Abstract
The energy profile for the water–gas-shift reaction has been calculated on the active sites of the industrially used Cu/ZnO/Al2O3 catalyst using the BEEF-vdW functional. Our theoretical results suggest that both active site motifs, a copper (211) step as well as a zinc decorated step, are equally active for the water–gas-shift reaction. We find that the splitting of water into surface OH* and H* constitutes the rate-limiting step and that the reaction proceeds through the carboxyl mechanism. Our findings also suggest that mixed copper-zinc step sites are most likely to exhibit superior activity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Main Text
The water–gas-shift (WGS) reaction (Eq. 1) is industrially relevant for several large-scale processes such as H2 production, ammonia synthesis, and methanol synthesis [1]. It is also a promising CO clean-up step for H2 streams in decentralized units for fuel cell applications [2]. The reaction is slightly exothermic (−41 kJ/mol), lower temperatures are hence advantageous, pushing the equilibrium towards high H2 and low CO contents. Industrially, the reaction often takes place in two steps, a high temperature and a low temperature step. The focus of this work is on the low-temperature WGS where copper-zinc catalysts are employed as they exhibit high WGS activity at moderate temperatures (200−270 °C) [3, 4]. The same class of catalysts is also used to convert synthesis gas, a mixture of CO, CO2 and H2, to methanol [5]. Here, CO2 is converted to methanol and water. Water subsequently reacts with CO to form CO2 and H2 via the WGS. Importantly, the WGS reaction is significantly faster than CO2 hydrogenation to methanol and hence equilibrated under industrial methanol synthesis conditions [6].
Owing to the industrial importance of the WGS reaction, a number of theoretical studies based on density functional theory have been performed on model catalysts [7–13]. Most commonly, the (111) facet of copper has been investigated [7–10]. Surface science experiments have shown, however, that the WGS reaction on copper is extremely structure sensitive, with the Cu(110) surface being more active than Cu(111) [14–16]. Also, experimental studies with Cu nanoparticles deposited on a SiO2 support by atomic layer epitaxy showed that defect sites on the Cu surface play a major role for the WGS activity by helping water activation and CO binding [17, 18]. The structure sensitivity has also been confirmed theoretically using stepped (110) and (321) surfaces [12, 13]. Most of these density functional theory studies have been performed using the PW91 functional [19], effects of the exchange–correlation functional on the H2O dissociation step using the PBE [20] and RevPBE [21] functional have also been considered [22]. We have shown recently that the BEEF-vdW [23] functional yields a more quantitative description of the conversion of CO2 and hydrogen to methanol over copper and copper-zinc surfaces [24, 25]. This can be attributed to the inclusion of van der Waals (vdW) forces in the description of adsorbate–surface interactions for larger adsorbates with dangling bonds. The intermediates of the WGS reaction are similar to those involved in CO2 hydrogenation and we therefore employ the BEEF-vdW functional in order to develop a quantitative picture of the WGS reaction. There is an old [26] and ongoing [2] debate about the mechansim of the low temperature WGS reaction, where mainly an associative mechanism via decomposition of a formate intermediate or a regenerative mechanism through complete (redox) or partial (carboxylate) dissociation of water have been discussed. For ceria supported Cu catalysts, there is recent ample experimental evidence that formate is a spectator species rather than an intermediate [27–30]. Herein, we consider a range of possible reactions involved in the WGS reaction network in the context of the redox and carboxylate mechanismsFootnote 1 that have also been described in earlier studies: [10].
where intermediates with an asterix are adsorbed on the surface. An asterix denotes a free site on the surface.
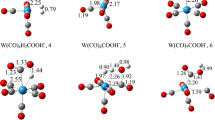
Recent experimental and theoretical work has identified the active site motif of the Cu/ZnO/Al2O3 catalyst that is used industrially to convert a mixture of CO2, CO and H2 to methanol [31]. Like the WGS reaction, methanol synthesis was found to be structure sensitive, here defects such as step sites are responsible for the high activity of the catalysts. It was further possible to identify the active sites for CO (a clean Cu(211) step) as well as CO2 hydrogenation (a Zn-doped Cu(211) step), and the industrially used methanol catalyst is thought to contain mainly the latter [25, 32]. Herein we report a detailed theoretical analysis of the WGS reaction using these two active site motifs (see Fig. 1). Employing the BEEF-vdW functional in connection with these active site motifs should give us a quite realistic description of the WGS reaction for the industrially employed Cu/ZnO/Al2O3 catalyst.
All DFT calculations were performed using the Quantum-Espresso code [33] using the ASE interface [34]. The active site motifs are identical to those reported earlier [25] and gas-phase CO2 and H2 energies were corrected as described elsewhere [25, 35]. Details about the calculational setup can be found in the experimental section. Figure 2 shows the calculated free energy diagram of the WGS reaction on a stepped Cu(211) surface via the (a) redox and (b) carboxyl mechanism.
Gibbs-free energy diagram of the water–gas-shift reaction via the redox (a) and carboxyl (b) mechanism on the Cu(211) surface (denoted here as the Cu-site). Reactions including hydrogen abstraction via adsorbed OH are depicted in red. All energies are relative to CO + H2O in the gas phase and the clean surfaces. Gibbs free energies were calculated at a temperature of 500 K, and standard pressure of all molecules
We will start by discussing the redox mechanism of the WGS reaction (Fig. 2a). Here the highest free energy barrier is the dissociation of OH* to O* and H*. This barrier is 2.16 eV above gas-phase CO and H2O, thus being prohibitively high. O* can also be produced via reaction of two OH* to yield water and O*. The corresponding free energy barrier is significantly lower being only 1.51 eV in terms of free energy. Both splitting of water to OH* and H* and the oxidation of CO* to CO2 have a lower free energy.
The bottleneck of the carboxyl mechanism (Fig. 2b) lies in the decomposition of COOH* to CO2 and H*. This transition state is located at 2.26 eV. Decomposition with the help of surface OH* (similar to that described for O–H splitting in the redox mechanism) substantially reduces the barrier to 1.20 eV. The rate determining step for the carboxyl mechanism is thus splitting of water to yield OH* and H*. The free energy barrier is 1.39 eV in. The results for both, the redox and carboxyl mechanism are in qualitative agreement with earlier studies on the (111) surface of Cu [10]. Based on the free energy diagram one would expect that the carboxyl mechanism dominates the total rate of the WGS reaction as the pathway proceeds via a free energy pathway that is about 0.1 eV lower than that of the redox pathway. H2O activation seems to constitute the rate-determining step. Both observations are in agreement with theoretical and kinetic studies on the Cu(111) surface [10].
Under the reaction conditions chosen here (see figure caption of Fig. 2), the H2O splitting free energy barrier is 1.39 eV above the educts, H2O (and CO). Given no other limitations this barrier would roughly correspond to a reaction rate of 10−1 per site and second using harmonic transition state theory. This highest free energy barrier is about 0.1 eV below those found for the hydrogenation of CO2 to methanol when employing the BEEF-vdW functional [24, 25], and in fair agreement with experimentally reported turnover rates of 10−2 s−1 [10, 36]. These rates are reported per total copper surface area, whereas steps usually only comprise 5 % of the total surface. Importantly, the difference of 0.1 eV between WGS and methanol synthesis would indicate that the WGS reaction is about 1 order of magnitude faster than methanol synthesis and hence equilibrated as observed experimentally [6, 14].
Figure 3 shows the free energy diagram of the WGS reaction over a Zn-doped Cu(211) step (denoted Zn-site, see also Fig. 1). We employ a fully Zn-doped copper step in our model here. We note that such sites only cover a fraction of active sites in a Cu/ZnO catalyst [25], as the extend of Zn surface alloying depends on the reduction potential of the reaction gas mixture [32]. Depicted are both the redox and carboxyl mechanism in analogy to what was discussed above for the Cu-site (see Fig. 2). Despite the different active site motif, the main conclusions that can be drawn for the energetics of the WGS reaction do not change significantly compared to the Cu-site. The carboxyl mechanism is the dominating pathway, surface OH helps reducing the barrier of the COO-H transition state significantly, and dissociation of water constitutes the rate-determining step. In fact, dissociation of H2O on the Zn-site has essentially the same barrier as dissociation on the Cu-site (1.38 vs. 1.39 eV). One would therefore expect that the rate of the WGS reaction is very similar on the Cu- and the Zn-site and certainly within the error of DFT. Indeed, there are experimental results that show that Zn-addition to Cu nanoparticles supported on different irreducible oxides clearly promotes CO2 conversion reactions like methanol synthesis and the reverse WGS reaction, but has a much lower effect on the forward WGS reaction or methanol steam reforming [37, 38], where the rate limiting step is related to water activation.
Gibbs-free energy diagram of the water–gas-shift reaction via the redox (a) and carboxyl (b) mechanism on then a Zn covered copper step (see Fig. 1). Reactions including hydrogen abstraction via adsorbed OH are depicted in red. All energies are relative to CO + H2O in the gas phase and the clean surfaces. Gibbs free energies were calculated at a temperature of 500 K
One can also speculate about the activity of mixed Cu–Zn-sites as these are likely present on the Cu/ZnO catalyst. The amount of Cu–Zn-sites depends on the preparation of the catalyst as well as the applied reaction conditions. Interestingly, atomic hydrogen and intermediates bound through carbon bind stronger on the Cu-site, whereas intermediates bound through oxygen bind stronger on the Zn-site [25]. This can be seen for example for the case of water splitting (see Figures, 2 and 3). Here, OH* binds 0.14 eV stronger whereas H* binds 0.33 eV weaker on the Zn-site. Both effects compensate each other so that the combined OH* + H* binding energy and the corresponding H2O dissociation barrier are almost the same for the two surfaces. Mixed Cu–Zn-sites should in principle have lower transition states as water splitting would result in H* binding to Cu and OH* binding to Zn, so that both species are stabilized to a maximum degree. The Cu/ZnO catalyst can therefore be thought of being comprised of Cu- and Zn-sites having similar activity as well as extremely active mixed Cu–Zn-sites. Performance catalysts will expose a high number of these mixed Cu–Zn-sites to maximize their beneficial interplay.
Support effects are well known to play an important role in Cu-catalyzed WGS. It was found that oxides help to dissociate H2O and that in particular oxygen vacancies in the supports are active in this respect and lower the activation energy of WGS [39]. In addition to Cu/ZnO, many recent reports focused on Cu/CeO2, for which an even higher promotion effect was detected [40]. Surface science studies revealed that Cu surfaces with a decoration of ceria nanoparticles do not only promote the reaction by facilitating H2O dissociation, but that in addition a substantial surface reconstruction of Cu was triggered by the presence of ceria [41]. This leads to the formation of many micro-terraces, which according to the structure-sensitivity of the reaction represents an additional independent oxide promotion effect. We note that our model of the active site(s) accounts for both effects, the reactivity of surface defect sites and the presence of reduced support species, by the stepped nature of the (211) model surface and by the (partial) decoration of the step with reduced Zn atoms, respectively. Thus, our results are largely consistent with the experimental evidence that defects and metal-support interaction plays a major role in activating H2O.
In summary we employed the BEEF-vdW functional to show how the WGS reaction is catalyzed over the Cu- and Zn-site of the industrial Cu/ZnO/Al2O3 catalyst. The reaction is found to proceed via the carboxyl pathway with H2O dissociation being the rate-determining step, similar to what has been observed for Cu(111) surfaces. This is also in agreement with recent experimental evidence supporting the carboxyl mechanism [42]. Based on calculated barriers for H2O dissociation on would expect turnover frequencies in the range of 10−1 per site and second in agreement with experimental data. We note here, however, that in order to make quantitative predictions about the reaction mechanism, possible rate-determining steps, and the reaction rates one would need to employ microkinetic or kinetic Monte Carlo simulations of the WGS reaction.
2 Methods
All calculations have been performed with the Quantum Espresso code [33] using a plane-wave basis set in the generalized gradient approximation. The plane-wave cutoff used in all calculations was 500 eV and the density cutoff was 5000 eV. The calculations were performed using the BEEF-vdW exchange–correlation functional, a functional that explicitly takes long-range dispersion forces into account [23]. The stepped Cu surface and the Zn substituted Cu surface (see Fig. 1) used to calculate the water–gas-shift energetics have been modeled using a 12 layered (3 × 1) unit cell resulting in a slab with 4 layers in the (111) direction having monoatomic steps between (111) facets with a (100) geometry. More than 12Å of vacuum have been included to separate periodic images and the Brillouin zone has been sampled using a (4 × 4 × 1) Monkhorst–Pack grid [43]. In all calculations the adsorbates and the atoms in the two topmost (111) layers were allowed to relax until forces became smaller than 0.05 eV/Å. The vibrational frequencies used to determine the zero-point energy and entropic contributions to the free energy have all been calculated within the harmonic approximation. Tables S1 and S2 list the total energies, zero-point energies, entropies and heat capacities of intermediates and transition states. For all transition state structures on the stepped Cu surface we found a single imaginary frequency thus identifying the geometry as a first-order saddle-point on the potential energy surface.
3 Supporting Information
Supporting information contains total energies of intermediates and transition states. This material is available free of charge via the Internet at http://pubs.acs.org
Notes
The term “redox mechanism” was originally introduced as “surface redox mechanism” in order to distinguish it from the true “redox mechanism” taking place on high-temperature iron oxide catalysts.
References
Hinrichsen KO, Kochloefl K, Muhler M (2008) In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis. Wiley, Weinheim
Ratnasamy C, Wagner JP (2009) Catal Rev Sci Eng 51:325
Ovesen CV, Stoltze P, Nørskov JK, Campbell CT (1992) J Catal 134:445
Ovesen CV, Clausen BS, Hammershøi BS, Steffensen G, Askgaard T, Chorkendorff I, Nørskov JK, Rasmussen PB, Stoltze P, Taylor P (1996) J Catal 158:170
Hansen JB, Nielsen PEH (2008) In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis. Wiley, Weinheim
Lee S (2007) In: Lee S, Speight JG, Loyalka SK (eds) Handbook of alternative fuel technologies. CRC Press, Boca Raton
Tang QL, Chen ZX, He X (2009) Surf Sci 603:2138
Lin CH, Chen CL, Wang JH (2011) J Phys Chem C 115:18582
Huang SC, Lin CH, Wang JH (2010) J Phys Chem C 114:9826
Gokhale AA, Dumesic JA, Mavrikakis M (2008) J Am Chem Soc 130:1402
Liu P, Rodriguez JA (2007) J Chem Phys 126:164705
Fajín JLC, Cordeiro MNDS, Illas F, Gomes JRB (2009) J Catal 268:131
Wang GC, Nakamura J (2010) J Phys Chem Lett 1:3053
Yoshihara J, Campbell CT (1996) J Catal 161:776
Campbell CT, Daube KA (1987) J Catal 104:109
Nakamura J, Campbell JM, Campbell CT (1990) J Chem Soc, Faraday Trans 86:2725
Chen CS, Lin JH, Lai TW, Liu BH (2009) J Catal 263:155
Chen CS, Lai TW, Chen CC (2010) J Catal 273:18
Perdew JP, Wang Y (1992) Phys Rev B 45:13244
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Zhang Y, Yang W (1998) Phys Rev Lett 80:890
Fajín JLC, Illas F, Gomes JRB (2009) J Chem Phys 130:224702
Wellendorff J, Lundgaard KT, Møgelhøj A, Petzold V, Landis DD, Nørskov JK, Bligaard T, Jacobsen KW (2012) Phys Rev B 85:235149
Studt F, Abild-Pedersen F, Varley JB, Nørskov JK (2013) Catal Lett 143:71
Studt F, Behrens M, Kunkes EL, Thomas N, Zander S, Tarasov A, Schumann J, Varley JB, Abild-Pedersen F, Nørskov JK, Schlögl R (2014) submitted
Rhodes C, Hutchings GJ, Ward AM (1995) Catal Today 23:43
Cámara AL, Chansai S, Hardacre C, Martínez-Arias A (2014) Int J Hydr Ener 39:4095
Barrio L, Estrella M, Zhou G, Wen W, Hanson JC, Hungria AB, Hornés A, Férnadez-García M, Martínez-Arias A, Rodriguez JA (2010) J Phys Chem C 114:3580
Wang X, Rodriguez JA, Hanson JC, Gamarra D, Martínez-Arias A, Fernández-García M (2006) J Phys Chem B 110:428
Yang Y, Mims CA, Disselkamp RS, Kwak JH, Peden CHF, Campbell CT (2010) J Phys Chem C 114:17205
Behrens M, Studt F, Kasatkin I, Kühl S, Hävecker M, Abild-Pedersen F, Zander S, Girgsdies F, Kurr P, Kniep BL, Tovar M, Fischer RW, Nørskov JK, Schlögl R (2012) Science 336:893
Kuld S, Conradsen C, Moses PG, Chorkendorff I, Sehested J (2014) Angew Chem Int Ed 53:5941
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M et al (2009) J Phys: Condens Matter 21:395502
Bahn SR, Jacobsen KW (2002) Comput Sci Eng 4:56
Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Nørskov JK (2010) Energy Environ Sci 3:1311
Koryabkina NA, Phatak AA, Ruettinger WF, Farrauto RJ, Ribeiro FH (2003) J Catal 217:233
Saito M, Wu J, Tomoda K, Takahara I, Murata K (2002) Catal Lett 83:1
Fujitani T, Nakamura I, Uchijima T, Nakamura J (1997) Surf Sci 383:285
Rodriguez JA, Liu P, Wang X, Wen W, Hanson J, Hrbek J, Pérez M, Evans J (2009) Catal Today 143:45
Rodriguez JA, Liu P, Hrbek J, Evans J, Pérez M (2007) Angew Chem Int Ed 46:1329
Senanayake SD, Stacchiola D, Rodriguez JA (2013) Acc Chem Res 46:1702
Yang Y, Mims CA, Mei DH, Peden CHF, Campbell CT (2013) J Catal 298:10
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Acknowledgments
We gratefully acknowledge the support from the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences to the SUNCAT Center for Interface Science and Catalysis. The authors would like to thank Jens K. Nørskov for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Studt, F., Behrens, M. & Abild-Pedersen, F. Energetics of the Water–Gas-Shift Reaction on the Active Sites of the Industrially Used Cu/ZnO/Al2O3 Catalyst. Catal Lett 144, 1973–1977 (2014). https://doi.org/10.1007/s10562-014-1363-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1363-9