and Wnt signaling pathways (Sasai and Briscoe 2012; Wallingford and Mitchell 2011). Moreover, a previous study suggested that the primary cilium of TCs in the vasculature may be particularly important in signaling processes within the vascular niche (Cantarero et al. 2011).
Future morphological and functional studies are required to demonstrate if TCs establish connections with glial cells and how their secretome acts upon the surrounding cells, especially in inflammatory conditions.
In conclusion, TCs represent a newly described population in the trigeminal ganglion, probably able to react differently in physiological homeostasis and in injury repair. Their close vicinity with microvasculature might be, in fact, an adaptation to facilitate an immediate response to tissue injury.
References
Albulescu R, Tanase C, Codrici E, Popescu DI, Cretoiu SM, Popescu LM. The secretome of myocardial telocytes modulates the activity of cardiac stem cells. J Cell Mol Med. 2015;19:1783–94.
Bei Y, Wang F, Yang C, Xiao J. Telocytes in regenerative medicine. J Cell Mol Med. 2015a;19:1441–54.
Bei Y, Zhou Q, Fu S, Lv D, Chen P, Chen Y, et al. Cardiac telocytes and fibroblasts in primary culture: different morphologies and immunophenotypes. PLoS One. 2015b;10:e0115991.
Bei Y, Wang F, Yang C, Xiao J. Telocytes in regenerative medicine. J Cell Mol Med. 2015a.
Campeanu RA, Radu BM, Cretoiu SM, Banciu DD, Banciu A, Cretoiu D, et al. Near-infrared low-level laser stimulation of telocytes from human myometrium. Lasers Med Sci. 2014;29:1867–74.
Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600.
Chotinantakul K, Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012;2012:270425.
Cismasiu VB, Popescu LM. Telocytes transfer extracellular vesicles loaded with microRNAs to stem cells. J Cell Mol Med. 2015;19:351–8.
Cismasiu VB, Radu E, Popescu LM. miR-193 expression differentiates telocytes from other stromal cells. J Cell Mol Med. 2011;15:1071–4.
Cretoiu D, Cretoiu SM. Telocytes in the reproductive organs: current understanding and future challenges. Semin Cell Dev Biol. 2016.
Cretoiu SM, Popescu LM. Telocytes revisited. Biomolecular Concepts. 2014;5:353–69.
Cretoiu D, Cretoiu SM, Simionescu AA, Popescu LM. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012;27:1067–78.
Cretoiu SM, Cretoiu D, Marin A, Radu BM, Popescu LM. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145:357–70.
Cretoiu D, Hummel E, Zimmermann H, Gherghiceanu M, Popescu LM. Human cardiac telocytes: 3D imaging by FIB-SEM tomography. J Cell Mol Med. 2014;18:2157–64.
Cretoiu D, Gherghiceanu M, Hummel E, Zimmermann H, Simionescu O, Popescu LM. FIB-SEM tomography of human skin telocytes and their extracellular vesicles. J Cell Mol Med. 2015a;19:714–22.
Cretoiu SM, Radu BM, Banciu A, Banciu DD, Cretoiu D, Ceafalan LC, et al. Isolated human uterine telocytes: immunocytochemistry and electrophysiology of T-type calcium channels. Histochem Cell Biol. 2015b;143:83–94.
Diaz-Flores L, Gutierrez R, Saez FJ, Diaz-Flores Jr L, Madrid JF. Telocytes in neuromuscular spindles. J Cell Mol Med. 2013;17:457–65.
Diaz-Flores L, Gutierrez R, Garcia MP, Saez FJ, Diaz-Flores Jr L, Valladares F, et al. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014;29:831–70.
Diaz-Flores L, Gutierrez R, Garcia MP, Gonzalez M, Saez FJ, Aparicio F, et al. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of alphaSMA+ cells during repair. Histol Histopathol. 2015a;30:615–27.
Diaz-Flores L, Gutierrez R, Garcia MP, Gonzalez M, Diaz-Flores L Jr, Madrid JF. Telocytes as a source of progenitor cells in regeneration and repair through granulation tissue. Curr Stem Cell Res Ther. 2015a
Diaz-Flores L, Gutierrez R, Diaz-Flores L, Gomez MG Jr, Saez FJ, Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol. 2016.
Fertig ET, Gherghiceanu M, Popescu LM. Extracellular vesicles release by cardiac telocytes: electron microscopy and electron tomography. J Cell Mol Med. 2014;18:1938–43.
Gherghiceanu M, Popescu LM. Cardiac telocytes—their junctions and functional implications. Cell Tissue Res. 2012;348:265–79.
Haslene-Hox H, Tenstad O, Wiig H. Interstitial fluid-a reflection of the tumor cell microenvironment and secretome. Biochim Biophys Acta. 2013;1834:2336–46.
Ibba-Manneschi L, Rosa I, Manetti M. Telocyte implications in human pathology: an overview. Semin Cell Dev Biol. 2016
Kai-Kai MA. Cytochemistry of the trigeminal and dorsal root ganglia and spinal cord of the rat. Comp Biochem Physiol A Comp Physiol. 1989;93:183–93.
Manetti M, Guiducci S, Ruffo M, Rosa I, Faussone-Pellegrini MS, Matucci-Cerinic M, et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17:482–96.
Manetti M, Rosa I, Messerini L, Guiducci S, Matucci-Cerinic M, Ibba-Manneschi L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J Cell Mol Med. 2014;18:253–62.
Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015;19:62–73.
Manole CG, Gherghiceanu M, Simionescu O. Telocyte dynamics in psoriasis. J Cell Mol Med. 2015
Maxwell DS. Fine structure of the normal trigeminal ganglion in the cat and monkey. J Neurosurg. 1967;26:127–31.
Milia AF, Ruffo M, Manetti M, Rosa I, Conte D, Fazi M, et al. Telocytes in Crohn’s disease. J Cell Mol Med. 2013;17:1525–36.
Old EA, Clark AK, Malcangio M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol. 2015;227:145–70.
Popescu LM, Faussone-Pellegrini MS. Telocytes—a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to telocytes. J Cell Mol Med. 2010;14:729–40.
Popescu LM, Fertig ET, Gherghiceanu M. Reaching out: junctions between cardiac telocytes and cardiac stem cells in culture. J Cell Mol Med. 2015.
Richter M, Kostin S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J Cell Mol Med. 2015;19:2597–606.
Roatesi I, Radu BM, Cretoiu D, Cretoiu SM. Uterine telocytes: a review of current knowledge. Biol Reprod. 2015;93:10.
Rusu MC. Skin telopodes. Romanian J Morphol Embryol = Rev Roum Morphol Embryol. 2014;55:723–4.
Rusu MC, Pop F, Hostiuc S, Dermengiu D, Lala AI, Ion DA, et al. The human trigeminal ganglion: c-kit positive neurons and interstitial cells. Ann Anat = Anat Anz: Off Organ Anat Ges. 2011;193:403–11.
Rusu MC, Jianu AM, Mirancea N, Didilescu AC, Manoiu VS, Paduraru D. Tracheal telocytes. J Cell Mol Med. 2012a;16:401–5.
Rusu MC, Jianu AM, Pop F, Hostiuc S, Leonardi R, Curca GC. Immunolocalization of 200 kDa neurofilaments in human cardiac endothelial cells. Acta Histochem. 2012b;114:842–5.
Rusu MC, Nicolescu MI, Jianu AM, Lighezan R, Manoiu VS, Paduraru D. Esophageal telocytes and hybrid morphologies. Cell Biol Int. 2012c;36:1079–88.
Rusu MC, Pop F, Hostiuc S, Curca GC, Jianu AM, Paduraru D. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012d;27:807–16.
Rusu MC, Hostiuc S, Loreto C, Paduraru D. Nestin immune labeling in human adult trigeminal ganglia. Acta Histochem. 2013;115:86–8.
Rusu MC, Folescu R, Manoiu VS, Didilescu AC. Suburothelial interstitial cells. Cells Tissues Organs. 2014a;199:59–72.
Rusu MC, Loreto C, Manoiu VS. Network of telocytes in the temporomandibular joint disc of rats. Acta Histochem. 2014b;116:663–8.
Rusu MC, Mănoiu VM, Mirancea N, Nini G. Quiescent satellite glial cells of the adult trigeminal ganglion. Cent Eur J Med. 2014c;9:500–4.
Sasai N, Briscoe J. Primary cilia and graded Sonic Hedgehog signaling. Wiley Interdiscip Rev Dev Biol. 2012;1:753–72.
Song D, Cretoiu D, Zheng M, Qian M, Zhang M, Cretoiu SM, et al. Comparison of Chromosome 4 gene expression profile between lung telocytes and other local cell types. J Cell Mol Med. 2015
Sun X, Zheng M, Zhang M, Qian M, Zheng Y, Li M, et al. Differences in the expression of chromosome 1 genes between lung telocytes and other cells: mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells and lymphocytes. J Cell Mol Med. 2014;18:801–10.
Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–23. discussion 1024-1005.
Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L, Faussone-Pellegrini MS. Telocytes express PDGFRalpha in the human gastrointestinal tract. J Cell Mol Med. 2013;17:1099–108.
Vannucchi MG, Bani D, Faussone-Pellegrini M-S. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr Stem Cell Res Ther. 2015
Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13.
Wang J, Ye L, Jin M, Wang X. Global analyses of chromosome 17 and 18 genes of lung telocytes compared with mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells, and lymphocytes. Biol Direct. 2015;10:9.
Yang J, Chi C, Liu Z, Yang G, Shen ZJ, Yang XJ. Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis. J Cell Mol Med. 2015
Zhang HQ, Lu SS, Xu T, Feng YL, Li H, Ge JB. Morphological evidence of telocytes in mice aorta. Chin Med J. 2015;128:348–52.
Zheng M, Sun X, Zhang M, Qian M, Zheng Y, Li M, et al. Variations of chromosomes 2 and 3 gene expression profiles among pulmonary telocytes, pneumocytes, airway cells, mesenchymal stem cells and lymphocytes. J Cell Mol Med. 2014;18:2044–60.
Zhou Q, Wei L, Zhong C, Fu S, Bei Y, Huica RI, et al. Cardiac telocytes are double positive for CD34/PDGFR-alpha. J Cell Mol Med. 2015;19:2036–42.
Zhu Y, Zheng M, Song D, Ye L, Wang X. Global comparison of chromosome X genes of pulmonary telocytes with mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells, and lymphocytes. J Transl Med. 2015;13:318.
Acknowledgments
This work was partially supported by grants of the Romanian National Authority for Scientific Research, CNCS—UEFISCDI, project numbers 82/2012 and 194/2014.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authers declare that they have no conflicts of interest.
Additional information
Mugurel Constantin Rusu and Dragos Cretoiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rusu, M.C., Cretoiu, D., Vrapciu, A.D. et al. Telocytes of the human adult trigeminal ganglion. Cell Biol Toxicol 32, 199–207 (2016). https://doi.org/10.1007/s10565-016-9328-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-016-9328-y