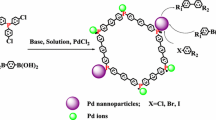
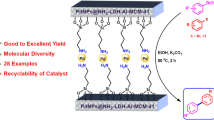
Abstract
An outstanding heterogeneous catalyst was successfully prepared by immobilization of palladium nanoparticles (Pd NPs) with polymer containing 4′‐(4‐hydroxyphenyl)‐2,2′:6′,2″‐terpyridine (HPTPy) ligand. The polymer cross‐linked with trimethylolpropane triacrylate (TMPTA) units was synthesized by polymerization of itaconic acid-HPTPy (ITC-HPTPy) monomer (so-called cross‐linked poly (ITC- HPTPy)). The cross‐linked poly (ITC-HPTPy) can stabilize the Pd NPs effectively against aggregation, thereby improving the catalytic efficiency of Pd NPs. The presence of Pd NPs on the polymer was confirmed by various physicochemical techniques. The resulting cross‐linked poly (ITC-HPTPy)-Pd was applied as a highly effective recyclable catalyst in Suzuki–Miyaura and Mizoroki–Heck coupling reactions under low Pd-loading conditions and straightforward methods, and provided the corresponding products with excellent yields (up to 98%), high catalytic activities (TOF up to 213 h−1). The catalyst can be separated from the reaction mixture by centrifugation and can be reused consecutive six times with slight reduction in catalytic activity.
Graphic Abstract
Pd NPs immobilized with polymer containing terpyridine ligand are highly active heterogeneous catalysts for Suzuki–Miyaura and Mizoroki–Heck coupling reactions.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cross-coupling reactions have been identified as outstanding tools to available to synthetic chemists to create or manipulate intricate organic molecules. Interestingly, the cross-coupling reactions provide straightforward possibilities for joining two organic fragments via carbon–carbon bond-forming reactions [1]. The cross-coupling reactions have been used as key steps in the synthesis of many medicines, natural products and valuable starting materials [2, 3]. Therefore, development of these reactions provides access to a diverse range of intricate organic scaffolds.
Since the heterogeneous nanocatalysts have the potential to develop more environmentally-friendly and economical catalytic processes, one of the most significant aspects of chemistry investigation has been the development of efficient, selective, stable and recyclable nanocatalysts. Palladium (Pd)-based nanocatalysts have found widespread use for catalytic applications, especially in carbon–carbon coupling reactions due to their superior catalytic performance [1]. As are well known, small metal nanoparticles (MNPs) have high surface energies and large surface area, which these properties could often lead to aggregation of the naked MNPs and decreasing of catalytic activity [4]. In particular, highly active palladium nanoparticles are prone to aggregate easily to form larger particles [5]. Recently, many stabilizing methods have been developed to make Pd-based nanocatalysts with small size, high thermodynamically stability and catalytic efficiency. In the case of noble metals such as palladium, the common stabilizing methods to prevent nanoparticles aggregation are to use polymers or ligands [6]. The use of various organic‐ligand stabilizers such as amines, phosphines, and thiols has been extensively developed [7]. It is interesting to note that dispersion and stability of the MNPs can be obtained employing tridentate nitrogen ligands due to the strong interaction metal-nitrogen and the formation of two five-membered metallacycles [8,9,10].
Since the early 1970s, functionalized polymers offer a broad range of applications in areas such as catalysis, ultrafiltration, separation methods, membrane technologies, medicine, pharmacy and others [11]. Use of polymer-immobilized MNPs as catalyst provides essential technique whereby classical organic synthetic process can shift towards green chemistry. In addition to the aspect of simplicity of catalyst recovery and recycling, it is even possible to apply the polymeric catalysts to the continuous flow system which can lead to an economical automation system [12,13,14].
Various methods, including chemical methods and electrochemical methods, have been developed to prepare the polymers containing MNPs. In most of the chemical techniques, monomer or polymer plays the role of reducing agent for the metal species. The chemical techniques include the following methods: polymerization of the monomer in the presence of the MNPs, in situ reduction of metal ions from their salt solution in the presence of the polymer, simultaneous formation of MNPs and polymerization of the monomer [15].
Our early interest in the use of noble metal nanoparticles as catalyst for organic transformations prompted us to examine various methods for the immobilization these nanoparticles [10, 17]. As part of our continuing investigations and considering potential advantages of using polymers to immobilize MNPs, herein, we report the synthesis of a new polymer containing HPTPy ligand for the stabilization of Pd NPs (cross‐linked poly (ITC-HPTPy)-Pd). The catalytic activity of the cross‐linked poly (ITC-HPTPy)-Pd was evaluated in the Suzuki–Miyaura and Mizoroki‐Heck coupling reactions (Scheme 1).
2 Experimental
2.1 Materials and Methods
The materials were purchased from Merck and Fluka and were used without any additional purification. Melting points were determined using a Stuart Scientific SMP2 apparatus. FT‐IR spectra were determined with a Perkin Elmer 683 instrument. Thermogravimetric analysis (TGA) was carried out with a STA PT- 1000 Linseis instrument (Germany) under air atmosphere at a heating rate of 10 °C min−1. SEM and energy‐dispersive X‐ray (EDX) measurements were performed using a TESCAN‐ MIRA3 operated at 26 kV with the electron gun filament: tungsten. TEM observations were carried out with a Zeiss‐EM10C (Germany) operating at 100 kV with samples on formvar carbon‐coated Cu grid (mesh 300). The elemental palladium content of nanocatalyst was determined by Perkin Elmer Optima 7300D inductively coupled plasma (ICP). X‐rays diffraction (XRD) patterns were obtained using STOE STADI-P diffractometer (Cu K-α radiation λ = 1.54060 Å). N2 adsorption study of nanocatalyst was done using BET (BELSORP Mini, Microtrac Bel Corp). The chemical compositions of the catalysts were performed using XPS a Kratos Axis Ultra DLS spectrometer with an Al Kα as a source.
2.2 Preparation of HPTPy- ITC
First, HPTPy was prepared according to recipes investigated in the literature [16, 17], then a mixture of HPTPy (0.325 g, 1 mmol) and ITC (0.065 g, 0.5 mmol) in DMSO (4 mL) was stirred at 100 °C for 48 h. After this time, EtOH (10 mL) and H2O (5 mL) were added into the reaction mixture, and the precipitate collected by filtration and dried at 70 °C.
2.3 Preparation of Cross‐Linked Poly (ITC-HPTPy)
Briefly, HPTPy- ITC (0.033 g) and TMPTA (0.9 mL) were added to a 40:60 mixture of MeOH/CH3CN (5 mL) and the mixture was sonicated for 2 min then 0.06 g of 2,2-azobisisobutyronitrile (AIBN) was added. The final volume is adjusted with MeOH/CH3CN (40:60) to 35 mL. The mixture was sonicated for 2 min and then purged with nitrogen to remove dissolved oxygen. The resulting mixture was refluxed at 60–70 °C for 24 h. Finally, after air cooling, the mixture was centrifuged and the solid obtained was dried in vacuum at 50 °C.
2.4 Preparation of Cross‐Linked Poly (ITC-HPTPy)-Pd
0.1 g of the cross‐linked poly (ITC-HPTPy) was sonicated in 5 mL EtOH for 10 min. To the resulting mixture PdCl2 (0.005 g, 0.028 mmol) was added and refluxed for 24 h. Then, the mixture was filtered and the solid catalyst was washed with EtOH to remove the excess of PdCl2 and dried under vacuum for 24 h. ICP showed 0.023 mmol of palladium loaded on the 0.1 g of cross‐linked poly (ITC-HPTPy)-Pd (2.48 wt%).
2.5 General Procedure for the Suzuki–Miyaura Cross‐Coupling Reactions
ArX (1.2 mmol), ArB(OH)2 (1 mmol) and K2CO3 (1.5 mmol) were added into a flask containing cross‐linked poly (ITC-HPTPy)-Pd (0.01 g, 0.23 mol%) and EtOH/H2O (2/1). The mixture was then stirred in an 80 °C oil bath for an appropriate reaction time (Table 2). The progress of the reaction was monitored by TLC. After completion of the reaction, ethyl acetate (10 mL) was added into the reaction mixture, the catalyst was separated by using centrifugation and the organic solvent was evaporated to obtain biaryl products in excellent yields.
2.6 General Procedure for the Mizoroki–Heck Cross‐Coupling Reactions
A mixture of aryl halide (1 mmol), styrene (1.2 mmol), Et3N (3 mmol) and the cross‐linked poly (ITC-HPTPy)-Pd (0.012 g, 0.27 mol%) was stirred at 100 °C (oil bath temperature) under solvent-free conditions. After completion of the reaction, which was monitored by TLC, ethylacetate (10 mL) was added to the reaction mixture. The catalyst was separated using centrifugation. Water (3 × 15 mL) was added to the ethylacetate phase and decanted. After evaporation of the solvent, the resulting crude products were purified from n-hexane/ethylacetate giving the pure products in high to excellent yields (Table 4).
3 Results and Discussion
3.1 Synthesis and Characterization of Cross‐linked poly (ITC-HPTPy)-Pd
The steps of synthesis of cross‐linked poly (ITC-HPTPy)-Pd are summarized in Scheme 2. HPTPy-ITC was initially prepared via esterification reaction of ITC and HPTPy. Then, polymerization of HPTPy-ITC as a monomer and TMPTA as a cross-linker was achieved in the presence of AIBN as an initiator in a mixture of MeOH/CH3CN affording the final polymer. The cross‐linked poly (ITC-HPTPy)-Pd was obtained by interaction of PdCl2 with cross‐linked poly (ITC-HPTPy) in EtOH as a solvent and green reducing agent (Scheme 2). Moreover, Pd NPs can be successfully prepared and stabilized on Polymer-TPy without the addition of external reducing agents because HPTPy ligands serve also as a reducing agent due to its nitrogen atoms [18, 19].
The cross‐linked poly (ITC-HPTPy)-Pd was extensively analyzed by several characterization techniques including, FT-IR, TEM, SEM, BET, TGA, EDX, XRD, XPS, UV–Vis and ICP.
At outset, influence of cross-linker concentration, on the resulting polymer porosity was investigated. For this purpose, two of polymeric samples using different TMPTA concentrations and the same degree of ITC-HPTPy dilution have been prepared under the same conditions. Porous properties of the polymeric samples were investigated by BET. The N2 adsorption/desorption isotherms of the polymers declare typical type IV isotherms (Fig. 1a and c). Furthermore, it is found that porosity, pore volume, surface area and mean pore diameter are greatly influenced by varying the concentration of cross-linker (Table 1) and using higher concentration of cross-linker (0.9 mL of TMPTA) has provided a better result (Table 1, entry 1). These findings are consistent with the literature results which confirm increasing amount of cross-linker in the polymer structure leads to increase of the pores number and decrease of their size [20].
In previous studies, EtOH has been suggested as a green reducing agent in the synthesis of Pd NPs [18]. Moreover, the nitrogen-based ligands have been employed to work as both reducing and stabilizing reagent in the reaction for preparation of Pd NPs [19]. UV–Visible spectroscopic technique is used to investigate the reduction of Pd(II) ions to Pd NPs (Fig. 2). The UV–visible spectrum of a solution of PdCl2 in EtOH at room temperature showed a distinct peak approximately at 425 nm, which is related to the existence of Pd(II) ion [21]. During the formation of Pd NPs on the polymer containing HPTPy ligand, the peak was removed indicating reduction of Pd(II) ions to Pd NPs.
The immobilization of Pd NPs on cross‐linked poly (ITC-HPTPy) was also confirmed by the color changes of the polymer from white into dark grey during the preparation of the catalyst in EtOH within 24 h (Fig. 3). The color changes are due to reduction of Pd(II) to Pd(0) [22] and immobilization of Pd NPs on cross‐linked poly (ITC-HPTPy).
Grafting of the HPTPy ligand to the ITC monomer and the synthesis of cross‐linked poly (ITC-HPTPy)-Pd was further confirmed through FT-IR spectra (Fig. 4). In the FT-IR spectrum of HPTPy (Fig. 4a), the following functional groups were identified: OH stretching vibration (3386 cm−1), sp2 C–H stretching vibration (3036 cm−1), and C=N stretching frequency (1595 cm−1). In the case of HPTPy-ITC (Fig. 4b), the band at 1740 cm−1 corresponds to C=O stretching of ester groups. The weak absorption peak at 2856 cm−1 and 2926 cm−1 are attributed to the sp3 C–H stretching vibrations. The bands observed at 1592 cm−1 and 3055 cm−1 can be attributed to the C=N stretching frequency and C–H aromatic (pyridine rings) stretching vibration, respectively. The C=N bands of complex TPy‐Pd in cross‐linked poly (ITC-HPTPy)-Pd is shifted to a lower frequency (1583 cm−1) in the FT‐IR spectrum compared to that of TPy (1595 cm−1). The lowering in frequency of the C=N peak is indicative of the interaction of TPy with Pd (Fig. 4c).
Figure 5a and b display SEM images of cross‐linked poly (ITC-HPTPy)-Pd with different magnifications. The size of the Pd NPs produced on the polymer was analysed by TEM. The image in Fig. 5c indicates the presence of uniform and small sized Pd NPs on the cross‐linked poly (ITC-HPTPy)-Pd. The particle size distribution of Pd NPs on the polymer was evaluated using TEM and showed that the maximum numbers of Pd NPs were around 9 nm in diameter (Fig. 5d).
The thermal behaviour of the prepared polymer was investigated by TGA in oxidative environment (air). TGA thermogram for the cross‐linked poly (ITC-HPTPy) is shown in Fig. 6. The TGA result can prove that the polymer has excellent thermal stabilities up to 320 °C in air. Maximum weight loss occurred between 350 and 470 °C and above 600 °C, virtually no mass remains.
Figure 7 presents XRD pattern of the MP-TPy/Pd. The diffraction peaks at the Bragg angles of 40.10°, 46.66°, and 68.14° correspond to the 111, 200, and 220 facets of elemental palladium in the cross‐linked poly (ITC-HPTPy)-Pd [23, 24].
A sample of the cross‐linked poly (ITC-HPTPy)-Pd was studied using EDX technique for precise elemental analysis, and successful synthesis of the cross‐linked poly (ITC-HPTPy)-Pd was inferred from this technique. EDX spectra show the presence of C, O, N and Pd in the cross‐linked poly (ITC-HPTPy)-Pd (Fig. 8).
The cross‐linked poly (ITC-HPTPy)-Pd was also studied by XPS, the observed characteristic peaks in XPS are attributed to C 1 s, O 1 s and Pd 3d (Fig. 9a). Furthermore, high resolution XPS results confirmed the presence of Pd (0) and Pd (II) on the catalyst (Fig. 9b). The peaks at 335.5 eV (Pd 3d5/2) and 340.5 eV (Pd 3d3/2) regard to Pd (0), whereas peaks at 336.5 eV (Pd 3d5/2) and 341.5 eV (Pd 3d3/2) are assigned for Pd (II) species present on the polymer [25, 26]. The XPS results also allow us to determine the percentage of the respective oxidation states of Pd by measuring the area under the peaks: 41% as Pd(0) and 59% as Pd(II) in the sample.
3.2 Catalytic Studies
The performance of cross‐linked poly (ITC-HPTPy)-Pd catalyst was initially tested for the Suzuki–Miyaura coupling reaction. Phenyl boronic acid and iodobenzene were selected as a model substrate for optimization of the Suzuki C‐C coupling reaction (Table 2). As shown in the Table, the catalytic activity of cross‐linked poly (ITC-HPTPy)-Pd catalyst was studied in different temperatures, solvents, bases, and in the presence of various mol% of the cross‐linked poly (ITC-HPTPy)-Pd for 2 h. As the first parameter of these set, the role of cross‐linked poly (ITC-HPTPy)-Pd as a catalyst was investigated. In the absence of any catalyst and only in the presence of K2CO3 (1.5 mmol) at 80 °C, the product was not formed (Table 2, entry 1). However, the use of a small amount (0.12, 0.16 and 0.23 mol%) of cross‐linked poly (ITC-HPTPy)-Pd gave the desired product in good (65%) to excellent (98%) yields (Table 2, entries 2-4). It was found that applying 0.01 g of cross‐linked poly (ITC-HPTPy)-Pd (0.23 mol%) and 1.5 mmol of K2CO3 as base at 80 °C for 2 h in a mixture of EtOH/H2O (2/1) as solvent, is the best conditions for the reaction of iodobenzene (1 mmol) with phenylboronic acid (1.2 mmol) (Table 2, entry 4).
The generality and scope of this approach were illustrated in the reaction of commercially available aryl halides with arylboronic acids (Table 3). The cross‐linked poly (ITC-HPTPy)-Pd effectively catalyzed the coupling reaction between aryl halides with arylboronic acids to afford the corresponding biaryl products in excellent yields. The aryl iodides, bromides and chlorides are effectively treated as substrates with this procedure, though, the reaction of aryl chlorides required longer reaction times.

As an extension to use of the catalyst, we also investigated the catalytic activities of the cross‐linked poly (ITC-HPTPy)-Pd for the Mizoroki–Heck coupling reaction. The iodobenzene and styrene were selected as substrates to optimization of condition in the presence of different mol% of the cross‐linked poly (ITC-HPTPy)-Pd and various bases and solvents at 100 °C (Table 4). As can be seen from the Table 4, the best result was obtained in 98% yield by carrying out the reaction with aryl iodide (1 mmol), styrene (1.2 mmol) in the presence of 0.27 mol% of cross‐linked poly (ITC-HPTPy)-Pd and Et3N (3 mmol) as base, under solvent-free condition at 100 °C (Table 4, entry 4).
Using the optimized reaction conditions, in a simple experimental procedure, several trans-stilbene compounds were prepared and the results are presented in Table 5. As the table shows electronic effect of the substituents was not generally observed and donor- and acceptor substituted aryl iodides have been reacted with styrene in excellent yields. However, when iodobenzene was replaced by bromobenzene, the reactions require longer reaction times. However, the scope of this methodology was found not to be effective to chlorobenzene (Table 5, entry 7).

The performance of the present catalyst has been compared with some Pd-based nanocatalysts. Table 6 shows the comparison between cross‐linked poly (ITC-HPTPy)-Pd as a catalyst for Suzuki coupling of iodobenzene and phenylboronic acid with some Pd-based nanocatalysts in terms of mol% of Pd, reaction time, and yields of the product. The results of comparison show that this catalyst is superior to some previously reported nanocatalysts in terms of mol% of Pd used, product yield and reaction time.
Reusability is important characteristic of the heterogeneous catalyst to be used for industrial applications, due to the reducing production cost and environmental pollution substantially. We investigated recyclability of the cross-linked poly (ITC-HPTPy)-Pd by using the reaction of iodobenzene and phenylboronic acid. This heterogeneous catalyst easily separated from the reagents and products, and washed with ethanol and followed by water, dried for the next run. It is interesting to note that, recycling experiments confirmed the chemical stability and reusability of the catalyst and the recovered catalyst can be used during five runs (Fig. 10). To confirm this further, leaching of palladium during the course of the catalytic reactions was exactly evaluated by ICP analysis. ICP of the recovered catalyst after six catalytic cycles indicated that 26% of palladium was leached into the reaction medium (0.1 g of the manufactured catalyst and the catalyst after 6th run, contained 0.023 and 0.017 mmol of Pd, respectively).
The EDX spectrum of the recovered nanocatalyst is presented in Fig. 11, atoms such as C, O, N and Pd related to the catalyst structure, are seen in the spectrum. However the EDX spectra shows little leaching of Pd species into reaction solutions over multiple uses according to relative peak surfaces. Furthermore, The SEM images of the recovered catalyst after sixth run (Fig. 12) prove the stability of the nanocatalyst after several uses.
4 Conclusion
In summary, cross‐linked poly (ITC-HPTPy)-Pd has been prepared and fully characterized. As expected, cross‐linked poly (ITC-HPTPy)-Pd exhibited excellent activity in Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions under low palladium loading conditions, and provided the corresponding products with excellent yields (up to 98%) and high catalytic activities (TOF up to 213 h−1). It is noteworthy that the Pd NPs were prepared by the in situ reduction of the PdCl2 precursor in the presence of cross‐linked poly (ITC-HPTPy) in ethanol as a green reducing agent. Furthermore the terpyridine-based ligands existing in the polymer structure can be considered as agent to stabilize Pd NPs. Ultimately we believe that this work offers several advantages including simplicity of product workup and separation of the catalyst. In addition, nanocatalyst was reused consecutive six times with small drop in catalytic activity, considering the high cost of palladium, reuse of the catalysts could lead to economical automation system.
References
Fortman GC, Nolan SP (2011) N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem Soc Rev 40:5151–5169
Corbet JP, Mignani G (2006) Selected patented cross-coupling reaction technologies. Chem Rev 106:2651–2710
Nicolaou KC, Bulger PG, Sarlah D (2005) Palladium-catalyzed cross-coupling reactions in total synthesis. Angew Chem Int Ed 44:4442–4489
Aijaz A, Xu Q (2014) Catalysis with metal nanoparticles immobilized within the pores of metal–organic frameworks. J Phys Chem Lett 5:1400–1411
Jiang Y, Gao Q (2006) Heterogeneous hydrogenation catalyses over recyclable Pd (0) nanoparticle catalysts stabilized by PAMAM-SBA-15 organic–inorganic hybrid composites. J Am Chem Soc 128:716–717
Ramirez E, Jansat S, Philippot K, Lecante P, Gomez M, Masdeu-Bultó AM, Chaudret B (2004) Influence of organic ligands on the stabilization of palladium nanoparticles. J Organomet Chem 689:4601–4610
Tatumi R, Akita T, Fujihara H (2006) Synthesis of small palladium nanoparticles stabilized by bisphosphine BINAP bearing an alkyl chain and their palladium nanoparticle-catalyzed carbon–carbon coupling reactions under room-temperature. Chem Commun 31:3349–3351
Sobhani S, Zeraatkar Z, Zarifi F (2015) Pd complex of an NNN pincer ligand supported on γ-Fe2O3@ SiO2 magnetic nanoparticles: a new catalyst for Heck, Suzuki and Sonogashira coupling reactions. New J Chem 39:7076–7085
Kwak Y, Matyjaszewski K (2009) ARGET ATRP of methyl methacrylate in the presence of nitrogen-based ligands as reducing agents. Polym Int 58:242–247
Targhan H, Hassanpour A, Bahrami K (2019) Highly efficient polymer-stabilized palladium heterogeneous catalyst: synthesis, characterization and application for Suzuki-Miyaura and Mizoroki-Heck coupling reactions. Appl Organomet Chem. https://doi.org/10.1002/aoc.5121
Pomogailo AD (2004) Catalysis by heterogenized metal polymers: advances and prospects. Kinet Catal 45:61–104
Kobayashi S, Nagayama SA (1996) polymer-supported scandium catalyst. J Org Chem 61:2256–2257
Zhao D, Ding K (2013) Recent advances in asymmetric catalysis in flow. ACS Catal 3:928–944
Puglisi A, Benaglia M, Chiroli V (2013) Stereoselective organic reactions promoted by immobilized chiral catalysts in continuous flow systems. Green Chem 15:1790–1813
Folarin OM, Sadiku ER, Maity A (2013) Polymer-noble metal nanocomposites. Int J Phys Sci 6:4869–4882
Spahni W, Calzagerri G (1984) Synthese von para-substituierten phenyl-terpyridin liganden. Helv Chim Acta 67:450–454
Bahrami K, Targhan H (2019) A new strategy to design a graphene oxide supported palladium complex as a new heterogeneous nanocatalyst and application in carbon–carbon and carbon-heteroatom cross-coupling reactions. Appl Organometal Chem. https://doi.org/10.1002/aoc.4842
Putta C, Sharavath V, Sarkar S, Ghosh S (2015) Palladium nanoparticles on β-cyclodextrin functionalised graphene nanosheets: a supramolecular based heterogeneous catalyst for C-C coupling reactions under green reaction conditions. RSC Adv 5:6652–6660
Villa A, Wang D, Spontoni P, Arrigo R, Su D, Prati L (2010) Nitrogen functionalized carbon nanostructures supported Pd and Au–Pd NPs as catalyst for alcohols oxidation. Catal Today 157:89–93
Hulubei C, Vlad CD, Stoica I, Popovici D, Lisa G, Nica SL, Barzic AI (2014) New polyimide-based porous crosslinked beads by suspension polymerization: physical and chemical factors affecting their morphology. J Polym Res 21:514
Shaik M, Ali Z, Khan M, Kuniyil M, Assal M, Alkhathlan H, Al-Warthan A, Siddiqui M, Khan M, Adil S (2017) Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules 22:165–177
Gholinejad M, Hamed F, Biji P (2015) A novel polymer containing phosphorus–nitrogen ligands for stabilization of palladium nanoparticles: an efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water. Dalton Trans 44:14293–14303
Long Y, Liu Y, Zhao Z, Long Y, Liu Y, Zhao Z, Luo S, Wu W, Wu L, Wen H, Wang RQ, Ma J (2017) Distinctive morphology effects of porous-spherical/yolk-shell/hollow Pd-nitrogen-doped-carbon spheres catalyst for catalytic reduction of 4-nitrophenol. J Colloid Interfaces Sci 496:465–473
Bahrami K, Khodamorady M (2018) Design of BNPs-TAPC palladium complex as a reusable heterogeneous nanocatalyst for the O-arylation of phenols and N-arylation of amines. Catal Lett 149:688–698
Labulo AH, Omondi B, Nyamori VO (2018) Suzuki-Miyaura reaction and solvent free oxidation of benzyl alcohol by Pd/nitrogen-doped CNTs catalyst. J Mater Sci 53:15817–15836
Long Y, Zhao Z, Wu L, Luo S, Wen H, Wu W, Zhang H, Ma J (2017) Distinctive ligand effects of functionalized magnetic microparticles immobilizing palladium acetate as heterogeneous coordination catalysts for selective oxidation of styrene to acetophenone. Mol Catal 433:291–300
Peng YY, Liu J, Lei X, Yin Z (2010) Room-temperature highly efficient Suzuki-Miyaura reactions in water in the presence of Stilbazo. Green Chem 12:1072–1075
Zeng X, Zhang T, Qin Y, Wei Z, Luo M (2009) Synthesis of a carbene transfer organometallic polymer and application to forming a recyclable heterogeneous catalyst for the Suzuki reactions of aryl chlorides. Dalton Trans 39:8341–8348
Hey DH, Orman S, Williams GH (1965) Homolytic aromatic substitution. Part XXXII. Some reactions with meta-substituted phenyl radicals. J Chem Soc. https://doi.org/10.1039/JR9650000101
Xu HJ, Zhao YQ, Zhou XF (2011) Palladium-catalyzed Heck reaction of aryl chlorides under mild conditions promoted by organic ionic bases. J Org Chem 76:8036–8041
Iranpoor N, Firouzabadi H, Tarassoli A, Fereidoonnezhad M (2010) 1, 3, 2, 4-Diazadiphosphetidines as new P-N ligands for palladium-catalyzed Heck reaction in water. Tetrahedron 66:2415–2421
Kim S, Cho HJ, Shin DS, Lee SM (2017) Recyclable and eco-friendly Pd-complexed graphene oxide/N-heterocyclic carbene catalyst for various coupling reactions in aqueous phase. Tetrahedron Lett 58:2421–2425
Dong D, Li Z, Liu D, Yu N, Zhao H, Chen H, Liu J, Liu D (2018) Postsynthetic modification of single Pd sites into uncoordinated polypyridine groups of a MOF as the highly efficient catalyst for Heck and Suzuki reactions. New J Chem 42:9317–9323
Khajehzadeh M, Moghadam M (2018) A new poly (N–heterocyclic carbene Pd complex) immobilized on nano silica: an efficient and reusable catalyst for Suzuki-Miyaura, Sonogashira and Heck-Mizoroki C-C coupling reactions. J Organomet Chem 863:60–69
Singh AS, Shelkar RS, Nagarkar JM (2015) Palladium (II) on functionalized NiFe2O4: an efficient and recyclable phosphine-free heterogeneous catalyst for Suzuki coupling reaction. Catal Lett 145:723–730
Bahrami K, Kamrani SN (2018) Synthesis, characterization and application of graphene palladium porphyrin as a nanocatalyst for the coupling reactions such as: suzuki-Miyaura and Mizoroki-Heck. Appl Organomet Chem 32:e4102
Kwon TH, Cho KY, Baek KY, Yoon HG, Kim BM (2017) Recyclable palladium–graphene nanocomposite catalysts containing ionic polymers: efficient Suzuki coupling reactions. RSC Adv 7:11684–11690
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Targhan, H., Hassanpour, A., Sohrabnezhad, S. et al. Palladium Nanoparticles Immobilized with Polymer Containing Nitrogen-Based Ligand: A Highly Efficient Catalyst for Suzuki–Miyaura and Mizoroki–Heck Coupling Reactions. Catal Lett 150, 660–673 (2020). https://doi.org/10.1007/s10562-019-02981-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02981-7