Abstract
Oxidation of the N–phosphonomethyl iminodiacetic acid (PMIDA) with hydrogen peroxide in a two-phase system (aqueous phase—organic phase) in the presence of the [(Octn)3NMe]3{PO4[WO(O2)2]4} catalyst was studied and the kinetic parameters of this reaction were determined. It was found that the PMIDA oxidation with aqueous H2O2 proceeds only in the presence of the catalyst giving PMIDA N-oxide as the main product. Under the studied conditions, the reaction orders with respect to the reagents (PMIDA and H2O2) and the catalyst were found to be the first. The apparent activation energy of the reaction for the temperature range of 313–343 K is 37 ± 3 kJ/mol.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chemistry of amine N-oxides is the field of growing interest in recent years due to the synthetic and biomedical importance of these compounds. Aliphatic and aromatic N-oxides are used as pharmaceutical agents [1], essential components in cosmetic products [2, 3] and detergents [4]. Tertiary amine N-oxides are considered as valuable intermediates in synthetic organic chemistry because of their ability to undergo various transformations: Polonovsky [5, 6], Boekelheide [7], Cope [8] reactions, C-functionalization of aromatic N-oxides [9], sigmatropic rearrangements [10], intramolecular oxidation [11], and also to act as catalysts for asymmetric reactions [12].
Since the amine N-oxides (especially the aliphatic ones) are labile compounds, they need to be synthesized at temperatures below 100 °C using mild oxidants—organic or inorganic peroxides [13,14,15]. The environment friendly methods for N-oxides synthesis are based on the catalytic oxidation of the tertiary amines with aqueous hydrogen peroxide in the presence of polyoxometallates (for example, Mo/P—POM) [16] or tungsten peroxo-polyoxo complexes [17]. PMIDA (1) oxidation with hydrogen peroxide in the presence of water-soluble tungsten and/or molybdenum compounds is the key step in the several manufacturing processes of the most common herbicide, glyphosate (N-phosphonomethyl glycine), production [18]. This reaction proceeds with the formation of PMIDA N-oxide (2) as an intermediate. The significant disadvantages of this process are the long reaction time (up to 2.0 h) required to achieve the practically significant yields of the PMIDA N-oxide and the contamination of the target product with catalyst, which cannot be separated from the reaction mixture [19].
The above problems can be solved by realization of the process in two-phase (organic-aqueous) liquid system using peroxotungstate-based bifunctional oxidation and phase-transfer catalysts which combine both efficiency and ease of separation from the product. Earlier, we have found that the best result in the oxidation of PMIDA to its N-oxide with hydrogen peroxide was obtained with the use of the tetra nuclear tungsten complex—tetra(oxodiperoxotungsto)phosphate in combination with methyltri-n-octylammonium as a catalyst [17, 20].
In this work, we report the kinetic studies of the PMIDA oxidation with aqueous hydrogen peroxide in a two-phase system in the presence of the [(Octn)3NMe]3{PO4[WO(O2)2]4} catalyst aimed at the optimization the conditions for the N-oxide producing.
2 Experimental
2.1 Chemicals
N-phosphonomethyl iminodiacetic acid (2,2'-((phosphonomethyl)azanediyl)diacetic acid, 97%, «Aldrich»), KCl (99 + %, «Acros»). Other chemicals were of reagent purity grade, obtained from commercial sources, and used without further purification. Water used as a solvent was previously distilled.
The sample of N-phosphonomethyl iminodiacetic acid N-oxide (PMIDA N-oxide, 2) was prepared according to the described procedure [21]. Yield 0.25 g (75%), white crystals, m.p. 146 °C. FT-IR: vmax/cm−1 3013, 2967.4, 2554.6, 1888.5, 1742.7 (COOH), 1478.5, 1440.0, 1417.8, 1383.6, 1304.2, 1261.5, 1232.0, 1158.8, 1122.4, 1070.0, 1053.6, 1026.8, 981.0 (N → O, [22]), 923.7, 781.3, 712.8, 676.3, 599.8, 580.1. 1H NMR (300 MHz, D2O), δ (ppm): 4.84, s; 4.33, d, 12.4. 31P NMR (121.49 MHz, D2O), δ (ppm):4.77, t, 12.4.
2.2 Analytic Methods
IR measurements of the catalyst samples were performed using IR-Fourier IRAffinity-1 Shimadzu spectrometer. Spectra were collected in 400–4000 cm−1 with a resolution of 2 cm−1. Prior to the measurements the samples were mixed with dry KCl and pressed to form self-supporting homogeneous pellets.
1H and 31P NMR spectra were recorded at Bruker AV–300 spectrometer (300.13 MHz for 1H, 121.49 MHz for 31P) in D2O at room temperature. Proton chemical shifts were recorded relative to tetramethylsilane external standard. The 31P NMR spectra with and without the suppression of heteronuclear resonance were recorded using H3PO4 (0 ppm) as an external standard.
HPLC analysis of the reaction mixtures was conducted using Varian ProStar instrument equipped with a ProStar 335 Photodiode Array Detector with a variable wavelength ranging from 190 nm to 900 nm; ProStar 210 and PrepStar 218 Solvent Delivery pumps; ProStar410 auto sampler, Varian 500-LC thermostate, and a Column Volve Module for the column temperature control. For the analysis, HPLC column PRP-X100 (Hamilton Company USA, 5 μm, 150 × 4.6 mm) thermostated at 35 °C was used, 0.0137 M KH2PO4 (pH 1.9) used as a mobile phase at a fixed flow rate of 0.5 mL × min−1. Chromatograms were recorded at the detector wavelength of 193 nm. Residence time was as follows: for H2O2– 3.48 min; for PMIDA—12.64 min; for N-oxide PMIDA—18.25 min. Component concentration was determined using an external standard. For this purpose, it was found that at the concentration range from 0 to 5 × 10–2 M, the dependences of the components concentrations on the peak area are linear (R2 ≥ 0.998).
2.3 Catalyst Preparation and Characterization
The [(Octn)3NMe]3{PO4[WO(O2)2]4} catalyst was synthesized in accordance with the described procedure [23]. FT-IR: ν max/cm−1: 3523.9, 2961.7, 2860.2, 1726.4, 1565.9, 1483.6, 1467.1, 1378.0, 1091.5, 1058.5, 974.0, 888.9, 855.9, 846.2, 723.3, 650.9, 628.6, 590.9, 576.0, 549.3, 523.5. 1H NMR 300 MHz, C6D6, δ (ppm): 4.29 (s, 1H), 3.20 (m, 5H), 2.94 (m, 10H), 1.39 (m, 78H), 1.03 (m, 20H), 0.29 (s, 2H); 31P NMR (121.49 MHz, C6D6), δ (ppm): 4.41 (m).
2.4 Kinetic Study
Reactions were carried out in IKA RCT basic parallel synthesis unit equipped with an H 135.3 platform together with sections for IKA H. 135.312 reaction vessels (up to 16 pieces), in G075X-23Kit25-H reactors.
For the kinetic study of PMIDA N-oxide formation, a measured volume of 1.20 × 10–3 M solution of [(Octn)3NMe]3{PO4[WO(O2)2]4} in toluene was loaded into each reaction vessel. Then a measured volume of a 30% aqueous hydrogen peroxide was added and stirred for 5 min at 343 K. Then a measured volume of 5 × 10–2 M aqueous solution of PMIDA was added. Reactions were carried out at 343 K; agitation was performed with Teflon-coated magnetic stirring bars.
For the kinetic study of H2O2 decomposition, 1 ml of 1.20 × 10–3 M solution of [(Octn)3NMe]3{PO4[WO(O2)2]4} in toluene was loaded into the half of the reaction vessels used. Then 2 ml of 0.322 M aqueous H2O2 was added into each reaction vessel. Reaction vessels were carried out at 343 K; agitation was performed with Teflon-coated magnetic stirring bars, stirring speed n = 1000 rpm.
After the specified time intervals, each vessel was taken out from the reactor set and cooled down to room temperature in ice bath. The concentration of the PMIDA N-oxide in the reaction mixtures was determined by HPLC using the sample of PMIDA N-oxide (prepared as specified above) for the comparison. The H2O2 concentration was determined by permanganometry according to the described procedure [24].
The rate of PMIDA N-oxide formation was determined from the slope of tangent to the kinetic curve (Fig. 1) in the initial interval (in the beginning of the oxidation reaction) when the changes in the initial concentrations of the reagents can be neglected, considering them constant. The relative error of the reaction rate value does not exceed ± 10%, which was determined using the results of a series of 3–5 experiments.
3 Result and Discussion
The study of formal kinetics [25] of the catalytic oxidation of N-phosphonomethyl iminodiacetic acid (PMIDA, 1) was carried out under the conditions of phase transfer catalysis using 30% aqueous H2O2 as an oxidant and toluene as an organic solvent. Hydrogen peroxide and the substrate, PMIDA, were in the aqueous phase, and the catalyst, [(Octn)3NMe]3{PO4[WO(O2)2]4}, was in the organic phase.
First, we estimated the contribution of non-productive thermal and catalytic decomposition of H2O2 under the reaction conditions. Aqueous H2O2 and the mixture of the aqueous H2O2 with the catalyst were stirred up to 3 h at 343 K without addition of the substrate. Figure 2 shows that the amount of decomposed hydrogen peroxide does not exceed 6% even after 3 h of exposure. Based on this, in the kinetic studies, we consider that hydrogen peroxide is consumed only for the substrate oxidation.
3.1 Selection of the Mixing Regime
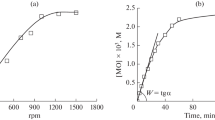
Influence of the agitation intensity on the rate of PMIDA oxidation in the biphasic reaction mixture was determined in experiments with the variation of stirring speed (n) in the range of 100–1200 rpm (revolutions per minute). As shown in the Fig. 3, the rate of the PMIDA N-oxide formation increases with the rise of the stirring speed up to 800 rpm, and then the curve approaches a plateau.
In this regard, further studies were carried out at n = 1000 rpm. Under these conditions. the stirring speed does not affect the reaction rate the and the expression for the rate of PMIDA N-oxide formation is:
where W—reaction rate, mole/(l·min); k0—rate constant of PMIDA N-oxide formation, l(a+b+c−1) × mole−(a+b+c−1) × min−1; Cat—methyltri-n-octylammonium tetrakis(oxodiperoxotungsto)phosphate, [(Octn)3NMe]3{PO4[WO(O2)2]4}; a—reaction order in catalyst, [(Octn)3NMe]3{PO4[WO(O2)2]4}”. b—reaction order in oxidant, H2O2: c—reaction order in substrate, PMIDA; Ea—apparent activation energy, kJ/mole.
3.2 Dependence of the PMIDA N-Oxide Formation Rate (W) on the Catalyst Concentration
To determine the optimal concentration of the catalyst, the kinetic dependence W = f ([Cat]) was measured. In this study, the catalyst concentration was varied in the range of (0.5–2.5) × 10–4 M. In order to reduce the possible influence of the hydrogen peroxide concentration on the reaction rate, the oxidation was carried out in excess of hydrogen peroxide compared to the stoichiometric amount, which was defined by the molar ratio: [PMIDA]/[H2O2] = 1 (Scheme 1). The reaction order (a) found from the slope of the logarithmic anamorphosis for catalyst concentrations below 2 × 10–4 M (Fig. 4) is first. Higher catalyst concentrations have practically no effect on the rate of PMIDA N-oxide formation. Based on this, the further investigations of the reaction rate dependence on various parameters were carried out considering the catalyst concentration of 2.5 × 10–4 M as the optimum one.
3.3 Dependence of the PMIDA N-Oxide Formation Rate on the Concentration of Hydrogen Peroxide
The dependence was studied in the range of H2O2 concentrations of 0.055–0.25 M. The kinetic data obtained (Fig. 5) show that, for the concentrations not exceeding 0.2 M, the observed reaction order in hydrogen peroxide (b) is first. Further increase in the hydrogen peroxide concentration does not cause the rise of the reaction rate. At the molar ratio [H2O2]/[PMIDA] ≥ 4, the zero order in hydrogen peroxide is observed.
3.4 Dependence of the PMIDA N-Oxide Formation Rate on the Substrate Concentration
The kinetic data were obtained for the PMIDA concentrations between 2 × 10–2 M and 5 × 10–2 M. In this range, the dependence of the logarithm of the PMIDA N-oxide formation rate on the logarithm of the PMIDA concentration is linear (Fig. 6) with the slope value close to unity. Therefore, in the studied interval of concentrations, the reaction order in the substrate (c) is first.
3.5 Temperature Dependence of PMIDA Catalytic Oxidation with H2O2
The study was carried out in the temperature range of 313–343 K in excess of hydrogen peroxide ([H2O2]/[PMIDA] = 4) and molar ratio of [PMIDA]/[Cat] = 250. The results obtained are presented in Fig. 7 as logarithmic plots (ln(1-([PMIDA]/[PMIDA]0) versus time). Linearity of the plots indicates the first order reaction. The reaction rate constants determined from the slopes of the plots are summarized in Table 1.
The value of apparent activation energy (Ea) is obtained from the slope of the plot in Arrhenius coordinates (Fig. 8), where Ea = −R [Δln kobs/Δ (T−1)]. The calculated value of Ea amounts to 37 ± 3 kJ/mol. The value of the pre-exponential factor (A) determined from the intercept (ln A) is equal to 8.9 × 105 min−1.
Based on the obtained results, the problem of PMIDA N-oxide (2) producing at the higher rate can be solved by using the substrate (Sub, PMIDA), oxidant (Ox, H2O2) and the catalyst in ratios: [Ox]/[Sub] = 4−5 and [Sub]/[Cat] ≤ 250 at the temperatures from 313 K to 343 K (Fig. 9). Under these conditions, at the PMIDA concentration in the interval of (10–50) × 10–3 M, the reaction rate of the PMIDA N-oxide formation (equation I) depends only on the reaction temperature and PMIDA concentration. Thus, the equation for the reaction rate is expressed in the following form:
where A = 8.9 × 105 min−1; Ea = 37 ± 3 kJ/mole.
In the expression (II), for the boundary conditions specified ([PMIDA]/[Cat] ≤ 250, [H2O2]/[PMIDA] > 4 and [PMIDA] ≤ 0.05 M), the functional contribution of the oxidant and catalyst concentrations does not limit the reaction rate of PMIDA N-oxide (2) formation and is included in the value of the pre-exponential factor (A):
This follows from the observed zero-order of the reaction in catalyst and oxidant under the selected conditions and the constant PMIDA concentration for all the experiments, when studying the temperature dependence.
Thus, the obtained results indicate that the PMIDA oxidation does not proceed in the absence of a catalyst; therefore, the reaction is multistage, for example, as in the case of oxidation of alkenes to carboxylic acids [26]. In the oxidation, the following stages can be distinguished:
-
(a)
The interaction of the catalyst and the substrate, resulting in the formation of the [Cat(O2)—PMIDA] complex, in which Cat(O2) contains active peroxo groups (ɳ2˗O2);
-
(b)
Subsequent formation of PMIDA N-oxide and inactive oxo-form of the catalyst (CatO) containing W = O groups;
-
(c)
Catalyst regeneration, which is the interaction of the oxo-form of the catalyst with hydrogen peroxide resulting in the active peroxo-form of the catalyst Cat(O2).
-
a.
Cat(O2) + PMIDA → [Cat(O2) − PMIDA]
-
b.
[Cat(O2) − PMIDA] → PMIDA N-oxide + Cat(O)
-
c.
Cat(O) + H2O2 → Cat(O2) + H2O
-
a.
It should be noted that the data obtained indicate the basic differences in the mechanism of PMIDA catalytic oxidation with hydrogen peroxide in a two-phase liquid system compared to the previously considered reactions, for example, oxidation of various unsaturated hydrocarbons with hydrogen peroxide [27]. In the PMIDA oxidation under the studied conditions, the substrate (PMIDA) and the oxidant (H2O2) are in the water phase. The catalyst, salt of the peroxotungstate anion and quarternary ammonium cation with the high lipophilicity, is dissolved in organic phase. In this case, as in the previously described reactions [28,29,30], cation acts as a phase-transfer catalyst and facilitates the oxygen transfer from hydrogen peroxide to the inactive oxo-form of the anion, which occurs at the interface and results in the catalyst regeneration. At the present stage of research, we suggest that, under the studied conditions, the oxygen transfer from the active peroxo-form of the anion dissolved in organic phase to the hydrophilic substrate (PMIDA) in water phase also occurs at the interface with the assistance of the cation as a phase-transfer catalyst (Scheme 2). Further studies of the mechanism of the catalytic oxidation of PMIDA to its N-oxide are currently underway.
4 Conclusion
Oxidation of N-phosphonomethyl iminodiacetic acid to its N-oxide with hydrogen peroxide in a two-phase liquid system in the presence of [(Octn)3NMe]3{PO4[WO(O2)2]4} bifunctional catalyst was studied. It was shown that the reaction proceeds only in the presence of a catalyst and has a complex mechanism. We demonstrated that [(Octn)3NMe]3{PO4[WO(O2)2]4} dissolved in organic phase is the efficient and selective catalyst for the oxidation of water-soluble amine to its N-oxide with aqueous hydrogen peroxide. In the reaction studied, the role of the tungsten peroxo complex in the catalyst is to transfer oxygen from the oxidant (hydrogen peroxide) to the substrate (N-phosphonomethyl iminodiacetic acid), and the role of the quarternary ammonium cation is to facilitate the interaction of hydrogen peroxide and the substrate with the tungsten complex anion in biphasic conditions.
Based on the found kinetics patterns of the influence of various process parameters, we have determined the conditions for the most efficient production of PMIDA N-oxide (2) without the formation of undesirable by-products. The oxidation of PMIDA (1) with hydrogen peroxide should be carried out in the presence of the catalyst, methyl-tri-n-octylammonium tetrakis(oxodiperoxotungsto)phosphate, in the ratio of [PMIDA]/[Cat] ≤ 250, with a fourfold excess of hydrogen peroxide and at temperature not exceeding 343 K.
References
Mfuh AM, Larionov OV (2015) Heterocyclic N-oxides - an emerging class of therapeutic agents. Curr Med Chem 22:2819–2857. https://doi.org/10.2174/0929867322666150619104007
Heffernan PL, Shaw PD, Wu MM, Davies CJ (2018) Personal cleansing composition. GB2552400, 26 p
Matharu NK (2015) Disinfectant composition. GB2518967, 31 p
Gaudreault R (2014) Hard surface cleaning composition. US2014066356, 7 p
Bur SK (2014) 6.17 Polonovski- and Pummerer-type Reactions and the Nef Reaction. In: Compr. Org. Synth. II. Elsevier, pp 755–801. https://doi.org/10.1016/B978-0-08-097742-3.00626-1
Do Pham DD, Kelso GF, Yang Y, Hearn MTW (2014) Studies on the oxidative N-demethylation of atropine, thebaine and oxycodone using a Fe III -TAML catalyst. Green Chem 16:1399–1409. https://doi.org/10.1039/C3GC41972J
Massaro A, Mordini A, Mingardi A, Klein J, Andreotti D (2011) A new sequential intramolecular cyclization based on the boekelheide rearrangement. European J Org Chem 2011:271–279. https://doi.org/10.1002/ejoc.201000936
Ellis GL, O’Neil IA, Ramos VE, Cleator E, Kalindjian SB, Chorlton AP, Tapolczay DJ (2007) The synthesis of functionalised chiral bicyclic lactam and lactone N-oxides using a tandem Cope elimination/reverse Cope elimination protocol. Tetrahedron Lett 48:1683–1686. https://doi.org/10.1016/j.tetlet.2007.01.045
Larionov OV, Stephens D, Mfuh A, Chavez G (2014) Direct, catalytic, and regioselective synthesis of 2-Alkyl-, Aryl-, and Alkenyl-substituted N -heterocycles from N-oxides. Org Lett 16:864–867. https://doi.org/10.1021/ol403631k
Commandeur C, Commandeur M, Bathany K, Kauffmann B, Edmunds AJF, Maienfisch P, Ghosez L (2011) Study of the oxidation of 3-hydroxypyrroloindoles to pyrrolobenzoxazine alkaloids. Tetrahedron 67:9899–9908. https://doi.org/10.1016/j.tet.2011.09.112
Cui L, Peng Y, Zhang L (2009) A two-step, formal [4 + 2] approach toward Piperidin-4-ones via Au catalysis. J Am Chem Soc 131:8394–8395. https://doi.org/10.1021/ja903531g
Oh K, Li J-Y, Ryu J (2010) Brucine N-oxide-catalyzed Morita–Baylis–Hillman reaction of vinyl ketones: a mechanistic implication of dual catalyst system with proline. Org Biomol Chem 8:3015. https://doi.org/10.1039/C003667F
Bull JA, Mousseau JJ, Pelletier G, Charette AB (2012) Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N -activated pyridines. Chem Rev 112:2642–2713. https://doi.org/10.1021/cr200251d
Yan G, Borah AJ, Yang M (2014) Recent advances in catalytic functionalization of N-oxide compounds via C-H bond activation. Adv Synth Catal 356:2375–2394. https://doi.org/10.1002/adsc.201400203
Cai X, Sha M, Guo C, Pan RM (2012) Synthesis of tertiary amine N-oxides - a review. Asian J Chem 24:3781–3784
Larionov OV, Stephens D, Mfuh AM, Arman HD, Naumova AS, Chavez G, Skenderi B (2014) Insights into the mechanistic and synthetic aspects of the Mo/P-catalyzed oxidation of N-heterocycles. Org Biomol Chem 12:3026–3036. https://doi.org/10.1039/C4OB00115J
Pai ZP, Yushchenko DY, Khlebnikova TB, Parmon VN (2015) N-phosphonomethyl iminodiacetic acid N-oxide synthesis in the presence of bifunctional catalysts based on tungsten complexes. Catal Commun 71:102–105. https://doi.org/10.1016/j.catcom.2015.08.021
Tian J, Shi H, Li X, Yin Y, Chen L (2012) Coupling mass balance analysis and multi-criteria ranking to assess the commercial-scale synthetic alternatives: a case study on glyphosate. Green Chem 14:1990–2000. https://doi.org/10.1039/C2GC35349K
Fields DL (1991) Process for producing N-phosphonomethyl glycine. US5023369, 4 p
Pai ZP, Chesalov YA, Berdnikova PV, Uslamin EA, Yushchenko DY, Uchenova YV, Khlebnikova TB, Baltakhinov VP, Kochubey DI, Bukhtiyarov VI (2020) Tungsten peroxopolyoxo complexes as advanced catalysts for the oxidation of organic compounds with hydrogen peroxide. Appl Catal A Gen 604:117786. https://doi.org/10.1016/j.apcata.2020.117786
Franz JE (1975) N-Organo-N-phosphonomethylglycine-N-oxides and plant growth regulant and phytotoxicant compositions containing same. US4062669, 13 p
Oswald AA, Guertin DL (1963) Organic nitrogen compounds. I. Peroxide intermediates of tertiary alkylamine oxidation by hydrogen peroxide. J Org Chem 28:651–657. https://doi.org/10.1021/jo01038a012
Pai ZP, Tolstikov AG, Berdnikova PV, Kustova GN, Khlebnikova TB, Selivanova NV, Shangina AB, Kostrovskii VG (2005) Catalytic oxidation of olefins and alcohols with hydrogen peroxide in a two-phase system giving mono- and dicarboxylic acids. Russ Chem Bull 54:1847–1854. https://doi.org/10.1007/s11172-006-0047-z
Huckaba CE, Keyes FG (1948) The accuracy of estimation of hydrogen peroxide by potassium permanganate titration. J Am Chem Soc 70:1640–1644. https://doi.org/10.1021/ja01184a098
Murzin DY, Salmi T (2016) Catalytic Kinetics: Chemistry and Engineering: Second Edition, 2nd ed. Catal Kinet Chem Eng Second Ed. https://doi.org/10.1016/C2013-0-18839-3
Pai ZP, Selivanova NV, Oleneva PV, Berdnikova PV, Beskopyl’nyi AM (2017) Catalytic oxidation of α-alkenes with hydrogen peroxide to carboxylic acids in the presence of peroxopolyoxotungstate complexes. Catal Commun 88:45–49. https://doi.org/10.1016/j.catcom.2016.09.019
Timofeeva MN, Pai ZP, Tolstikov AG, Kustova GN, Selivanova NV, Berdnikova PV, Brylyakov KP, Shangina AB, Utkin VA (2003) Epoxidation of cycloolefins with hydrogen peroxide in the presence of heteropoly acids combined with phase transfer catalyst. Russ Chem Bull 52:480–486. https://doi.org/10.1023/A:1023495824378
Ishii Y, Yamawaki K, Ura T, Yamada H, Yoshida T, Ogawa M (1988) Hydrogen peroxide oxidation catalyzed by heteropoly acids combined with cetylpyridinium chloride. Epoxidation of olefins and allylic alcohols, ketonization of alcohols and diols, and oxidative cleavage of 1,2-diols and olefins. J Org Chem 53:3587–3593. https://doi.org/10.1021/jo00250a032
Sato K, Hyodo M, Aoki M, Zheng X-Q, Noyori R (2001) Oxidation of sulfides to sulfoxides and sulfones with 30% hydrogen peroxide under organic solvent- and halogen-free conditions. Tetrahedron 57:2469–2476. https://doi.org/10.1016/S0040-4020(01)00068-0
Craven M, Yahya R, Kozhevnikova EF, Robertson CM, Steiner A, Kozhevnikov IV (2016) Alkylaminophosphazenes as efficient and tuneable phase-transfer agents for polyoxometalate-catalysed biphasic oxidation with hydrogen peroxide. ChemCatChem 8:200–208. https://doi.org/10.1002/cctc.201500922
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (project AAA-A21-121011390007-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yushchenko, D.Y., Pai, Z.P. & Khlebnikova, T.B. Kinetics of N-Phosphonomethyl Iminodiacetic Acid Catalytic Oxidation with Hydrogen Peroxide Under the Phase-Transfer Conditions. Catal Lett 152, 2025–2032 (2022). https://doi.org/10.1007/s10562-021-03798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03798-z