Abstract
WO3 role in NO reduction over V2O5-WO3/TiO2 was studied by comparing the kinetic parameters of the selective catalytic reduction (SCR) reaction, the non selective catalytic reduction (NSCR) reaction and the C–O reaction. The results show that the SCR reaction over V2O5/TiO2 was promoted after WO3 incorporation, while the NSCR reaction and the C–O reaction were both restrained.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Now, selective catalytic reduction (SCR) of NO by NH3 is the major technology to control the emission of nitrogen oxides from coal fired plants [1–3] and V2O5-WO3/TiO2 is widely used as the commercial catalyst [4, 5]. WO3 in V2O5-WO3/TiO2 can increase the SCR activity, widen the temperature range and improve N2 selectivity [6, 7]. So far, the mechanism of WO3 role in the SCR reaction over V2O5-WO3/TiO2 has been widely studied [8, 9] and the promotion was generally attributed to the increase of the acidity on V2O5/TiO2, the delay of the loss of the BET surface, the inhibition of the transformation of monomeric vanadyl to crystalline V2O5, and the improvement of the reducibility of V component [10].
The non selective catalytic reduction (NSCR) reaction and the catalytic oxidation of NH3 to NO (C–O reaction) can simultaneously happen during the SCR reaction over V2O5-WO3/TiO2 especially at higher temperatures, resulting in the formation of N2O and the decrease of NO conversion at higher temperatures [11]. Therefore, the SCR performance (including NO x conversion and N2 selectivity) depends on the competition of the SCR reaction, the NSCR reaction and the C–O reaction. Previous studies mainly aimed at WO3 role in the SCR reaction. However, WO3 role in the side reactions (i.e. the NSCR reaction and the C–O reaction) was seldom investigated. In our previous work, a global kinetics of NO reduction over V2O5-WO3/TiO2 (including the SCR reaction, the NSCR reaction and the C–O reaction) was built [12] and their kinetic parameters can be obtained from the steady-state kinetic study. In this work, WO3 role in NO reduction over V2O5-WO3/TiO2 was studied by comparing the kinetic parameters of NO reduction over V2O5/TiO2 and V2O5-WO3/TiO2. The results show that the SCR reaction over V2O5/TiO2 was promoted after WO3 incorporation due to the promotion of NH3 adsorption and the increase of oxidation ability. Meanwhile, the C–O reaction was restrained due to the decrease of V5+ concentration. As the SCR reaction contributed to NO reduction while the C–O reaction contributed to NO formation, NO x conversion over V2O5/TiO2 obviously increased after WO3 incorporation. Furthermore, the NSCR reaction was restrained due to the decrease of V5+ concentration, resulting in an obvious increase of N2 selectivity.
2 Experimental
2.1 Catalyst Preparation
V2O5-WO3/TiO2 (with 5 wt% V2O5 and 10 wt% WO3) and V2O5/TiO2 (with 5 wt% V2O5) were prepared by the conventional impregnation method using NH4VO3, (NH4)10W12O41 (when used) as precursors, H2C2O4·2H2O as the dissolving promoter and P25 as support [7, 13].
2.2 Characterization
BET surface areas, H2-temperature programmed reduction (H2-TPR), X-ray diffraction patterns (XRD), X-ray photoelectron spectra (XPS) and in situ DRIFT spectra were performed on a nitrogen adsorption apparatus (Quantachrome, Autosorb-1), a chemisorption analyzer (Micromeritics, ChemiSorb 2720 TPx), an X-ray diffractionmeter (Bruker-AXS D8 Advance), an X-ray photoelectron spectroscopy (Thermo, ESCALAB 250) and a Fourier transform infrared spectrometer (Nicolet IS 50), respectively.
2.3 Catalytic Test
The SCR reaction and temperature programmed desorption of NH3 (NH3-TPD) were performed on a fixed-bed quartz tube micro-reactor [14]. 100 mg of catalyst with 40–60 mesh and 200 mL min−1 of the simulated flue gas containing 500 ppm of NO, 500 ppm of NH3, 2 % of O2, and balance of N2 were used and the corresponding gas hourly space velocity (GHSV) was 1.2 × 105 cm3 g−1 h−1. The concentrations of NO, NO2, NH3 and N2O in the inlet or outlet were determined online by an infrared spectrometer (Thermo SCIENTIFIC, ANTARIS, IGS Analyzer).
2.4 Steady State Kinetic Study
Gaseous NH3 concentration in the inlet was kept at 500 ppm, while gaseous NO concentration varied from 300 to 700 ppm [15, 16]. To minimize the inner diffusion and external diffusion, a very high GHSV of 4.8 × 106 − 1.2 × 107 cm3 g−1 h−1 was adopted to keep NO conversion <15 % [17].
3 Results
3.1 SCR Performance
Figure 1 shows that the SCR activity of V2O5-WO3/TiO2 was higher than that of V2O5/TiO2 at 150–500 °C. Meanwhile, N2O selectivity of NO reduction over V2O5/TiO2 was much higher than that over V2O5-WO3/TiO2. They suggest that the SCR performance of V2O5/TiO2 obviously improved after WO3 incorporation, which was consistent with the result of previous study [6]. Moreover, NH3 conversions of both V2O5/TiO2 and V2O5-WO3/TiO2 were close to NO conversions at 150–250 °C, and higher than NO conversions at 300–500 °C. It suggests that the C–O reaction happened at 300–500 °C over V2O5/TiO2 and V2O5-WO3/TiO2.
3.2 Characterization
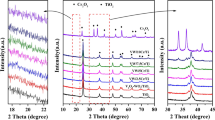
The characteristic peaks appeared in the XRD patterns of V2O5-WO3/TiO2 and V2O5/TiO2 mainly corresponded to anatase (JCPDS: 21-1272) and rutile (JCPDS: 21-1276) (shown in Fig. 2) [7]. Meanwhile, other characteristic peaks corresponding to tungsten oxides and vanadium oxides cannot be clearly observed. It suggests that the loaded tungsten oxides and vanadium oxides were well dispersed on P25. The BET surface areas of V2O5/TiO2 and V2O5-WO3/TiO2 were 41.6 and 49.3 m2 g−1, respectively.
XPS spectra of V2O5/TiO2 and V2O5-WO3/TiO2 over the spectral regions of V2p and W 4f are shown in Fig. 3. The binding energies of V 2p 3/2 on V2O5/TiO2 mainly appeared at 515.6 and 517.1 eV, which were attributed to V4+ and V5+ on V2O5/TiO2, respectively [18, 19]. After WO3 was loaded, no significant changes can be observed in the spectral region of V 2p. Meanwhile, the binding energies of W 4f species mainly appeared at 35.6 and 37.5 eV, which were assigned to W6+ on the surface [20]. The percentages of V, Ti, O and W species on V2O5/TiO2 and V2O5-WO3/TiO2 were calculated from the XPS spectra. As shown in Table 1, the percentages of V5+ on V2O5/TiO2 decreased from 5.9 to 3.0 % after WO3 incorporation.
H2-TPR profiles (Fig. 4) show a clear reduction peak at approximately 504 °C on V2O5/TiO2 and V2O5-WO3/TiO2, which was attributed to the reduction of V5+ on the surface [10, 21]. Some H2 consumption can be clearly observed on V2O5-WO3/TiO2 below 300 °C, while it cannot be observed on V2O5/TiO2. It suggests the oxidation ability of V5+ on V2O5-WO3/TiO2 was much higher than that over V2O5/TiO2 [10]. Figure 4 also shows that the area of the first reduction peak of V2O5-WO3/TiO2 was much less than that on V2O5/TiO2. It suggests that V5+ concentration on V2O5-WO3/TiO2 was much less than that of V2O5/TiO2, which was consistent with the result of XPS analysis.
Figure 5 shows NH3-TPD profiles of V2O5/TiO2 and V2O5-WO3/TiO2. The integration of NH3-TPD profiles (Fig. 5) shows that the capacities of V2O5/TiO2 and V2O5-WO3/TiO2 for NH3 adsorption at 200 °C were approximately 101 and 131 μmol g−1, respectively. It suggests that the adsorption of NH3 on V2O5/TiO2 was promoted after WO3 incorporation.
3.3 In situ DRIFT Study
After the introduction of NH3 at 250 °C for 30 min, V2O5/TiO2 was mainly covered by coordinated NH3 bound to the Lewis acid sites (at 1220, 1246 and 1602 cm−1) and ionic NH4 + bound to the Brønsted acid sites (1422 and 1675 cm−1) [13]. After NO+O2 were then introduced, adsorbed NH3 species (i.e. coordinated NH3 and ionic NH4 +) gradually decreased (shown in Fig. 6a). It suggests that the Eley–Rideal mechanism (i.e. the reaction between gaseous NO and adsorbed NH3 species) can contribute to NO reduction over V2O5/TiO2.
a In situ DRIFT spectra taken at 250 °C upon passing NO+O2 over NH3 presorbed V2O5/TiO2; b In situ DRIFT spectra taken at 250 °C upon passing NH3 over NO+O2 presorbed V2O5/TiO2; c In situ DRIFT spectra taken at 250 °C upon passing NO+O2 over NH3 presorbed V2O5-WO3/TiO2; d In situ DRIFT spectra taken at 250 °C upon passing NH3 over NO+O2 presorbed V2O5-WO3/TiO2
After the introduction of NO+O2 at 250 °C for 30 min, V2O5/TiO2 was mainly covered by monodentate nitrite (at 1610 cm−1) [22]. After NH3 was then introduced, adsorbed monodentate nitrite rapidly diminished. At last, V2O5/TiO2 was mainly covered by coordinated NH3 and ionic NH4 + (shown in Fig. 6b). It suggests that the Langmuir–Hinshelwood mechanism (i.e. the reaction between adsorbed NOx and adsorbed NH3 species) can contribute to NO reduction over V2O5/TiO2.
In situ DRIFT spectra taken at 250 °C upon passing NO+O2 over NH3 presorbed V2O5-WO3/TiO2 and those upon passing NH3 over NO+O2 presorbed V2O5-WO3/TiO2 were similar to those over V2O5/TiO2 (shown in Fig. 6c, d). Therefore, both the Langmuir–Hinshelwood mechanism and the Eley–Rideal mechanism can contribute to NO reduction over V2O5-WO3/TiO2.
3.4 Steady-State Kinetic Study
To investigate WO3 role in the SCR reaction, the NSCR reaction and the C–O reaction during NO reduction over V2O5-WO3/TiO2, the steady-state kinetic study was performed. Figures 7a–c and 8a–c show the dependences of the rates of NH3 conversion, NO x conversion and N2O formation on gaseous NO concentration during NO reduction over V2O5/TiO2 and V2O5-WO3/TiO2. The SCR reaction, the NSCR reaction and the C–O reaction all contributed to NH3 conversion [11]. The SCR reaction and the NSCR reaction contributed to NO reduction, while the C–O reaction contributed to NO formation [23]. Therefore, the rates of NH3 conversion (\(\delta _{{{\text{NH}}_{3} }}\)) and NO x conversion (\(\delta _{{{\text{NO}}_{{\text{x}}} }}\)) can be described as follows [24]:
where, δ SCR, δ NSCR and δ C–O were the rates of the SCR reaction, the NSCR reaction and the C–O reaction, respectively.
Then, δ C–O can be calculated as [25, 26]:
The rates of the SCR reaction and the NSCR reaction were equal to the rates of N2 formation and N2O formation, respectively. Figures 7 and 8 show that both δ NSCR and δ C–O of V2O5/TiO2 were much higher than those of V2O5-WO3/TiO2. However, δ SCR of V2O5/TiO2 was generally much less than that of V2O5-WO3/TiO2. It suggests that the SCR reaction over V2O5/TiO2 was promoted after WO3 incorporation, while both the NSCR reaction and the C–O reaction were restrained remarkably. Therefore, both NOx conversion and N2 selectivity of NO reduction over V2O5/TiO2 obviously increased after WO3 incorporation.
4 Discussion
The SCR reaction through the Eley–Rideal mechanism, the NSCR reaction and the C–O reaction during NO reduction over V2O5/TiO2 and V2O5-WO3/TiO2 can be approximately described as [27]:
Meanwhile, NO reduction through the Langmuir–Hinshelwood mechanism can be approximately described as [28]:
The kinetic equations of Reactions 6 and 8 can be described as [29]:
where, k 1, k 2, [NH2], [NH], [V5+=O] and [NO(g)] were the kinetic constants of Reactions 6 and 8, the concentrations of NH2, NH and V5+ on the surface, and gaseous NO concentration, respectively.
The kinetic equations of Reactions 5 and 7 can be described as:
where, k 3, k 4 and [NH3(ad)] were the kinetic constants of Reactions 5 and 7, and the concentration of NH3 adsorbed, respectively.
Furthermore, the kinetic of Reaction 9 can be approximately described as:
where, k 5 was the kinetic constant of Reaction 9.
NH concentration on the surface would not vary as the reaction reached the steady-state. Thus,
Therefore,
Then, Eqs. 15 and 18 can be transformed as follows [12]:
Furthermore, the kinetic equation of Reaction 13 can be described as:
where, k 6 and [V4+–O–NO–NH3] were the rate constant of NH4NO2 decomposition and NH4NO2 concentration on the surface, respectively.
Then, the SCR reaction rate can be described as:
Our previous study demonstrated that NH4NO2 concentration on the surface can be approximately regarded as a constant at the steady-state [12, 30], so the rate of the SCR reaction through the Langmuir–Hinshelwood mechanism was nearly independent of gaseous NO and NH3 concentrations. Our previous study also demonstrated that NH2 concentration on the surface at the steady-state was approximately independent of gaseous NO and NH3 concentrations [12, 31], so the rate of the SCR reaction through the Eley–Rideal mechanism was nearly in direct proportion to gaseous NO concentration. Therefore, the parameters of k 6[V3+–O–NO–NH3] and k 1[NH2] can be obtained after the linear regression of δ SCR with gaseous NO concentration. Furthermore, the parameters of k 4[NH2][V5+=O] and k 2/k 5 can also be obtained after the non-linear regression of δ C–O with gaseous NO concentration. δ c-o of V2O5-WO3/TiO2 and V2O5/TiO2 were both close to 0 at 200–350 °C, so k 5 was approximately 0. Then, k 4[NH2][V5+=O] at 200–350 °C can be approximately obtained from the average values of \(\delta _{{{\text{N}}_{2} {\text{O}}}}\) (hinted by Eq. 21) [12].
The parameters of k 6[V3+–O–NO–NH3], k 1[NH2], k 4[NH2][V5+=O] and k 2/k 5, which resulted from the regression of Figs. 7 and 8, were listed in Table 2. k 1 was the reaction kinetic constant of Reaction 6, so it of V2O5/TiO2 would be equal to that of V2O5-WO3/TiO2. [NH2] on V2O5/TiO2 was proportional to the products of k 3, [NH3(ad)] and [V5+=O] (hinted by Eq. 16). k 3 was related to the oxidation ability of V5+ on the surface, so it of V2O5/TiO2 was less than that of V2O5-WO3/TiO2 (hinted by the H2-TPR analysis). NH3-TPD analysis shows that the adsorption of NH3 on V2O5/TiO2 was promoted after WO3 incorporation. Although [V5+=O] on V2O5/TiO2 was higher than that on V2O5-WO3/TiO2, both k 3 and [NH3(ad)] of V2O5/TiO2 were less than those of V2O5-WO3/TiO2. Therefore, [NH2] on V2O5/TiO2 was much less than that on V2O5-WO3/TiO2. As a result, k 1[NH2] of V2O5/TiO2 obviously increased after WO3 incorporation (shown in Table 2), resulting in an obvious promotion of the SCR reaction through the Eley–Rideal mechanism (hinted by Eq. 14).
As V5+ concentration on V2O5-WO3/TiO2 was much less than that on V2O5/TiO2, the probability of two V5+ on the adjacent sites for the over-activation of NH3 to NH on V2O5-WO3/TiO2 was much less than that of V2O5/TiO2. It suggests that k 4 of V2O5-WO3/TiO2 was much less than that of V2O5/TiO2. Although [NH2] on V2O5-WO3/TiO2 was much higher than that of V2O5/TiO2, both k 4 and [V5+=O] of/on V2O5-WO3/TiO2 was much less than those of/on V2O5/TiO2. Therefore, k 4[NH2][V5+=O] of V2O5/TiO2 obviously decreased after WO3 incorporation (shown in Table 2), resulting in a remarkable inhibition of the NSCR reaction (hinted by Eq. 21).
As V5+ concentration on V2O5-WO3/TiO2 was much less than that on V2O5/TiO2, the probability of three V5+ on the adjacent sites for the deep oxidation of NH3 to gaseous NO on V2O5-WO3/TiO2 was much less than that of V2O5/TiO2. It suggests that k 5 of V2O5-WO3/TiO2 was much less than that of V2O5/TiO2. Meanwhile, k 2 was the reaction kinetic constant of Reaction 8, so it of V2O5/TiO2 would be approximately equal to that of V2O5-WO3/TiO2. Therefore, k 5/k 2 of V2O5/TiO2 obviously decreased after WO3 incorporation (shown in Table 2). Meanwhile, k 4[NH2][V5+=O] of V2O5/TiO2 obviously decreased after WO3 incorporation. Hinted by Eq. 22, the C–O reaction over V2O5/TiO2 was remarkably inhibited after WO3 incorporation.
5 Conclusion
The steady-state kinetic study demonstrated that the SCR reaction over V2O5/TiO2 was promoted after WO3 incorporation due to the promotion of NH3 adsorption and the increase of oxidation ability. Meanwhile, the C–O reaction was restrained due to the decrease of V5+ concentration. As the SCR reaction contributed to NO reduction while the C–O reaction contributed to NO formation, NOx conversion over V2O5/TiO2 obviously increased after WO3 incorporation. Furthermore, the NSCR reaction was restrained due to the decrease of V5+ concentration, resulting in an obvious increase of N2 selectivity.
References
Liu Z, Zhu J, Zhang S, Ma L, Woo SI (2014) Catal Commun 46:90–93
Peng Y, Li K, Li J (2013) Appl Catal B Environ 140–141:483–492
Liu F, He H, Zhang C (2008) Chem Commun 17:2043–2045
Chen L, Li J, Ge M (2009) J Phys Chem C 113:21177–21184
Qi G, Yang RT (2003) J Catal 217:434–441
Lietti L, Forzatti P, Bregani F (1996) Ind Eng Chem Res 35:3884–3892
Wang C, Yang S, Chang H, Peng Y, Li J (2013) Chem Eng J 225:520–527
Amiridis MD, Duevel RV, Wachs IE (1999) Appl Catal B Environ 20:111–122
Giraud F, Geantet C, Guilhaume N, Loridant S, Gros S, Porcheron L, Kanniche M, Bianchi D (2015) J Phys Chem C 119:15401–15413
Kompio PG, Brückner A, Hipler F, Auer G, Löffler E, Grünert W (2012) J Catal 286:237–247
Yang S, Qi F, Liao Y, Xiong S, Lan Y, Fu Y, Shan W, Li J (2014) Ind Eng Chem Res 53:5810–5819
Xiong S, Xiao X, Liao Y, Dang H, Shan W, Yang S (2015) Ind Eng Chem Res 54:11011–11023
Yang S, Wang C, Ma L, Peng Y, Qu Z, Yan N, Chen J, Chang H, Li J (2013) Catal Sci Technol 3:161–168
Yang S, Wang C, Li J, Yan N, Ma L, Chang H (2011) Appl Catal B Environ 110:71–80
Yang SJ, Xiong SC, Liao Y, Xiao X, Qi FH, Peng Y, Fu YW, Shan WP, Li JH (2014) Environ Sci Technol 48:10354–10362
Xiong S, Liao Y, Dang H, Qi F, Yang S (2015) RSC Adv 5:27785–27793
Yang S, Qi F, Xiong S, Dang H, Liao Y, Wong PK, Li J (2016) Appl Catal B Environ 181:570–580
Zhao S, Ma Y, Qu Z, Yan N, Li Z, Xie J, Chen W (2014) Catal Sci Technol 4:4036–4044
Zhao L, Li C, Zhang J, Zhang X, Zhan F, Ma J, Xie Ye, Zeng G (2015) Fuel 153:361–369
Chen H, Xu N, Deng S, Lu D, Li Z, Zhou J, Chen J (2007) Nanotechnology 18:205701
Tang F, Xu B, Shi H, Qiu J, Fan Y (2010) Appl Catal B Environ 94:71–76
Hadjiivanov K (2000) Catal Rev 42:71–144
Yang S, Liu C, Chang H, Ma L, Qu Z, Yan N, Wang C, Li J (2013) Ind Eng Chem Res 52:5601–5610
Roduit B, Wokaun A, Baiker A (1998) Ind Eng Chem Res 37:4577–4590
Xiao X, Xiong S, Shi Y, Shan W, Yang S (2016) J Phys Chem C 120:1066–1076
Huang H, Lan Y, Shan W, Qi F, Xiong S, Liao Y, Fu Y, Yang S (2013) Catal Lett 144:578–584
Busca G, Lietti L, Ramis G, Berti F (1998) Appl Catal B Environ 18:1–36
Tuenter G, van Leeuwen WF, Snepvangers L (1986) Ind Eng Chem Res 25:633–636
Yang S, Liao Y, Xiong S, Qi F, Dang H, Xiao X, Li J (2014) J Phys Chem C 118:21500–21508
Xiong S, Liao Y, Xiao X, Dang H, Yang S (2015) J Phys Chem C 119:4180–4187
Xiong S, Liao Y, Xiao X, Dang H, Yang S (2015) Catal Sci Technol 5:2132–2140
Acknowledgments
This study was financially supported by the National Natural Science Fund of China (Grant Nos. 21207067 and 41372044) and the Natural Science Fund of Jiangsu Province (Grant No. BK20150036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, X., Xiong, S., Li, B. et al. Role of WO3 in NO Reduction with NH3 over V2O5-WO3/TiO2: A New Insight from the Kinetic Study. Catal Lett 146, 2242–2251 (2016). https://doi.org/10.1007/s10562-016-1852-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1852-0