Abstract
Hydrogenation of CO and CO2 to methanol on a stepped copper surface has been calculated using the BEEF-vdW functional and is compared to values derived with RPBE. It is found that the inclusion of vdW forces in the BEEF-vdW functional yields a better description of CO2 hydrogenation as compared to RPBE. These differences are significant for a qualitative description of the overall methanol synthesis kinetics and it is suggested that the selectivity with respect to CO and CO2 is only described correctly with BEEF-vdW.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The production of methanol is one of the most important processes in the chemical industry. Produced at a scale of ~50 Mtons year−1, methanol is used for the production of a variety of chemicals like formaldehyde, acetic acid, and methyl-tertbutyl ether [1]. Industrially, methanol is produced from synthesis gas (a mixture of CO, CO2 and H2) at temperatures of 200–300 °C and pressures of 50–100 bar employing a Cu/ZnO/Al2O3 catalyst [2].
Despite several decades of research, there are still a number of open questions regarding the reaction mechanism and the nature of the active site [1, 3]. Recently, a combined experimental and theoretical study revealed the importance of defects in the active phase of the Cu catalyst as well as the proximity of ZnO [4]. The active site can be thought of being comprised of a Cu defect (e.g. a step) with Zn in its oxidized form close by. Density functional theory calculations showed that the intermediates involved in both, the hydrogenation of CO and CO2, bind stronger on the (211) facet than on the (111) facet of Cu [4, 5]. Alloying of Zn into the Cu steps further increases the bond strength [4]. The hydrogenation barriers follow the same trend explaining why Cu/ZnO exhibits such a high activity in methanol synthesis.
Another key mechanistic question concerns the carbon source. While CO was assumed to be the main carbon source in the early years [6], isotope labeling experiments clearly showed that CO2 is preferentially hydrogenated to methanol [1, 7]. CO can then react with water to form CO2 via the water–gas-shift reaction, which is easily facilitated on Cu surfaces. Theoretically, however, it is generally found using semi-local density functional theory calculations that the hydrogenation of CO2 involves intermediates and reaction barriers that are higher in free energy when compared to CO hydrogenation [4, 8]. This is quite puzzling and the question arises about underlying reasons for these discrepancies. We suggest in the present paper that van der Waals forces may play an important role in the interaction of CO2 with metal surfaces and that inclusion of them is needed to understand the difference between CO and CO2 hydrogenation.
We have performed DFT calculations for both CO and CO2 hydrogenation on Cu(211) using the newly developed BEEF-vdW functional [9]. This functional was specifically designed to address vdW forces reasonably well while maintaining an accurate description of chemisorption energies of molecules on surfaces. Calculations using the BEEF-vdW functional were carried out using the GPAW code [10, 11]. A grid-spacing of h = 0.18 was used. All other parameters are identical to those reported in Ref. [4], and further details can be found in the supplementary material. In order to make sure that gas phase reaction energies are treated in a reasonable way gas-phase CO2 and H2 energies were corrected using a procedure previously developed for the RPBE functional [12] (details can be found in the supplementary material).
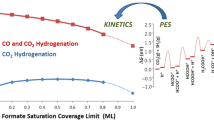
Figure 1 shows a comparison of the calculated free energy diagram of CO and CO2 hydrogenation under industrially relevant reaction conditions calculated with the RPBE [4, 13] and BEEF-vdW functional, respectively. As reported earlier, results using the RPBE functional suggest that hydrogenation of CO to methanol proceeds most efficiently via HCO, H2CO and H3CO [4, 8, 14]. The highest free energy barrier in this sequence is the second hydrogenation step, \( {\text{HCO}} + {\text{H}} \to {\text{H}}_{2} {\text{CO}} \). Under the reaction conditions chosen here (see caption of Fig. 1), this free energy barrier is 1.46 eV above the educts, CO and H2. Hydrogenation of CO2 proceeds via HCOO, HCOOH, and H2COOH. H2COOH is then split into H2CO and OH, hydrogenation of OH yields water while H2CO hydrogenation follows the sequence observed for CO hydrogenation and ultimately produces methanol. Interestingly, the RPBE functional predicts this reaction to go through a transition state that is 0.44 eV higher in free energy than CO hydrogenation (the highest barriers are the hydrogenation of HCOOH and H2CO with free energy barriers of 1.84 and 1.90 eV, respectively). Such a difference in the highest free energy transition state corresponds roughly to a difference in forward rates of about 4–5 orders of magnitude. This result is at odds with the experimental evidence suggesting that CO2 is the major source of carbon in methanol over Cu-based catalysts [7, 15]. The results using the BEEF-vdW functional (red line in Fig. 1) are quite different. Since this functional accounts for the dispersion forces of adsorbed species, one might expect a stabilization for larger intermediates, e.g. those involved in the hydrogenation of CO2, while smaller adsorbates are affected to a lesser extent. As can be seen in Fig. 1a, the energetics for the hydrogenation of CO to methanol are very similar for the two functionals. Intermediates and transition-states involved in the hydrogenation of CO2, on the other hand, are found to interact considerably stronger with the Cu(211) surface when using the BEEF-vdW functional instead of RPBE (see Fig. 1b). In fact, the highest free energy state in the hydrogenation of CO2 (\( {\text{HCOOH}} + {\text{H}} \to {\text{H}}_{2} {\text{COOH}} \)) is 1.56 eV, comparable to the highest free energy state for the hydrogenation of CO (\( {\text{HCO}} + {\text{H}} \to {\text{H}}_{2} {\text{CO}} \)), which is calculated to be 1.53 eV using BEEF-vdW. Since Zn has a much stronger effect on intermediates that bind to the surface through oxygen atoms, one would expect that the rate of CO2 hydrogenation increases significantly with the presence of ZnO [4] making CO2 hydrogenation considerably faster than CO hydrogenation for the Cu/ZnO system.
Gibbs-free energy diagram for CO (a) and CO2 (b) hydrogenation on Cu(211) calculated using the RPBE (black) and the BEEF-vdW (red) functional. All energies are relative to CO + 2H2 (CO2 + 3H2) in the gas phase and the clean surfaces. Intermediates marked with a star are adsorbed on the surface. Gibbs free energies were calculated at T = 500 K and p of 40 bar H2, 10 bar CO (10 bar CO2) and 1 bar of methanol (and water)
In summary we showed that the outcome of DFT calculations can be highly dependent on the functional used. In the case of CO2 hydrogenation to methanol we found that the highest free energy barrier is ~0.4 eV lower in energy when using BEEF-vdW as compared to RPBE. While trends from one metal to the next are still expected to be less dependent on the functional employed, [16] quantitative kinetic data can be highly functional dependent. We find that a functional explicitly including van der Waals interactions is needed to get the details of selectivity in methanol synthesis correctly. It should be noted, however, that not all differences that we find here can be assigned to the inclusion of vdW interactions, but are also due to the different nature of the functionals used. DFT calculations still have considerable problems of treating all molecules with a sufficient accuracy and the present results rely on a procedure to correct such errors. Until these problems are solved there will be uncertainties in our description of methanol synthesis, but we suggest that we may now have a description that is sufficient for getting the selectivity with respect to CO and CO2 hydrogenation qualitatively correct.
References
Hansen JB, Nielsen PEH (2008) In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis. Wiley, Weinheim
Lee S (1989) Methanol synthesis technology, CRC Press
Grunwaldt JD, Molenbroek AM, Topsøe NY, Topsøe H, Clausen BS (2000) J Catal 194:452
Behrens M, Studt F, Kasatkin I, Kühl S, Hävecker M, Abild-Pedersen F, Zander S, Girgsdies F, Kurr P, Kniep BL, Tovar M, Fischer RW, Nørskov JK, Schlögl R (2012) Science 336:893
Durand WJ, Peterson AA, Studt F, Abild-Pedersen F, Nørskov JK (2011) Surf Sci 605:1354
Klier K (1982) Adv Catal 31:243
Chinchen GC, Denny PJ, Parker DG, Spencer MS, Whan DA (1987) Appl Catal 30:333
Grabow LC, Mavrikakis M (2011) ACS Catal 1:365
Wellendorff J, Lundgaard KT, Møgelhøj A, Petzold V, Landis DD, Nørskov JK, Bligaard T, Jacobsen KW (2012) Phys Rev B 85:235149
Mortensen JJ, Hansen LB, Jacobsen KW (2005) Phys Rev B 71:035109
Enkovaara J, Rostgaard C, Mortensen JJ, Chen J, Dulak M, Ferrighi L, Gavnholt J, Glinsvad C, Haikola V, Hansen HA, Kristoffersen HH, Kuisma M, Larsen AH, Lehtovaara L, Ljungberg M, Lopez-Acevedo O, Moses PG, Ojanen J, Olsen T, Petzold V, Romero NA, Stausholm J, Strange M, Trisaris GA, Vanin M, Walter M, Hammer B, Häkkinen H, Madsen GKH, Nieminen RM, Nørskov JK, Puska M, Rantala TT, Schiøtz J, Thygesen KS, Jacobsen KW (2010) J Phys 22:253202
Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Nørskov JK (2010) Energy Environ Sci 3:1311
Hammer B, Hansen LB, Nørskov JK (1999) Phys Rev B 59:7413
Studt F, Abild-Pedersen F, Wu Q, Jensen AD, Temel B, Grunwaldt J-D, Nørskov JK (2012) J Catal 293:51
Sahibzada M, Metcalfe IS, Chadwick D (1998) J Catal 174:111
Nørskov JK, Abild-Pedersen F, Studt F, Bligaard T (2011) Proc Natl Acad Sci USA 108:937
Acknowledgments
Work supported by the U.S. Department of Energy under contract number DE-AC02-76SF00515.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Studt, F., Abild-Pedersen, F., Varley, J.B. et al. CO and CO2 Hydrogenation to Methanol Calculated Using the BEEF-vdW Functional. Catal Lett 143, 71–73 (2013). https://doi.org/10.1007/s10562-012-0947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0947-5