Abstract
A series of amino-functional imidazolium ionic liquids have been prepared and used as catalysts for cycloaddition of CO2 with epoxide. The reactions generated the cyclic carbonate even at room temperature under atmospheric pressure. Under the optimal reaction conditions, the propylene carbonate was yield to 98.0 % in the presence of [APbim]I, and the ionic liquids could be reused at least nine times without noticeable decrease in activity and selectivity. Besides, the reaction mechanism was proposed.
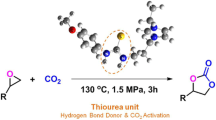
Graphical Abstract
Cycloaddition of CO2 with epoxides catalyzed by amino-functionalized imidazolium ionic liquid can achieve CO2 activation and conversion directly. The reactions generated the corresponding products even at room temperature under atmospheric pressure. Amino-functionalized imidazolium ionic liquid has showed excellent catalytic activity, reusability and general applicability.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbon dioxide is the largest contributor to the greenhouse effect. In order to prevent the risky reinforcement of the greenhouse effect, it is extremely important to reduce carbon dioxide emissions. At the same time, carbon dioxide is an easily available, non-toxic, inexpensive C1 resource [1–5], which can be used to produce valuable compounds such as organic carbonates, urea derivatives, oxazolidinones and formic acid. Under these circumstances, CO2 activation and subsequent conversion is the key to the use of carbon dioxide as a building block. One of the most promising ways for effective utilization of CO2 is the synthesis of five-membered cyclic carbonates via coupling carbon dioxide and epoxides (Scheme 1). Cyclic carbonates are valuable industrial raw materials, which are useful as aprotic polar solvents, electrolytes in lithium secondary batteries, intermediates for the pharmaceutical and fine chemical industries, and precursors for synthesizing polycarbonate materials [6–8].
In recent years, a large number of catalysts have been employed for the insertion of carbon dioxide into epoxides to form cyclic carbonates. Such examples vary from alkali metal salts [9, 10], metal oxides [11], molecular sieves [12], transition metal complexes [13–20], N-heterocyclic carbene [21], metal–organic frameworks [22–26], quaternary ammonium and phosphonium salts [27–31], gold nanoparticles [32], cross-linked polymeric nanoparticles [33] to ionic liquids [34–42]. Among the catalysts mentioned above, ionic liquid is one of the most important catalysts for the cycloaddition of CO2 with epoxides [43–46]. In recent years, the functionalized ionic liquids have been received attention and show much better catalytic efficiency than traditional ionic liquids toward the synthesis of cyclic carbonates with no use of a cocatalyst and organic solvent [47]. Sun et al. [48] found hydroxyl-functional ionic liquids showed highly catalytic activity in the coupling reaction of carbon dioxide and epoxides, and OH groups ionic liquid was crucial for the reaction to proceed smoothly due to its cooperation function of ring-opening of epoxide. However, a sharp decline in the yield was observed when the temperature dropped to 110 °C. Park and coworkers [49] developed Brønsted acidic ionic liquids as a catalyst for the cycloaddition of CO2 to epoxides and they found the low yield of propylene carbonate was obtained when the reaction temperature was below 90 °C. Although great strides have been made in synthesis of cyclic carbonates from CO2 and epoxides, the development of efficient, stable, and economical ionic liquid-based catalytic systems that facilitate the production of cyclic carbonates under mild conditions is highly desired.
Organic amine molecules have recently been shown to catalyze the direct reaction between CO2 and epoxides to form cyclic carbonates [50–52]. One major advantage of this type of catalyst system is that it would not introduce metal contaminant(s) to products and the environment [53–55]. Recently, Tsang and coworkers [56] investigated the role of amine in cycloaddition of carbon dioxide and epoxides and suggested a mechanism of coupling reaction of carbon dioxide and epoxides in the presence of organic amine catalyst. In context, we conceived that the amine-functional imidazolium ionic liquids have a great potential to accelerate the reaction in forward direction, due to its ability for activating and fixing carbon dioxide [57, 58]. In contrast to other imidazolium ionic liquids, amino-functional imidazolium ionic liquids could separate and fix the CO2 by way of ammonium carbamate formation [59]. Until now, a numerous of amino-functional imidazolium ionic liquids were synthesized and used for carbon dioxide capture [60, 61]. However, to the best of our knowledge, no literature reported amino-functional imidazolium ionic liquid was used as catalyst for conversion of carbon dioxide to cyclic carbonate [55]. In the present work, a series of amino-functional imidazolium ionic liquids were synthesized, characterized and employed as a catalyst for coupling reaction of epoxide and CO2 to form five-membered cyclic carbonate without using transition metal additives and co-solvent. The reactions generated the corresponding products even at room temperature under atmospheric pressure. Under the optimized reaction conditions, amino-functional imidazolium ionic liquids showed significant activity providing excellent yield of desired product with appreciable recyclability for nine consecutive recycles and a possible mechanism was proposed.
2 Experimental
All the chemicals were commercially available and were used without further purification. NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer and the FT-IR spectra were measured on a PerkinElmer spectrum 100 spectrometer. The products were analyzed by a HP 6890/5973 GC–MS, NMR and a gas chromatography (GC, Agilent 6820) equipped with a flame-ionized detector (FID).
2.1 Preparation of Amino-Functional Imidazolium Ionic Liquids
2.1.1 Synthesis of 1-(3-Aminopropyl)-3-methylimidazolium Chloride
The imidazolium ionic liquids used in this article were synthesized according to previous methods [57], N-methylimidazole (8.21 g, 100 mmol) and 3-chloropropylamine hydrochloride (13.0 g, 100 mmol) were added to 50 ml ethanol under stirring. The resulting solution was refluxed for 24 h. After removal of ethanol in vacuum, the residue was dissolved in water. Then the pH value of the solution was adjusted to ~8 by the addition of potassium hydroxide. The obtained solution was concentrated under vacuum and then extracted with ethanol-tetrahydrofuran. The combined extracts were concentrated to get the product 1-(3-aminopropyl)-3-methylimidazolium chloride ([APmim]Cl) as a pale yellow viscous liquid (Fig. 1).
2.1.2 Synthesis of 1-Propylamine-3-methylimidazolium Bromine
1-(3-Aminopropyl)-3-methylimidazolium bromine ([APmim]Br) was given by ion exchange through ([APmim]Cl) with potassium bromide. The mixture was performed in ethanol for 48 h at room temperature and then filtered to remove the chloride salt. The resulting solution was concentrated to remove ethanol in vacuum. The obtained solution was re-dissolved in ethanol-tetrahydrofuran and filtered to remove the inorganic salt. At last the filtrates were concentrated to give the product 1-(3-aminopropyl)-3-methylimidazolium bromine ([APmim]Br) (Fig. 1).
Different amino-functional imidazolium ionic liquids, such as 1-(3-aminopropyl)-3-methylimidazolium iodide ([APmim]I), 1-(3-aminopropyl)-3-ethylimidazolium chloride ([APeim]Cl), 1-(3-aminopropyl)-3-butylimidazolium chloride ([APbim]Cl) and 1-(3-aminopropyl)-3-butylimidazolium iodide ([APbim]I), were prepared by the similar way Fig. 1.
All the amino-functional imidazolium ionic liquids were determined by 1H NMR and FT-IR, and the data was provided as follows:
[APmim]Cl: 1H NMR (400 MHz, D2O): δ (ppm) 7.44 (s, 1H), 7.41 (s, 1H), 4.17 (t, 2H), 3.79 (s, 3H), 2.67 (t, 2H), 1.97 (m, 2H); FT-IR (KBr): 3422, 3151, 3097, 2959, 1625, 1573, 1462, 1339, 1170, 1022, 839, 756, 650, 622 cm−1.
[APmim]Br: 1H NMR (400 MHz, D2O): δ (ppm) 7.41 (s, 1H), 7.35 (s, 1H), 4.18 (t, 2H), 3.80 (s, 3H), 2.68 (t, 2H), 2.00 (m, 2H); FT-IR (KBr): 3427, 3153, 3095, 2951, 2857, 1639, 1569, 1462, 1383, 1334, 1169, 1111, 1020, 837, 754, 650, 620 cm−1.
[APmim]I: 1H NMR (400 MHz, D2O): δ (ppm) 7.42 (s, 1H), 7.37 (s, 1H), 4.19 (t, 2H), 3.81 (s, 3H), 2.68 (t, 2H), 2.02 (m, 2H); FT-IR (KBr): 3430, 3146, 3091, 2945, 2871, 1625, 1571, 1458, 1386, 1338, 1167, 1109, 1073, 1020, 831, 754, 650, 619 cm−1.
[APeim]Cl: 1H NMR (400 MHz, D2O): δ (ppm) 7.43 (s, 2H), 4.20 (t, 2H), 4.11 (t, 2H), 2.69 (t, 2H), 2.01 (m, 2H), 1.41 (t, 3H); FT-IR (KBr): 3426, 3145, 3090, 2948, 2868, 1639, 1565, 1462, 1384, 1339, 1165, 1110, 1079, 1030, 842, 762, 647 cm−1.
[APbim]Cl: 1H NMR (400 MHz, D2O): δ (ppm) 7.41 (s, 2H), 4.17 (t, 2H), 4.08 (t, 2H), 2.55 (t, 2H), 1.92 (m, 2H), 1.75 (m, 3H), 1.22 (m, 2H), 0.82 (m, 3H); FT-IR (KBr): 3420, 3142, 3091, 2959, 2874, 1645, 1562, 1462, 1382, 1330, 1165, 1110, 1059, 1030, 839, 753, 664, 644, 622 cm−1.
[APbim]I: 1H NMR (400 MHz, D2O): δ (ppm) 7.45 (s, 2H), 4.19 (t, 2H), 4.13 (t, 2H), 2.66 (t, 2H), 1.99 (m, 2H), 1.78 (m, 3H), 1.25 (m, 2H), 0.84 (m, 3H); FT-IR (KBr): 3445, 3137, 3084, 2957, 2872, 1637, 1564, 1461, 1385, 1332, 1163, 1110, 1077, 1024, 825, 752, 664, 636 cm−1.
2.2 Coupling Propylene Oxide and CO2 to Form Propylene Carbonate
The coupling reaction was carried out in a 50 ml stainless steel autoclave equipped with a magnetic stirrer. For each typical reaction process: imidazolium ionic liquid (0.71 mmol) and propylene oxide 1a (5.0 ml, 71.5 mmol) were charged into the reactor vessel without using any co-solvent and co-catalyst. The reactor vessel was placed under a constant pressure of carbon dioxide and then heated to 120 °C for 1.5 h. Then the reactor was cooled to ambient temperature, and the resulting mixture was transferred to a 50 ml round bottom flask. By distillation under vacuum, the product propylene carbonate 2a was then obtained as a colorless liquid. The cyclic carbonates were identified on GC–MS (HP6890/5973) and NMR. The catalyst was separated from the resulting mixture by distillation under vacuum and reused directly without further treatment.
The NMR characterizations of cyclic carbonates were shown as follows:
4-Methyl-1,3-dioxolan-2-one: 1H NMR (400 MHZ, CDCl3): δ (ppm) 4.84 (m, 1H), 4.54 (dd, 1H), 4.01 (dd, 1H), 1.47 (d, 3H); 13C NMR (100 MHz, D2O): δ (ppm) 155.05, 73.54, 70.66, 19.42;
4-(Chloromethyl)-1,3-dioxolan-2-one: 1H NMR (400 MHZ, CDCl3) δ (ppm) 4.97 (mm, 1H), 4.58 (t, 1H), 4.40 (dd, 1H), 3.75 (mm, 2H);13C NMR (100 MHz, D2O): δ (ppm) 154.19, 74.30, 66.99, 43.69;
4-(Phenoxymethyl)-1,3-dioxolan-2-one: 1H NMR (400 MHZ, CDCl3) δ (ppm) 7.31 (m, 2H), 7.02 (t, 1H), 6.91 (dd, 2H), 5.03 (mm, 1H), 4.62 (t, 1H), 4.54 (dd, 1H), 4.24 (dd, 1H), 4.15 (dd, 1H);13C NMR (100 MHz, D2O): δ (ppm) 157.76, 154.60, 129.71, 122.03, 114.63, 74.07, 66.90, 66.26;
4-Phenyl-1,3-dioxolan-2-one: 1H NMR (400 MHZ, CDCl3) δ (ppm) 7.46 (d, 3H), 7.38 (m, 2H), 5.69 (t, 1H), 4.82 (t, 1H), 4.36 (dd, 1H); 13C NMR (100 MHz, D2O): δ (ppm) 154.83, 135.84, 129.74, 129.25, 125.88, 78.00, 71.17;
Cis-hexahydrobenzo [1, 3] dioxol-2-one: 1H NMR (400 MHZ, CDCl3) δ (ppm) 4.69 (m, 2H), 1.91 (m, 4H), 1.64 (m, 2H), 1.42 (m, 2H),13C NMR (100 MHz, D2O): δ (ppm) 155.39, 75.78, 26.63, 19.03.
3 Results and Discussions
3.1 Catalytic Performance of Different Catalysts
Propylene carbonate synthesis from CO2 and propylene oxide was carried out in the presence of a series of amino-functional imidazolium ionic liquids under identical reaction conditions (catalyst loading 1 mol%, CO2 pressure 1.5 MPa, temperature 120 °C, time 1.5 h) and the results were listed in Table 1. As shown in Table 1, both the cation and anion of the imidazolium ionic liquids have strong impact on the catalytic activities (Table 1, entries 1–6). When [APmim]Cl was used as a catalyst, the propylene carbonate was yield to 58.9 %, and the yield of propylene carbonate was increased to 91.5 % in the presence of [APmim]I, and these results were indicated the catalytic activity of the halide anions was decreased in the order of I− > Br− > Cl− (Table 1, entries 1–3). It was due to their good leaving ability and nucleophilicity [51]. It is worth noting that the structure of the cation had a large influence on catalytic activity towards the synthesis of propylene carbonate. The long N-alkyl chain in amino-functional imidazolium ionic liquids was beneficial for the increase in its catalytic activity (Table 1, entries 1, 4, 5) and a similar result was observed over other imidazolium ionic liquid catalyst [62]. The yield of propylene carbonate was achieved at 94.3 % with using [APbim]I as a catalyst (Table 1, entry 6). For comparison, imidazole and metal halide were used as a sole catalyst in this reaction, but no product was founded in the reaction mixture (Table 1, entries 8 and 9). In the literatures, the propylene carbonate was yield to 99 % under 2.0 MPa when hydroxyl-functionalized ionic liquid (1.6 mol%) was used as a catalyst in this reaction [48]. Comparing to hydroxyl group, carboxyl group is a stronger Brønsted acid and hydrogen bond donor, Park and coworkers [49] used carboxyl functionalized imidazolium-based ionic liquid as a catalyst for synthesis of styrene carbonate, the yield was achieved at 95 % with 1.5 mol% ionic liquid catalyst. So, the catalytic activity of carboxyl functionalized imidazolium-based ionic liquid was investigated and the results were listed in Table 1. The propylene carbonate was yield to 84.0 and 84.4 % when 1 mol% [CEmim]Br or [CPmim]Br was used as a catalyst (Table 1, entries 7 and 10). Compared to the carboxyl functionalized ionic liquid, the propylene carbonate was yield to 88.6 % in the presence of 1 mol% amino-functional imidazolium ionic liquid and it indicated that the catalytic activity of amino-functional imidazolium ionic liquids was higher than that of carboxyl-functional imidazolium ionic liquid (Table 1, entries 2, 7, 10). Under the same reaction conditions, the middle yield of propylene carbonate was obtained when the [Emim]Br was used as a catalyst (Table 1, entry 11). These results suggested that the highly catalytic activity may attribute to the amine tethered to the cation of the ionic liquid. It was because an amine group strongly increased the reactivity of CO2 by forming ammonium carbamate [50] and ammonium-group, which was formed by the reaction of carbon dioxide and amino-functional imidazolium ionic liquid [57], could help the ring-opening reaction of propylene oxide through hydrogen bond interaction with oxygen, and halogen anion can nucleophilic attack the β-carbon atom with small steric hindrance [35]. Therefore, [APbim]I was identified as the most effective catalyst, and was thus chosen as the model catalyst for further investigation.
3.2 Optimum Reaction Conditions
The influence of reaction time on the synthesis of propylene carbonate was given in Fig. 2. From Fig. 2, it can be seen that the yield of propylene carbonate was increased rapidly within the first 2.0 h, and almost quantitative yield could be achieved with >99 % selectivity. When another half an hour was employed to synthesize of propylene carbonate, only a slight raising (<2 %) on the yield of propylene carbonate was observed. That is to say, [APbim]I could be an effective catalyst for converting CO2 into cyclic carbonate in 2.0 h. Therefore, the suitable reaction time would be 2.0 h.
Furthermore, we found that the yield of propylene carbonate was strongly affected by the reaction temperature while the selectivity was kept more than 99 % (Fig. 3). As shown in Fig. 3, the yield of propylene carbonate was increased with increasing the reaction temperature and the optimal performance was achieved at 120 °C. These results suggested that higher temperature favors the insertion of carbon dioxide into the C–O bond of epoxide leading to the rapid conversion of epoxide to cyclic carbonate. But when a higher temperature was used for this reaction and only a tiny change was observed. Besides, in order to investigate the activity of [APbim]I at low temperature, the reaction was conducted at room temperature without further energy input and the 41.3 % propylene carbonate was obtained when the reaction time was prolonged to 24 h. It was indicated amino-functional imidazolium ionic liquid could activated and converted carbon dioxide.
The effect of CO2 pressure was also studied for the reaction and the results showed that the pressure had a great impact on the yield of cyclic carbonate (Fig. 4). The yield of propylene carbonate was increased by an increase in the pressure in the ranking up to 1.5 MPa and further increase of the pressure causes a decline in the yield of propylene carbonate. Such an effect of pressure on the reaction has also been observed in other catalytic systems. Based on these reports, it could be explained that propylene carbonate was in its liquid form under the adopted reaction conditions. A high pressure mainly caused by CO2 would reduce propylene oxide conversion because a lowered propylene oxide concentration in the vicinity of the catalyst was not favorable to the reaction since propylene oxide was also a reactant. To our astonishment, even at room temperature under atmospheric pressure yield of propylene carbonate was up to 31.5 after 24 h. It was probably because amino-functional imidazolium ionic liquid can sequestrate and transport CO2.
3.3 Coupling Carbon Dioxide and Other Epoxides
Under the optimized reaction conditions, the utility and generality of catalyst were examined. Various epoxides were used to react with CO2 to produce the corresponding products and the results were shown in Table 2. All the epoxides could be converted to their corresponding cyclic carbonates in excellent yield (Table 2, entries 1–4). It should be noted that internal cyclohexene oxide showed poor reactivity and only 76 % of the corresponding cyclic carbonate was obtained even after 24 h. It was because the steric effect of cyclohexene oxide (Table 2, entry 5). When the stereochemistry of 2e was determined by the 1H, 13C and NOESY NMR, we found it was cis-stereochemistry and this result was according to our previous result [39]. Compared to cyclohexene oxide, the other epoxides with less steric hindrance were easy that the ring-opening reaction and epoxides take place with an electron-withdrawing group that are able to stabilize the ring-opened structure of epoxides, thus resulting in a higher activity.
3.4 The Reusability of the Catalyst
As we all know, the stability and reusability of a catalyst system are the two keys for its potentially practical application in industry. In this study, the catalyst reusability of [APbim]I was tested under the optimal conditions. The catalyst was recovered after separation of propylene carbonate from the reaction mixture by distillation and reused directly for the subsequent reaction. As shown in Fig. 5, the yield of propylene carbonate in subsequent runs was similar to the fresh catalyst and the catalyst could be reused at least nine cycles without significant loss of activity.
Moreover, we characterized the recovered catalyst (after six runs) by FT-IR (Fig. 6). Compared to the fresh one, the recovered catalyst exhibited a new peak at 1,691 cm−1 (the peak at 1,789 cm−1 was assigned to C=O of propylene carbonate), which corresponds to the new COOH moiety formed from the reaction of CO2 with the amine tethered to the cation of the ionic liquid [63]. It can be deduced that this catalyst has excellent reusability and stability for the cycloaddition reaction of carbon dioxide and propylene oxide.
3.5 Possible Reaction Mechanism
Based on previous reports [50–54, 56] and the results in our study, a probable catalytic cycle was proposed for the cycloaddition of CO2 to propylene oxide using amino-functional imidazolium ionic liquids as catalysts, as shown in Scheme 2. In this reaction process, the most marked characteristic was achieving CO2 activation and subsequent conversion by one step. Firstly, the primary nitrogen atom of the catalyst reacted reversibly with CO2 to afford the ammonium carbamate 1 in which CO2 was activated. Then, the proton was coordinated with the oxygen of the propylene oxide through a hydrogen bond, resulting in activation of an propylene oxide, and simultaneously, the nucleophilic attack of iodide anion on the less sterically hindered β-carbon atom of the propylene oxide generates the ring-opened intermediate 2. Followed by the formation of ring-opened intermediate, insertion of the activated CO2 into C–I bond produces intermediate 3. Finally, the intermediate 3 converted to the propylene carbonate through intramolecular ring closing. Based on the literature [47–49, 64, 65], we proposed another way which may lead to the formation of cyclic carbonate. The insertion of CO2 into the ring-opened intermediate 4 forms an alkyl carbonate anion 5. Then, the halocarbonate further converted to the corresponding cyclic carbonate through ring closing, and the catalyst is regenerated.
4 Conclusions
In summary, the amino-functional imidazolium ionic liquid was found to be an effective catalyst for the synthesis of cyclic carbonates from carbon dioxide and epoxides under mild conditions without any co-solvent. Various epoxides were used to react with CO2 to produce the corresponding products in excellent yield. And the catalytic system can be reused at least nine times without noticeable decrease in activity and selectivity. Notable among these are that CO2 activation and subsequent conversion can be achieve in one step in the place of amino-functional imidazolium ionic liquid at room temperature under atmospheric pressure. Nowadays, further efforts to extend the next application of the system are underway in our laboratory.
References
Behr A (1988) Angew Chem Int Ed 27:661–678
Mikkelsen M, Jorgensen M, Krebs FC (2010) Energy Environ Sci 3:43–81
Kuwahara Y, Yamashita H (2013) J CO2 Util 1:50–59
Omae I (2012) Coord Chem Rev 256:1384–1405
Razali NAM, Lee KT, Bhatia S (2012) Renew Sustain Energy Rev 16:4951–4964
Shaikh AAG (1996) Chem Rev 96:951–976
Zhang LF, Fu XL, Gao GH (2011) ChemCatChem 3:1359–1364
Gao GH, Zhang LF, Wang BS (2013) Chin J Catal 34:1187–1191
Kihara N, Hara N, Endo T (1993) J Org Chem 58:6198–6202
Huang JW, Shi M (2003) J Org Chem 68:6705–6709
Yamaguchi K, Ebitani K, Yoshida T, Yoshida H, Kaneda K (1999) J Am Chem Soc 121:4526–4527
Xie ZZ, Zhu MQ, Nambo A, Jasinski JB, Carreon MA (2013) Dalton Trans 42:6732–6735
Castro-Gomez F, Salassa G, Kleij AW, Bo C (2013) Chem Eur J 19:6289–6298
Yan P, Jing HW (2009) Adv Synth Catal 351:1325–1332
Li FW, Xia CG, Xu LW, Sun W, Chen GX (2003) Chem Commun 2003:2042–2043
Huang ZJ, Li FB, Chen BF, Lu T, Yuan Y, Yuan GQ (2013) Appl Catal A Gen 136–137:269–277
Saunders LN, Ikpo N, Petten CF, Das UK, Dawe LN, Kozak CM, Kerton FM (2012) Catal Commun 18:165–167
Bai DS, Duan SH, Hai L, Jing HW (2012) ChemCatChem 4:1752–1758
Wu ZL, Xie HB, Yu X, Liu EH (2013) ChemCatChem 5:1328–1333
Kumar S, Jain SL, Sain B (2012) Catal Lett 142:615–618
Zhou H, Wang YM, Zhang WZ, Qu JP, Lu XB (2011) Green Chem 13:644–650
Xie Y, Wang TT, Liu XH, Zou K, Deng WQ (2013) Nat Commun 4:1960
Zalomaeva OV, Chibiryaev AM, Kovalenko KA, Kholdeeva OA, Balzhinimaev BS, Fedin VP (2013) J Catal 298:179–185
Lescouet T, Chizallet C, Farrusseng D (2012) ChemCatChem 4:1725–1728
Gao WY, Wojtas L, Ma SQ (2014) Chem Commun. doi:10.1039/c3cc47542e
Huang XQ, Chen YF, Lin ZG, Ren XQ, Song YN, Xu ZZ, Dong XM, Li XG, Hu CW, Wang B (2014) Chem Commun. doi:10.1039/c3cc49187k
Caló V, Nacci A, Monopoli A, Fanizzi A (2002) Org Lett 4:2561–2563
Zhang YY, Chen L, Yin SF, Luo SL, Au CT (2012) Catal Lett 142:1376–1381
Tharun J, Kim DW, Roshan R, Hwang Y, Park DW (2013) Catal Commun 31:62–65
Zhou X, Zhang Y, Yang XG, Zhao LZ, Wang GY (2012) J Mol Catal A Chem 362:12–16
Wang JQ, Dong K, ChenG WG, Sun J, Zhang SJ (2012) Catal Sci Technol 2:1480–1484
Shi F, Zhang Q, Ma Y, He Y, Deng Y (2005) J Am Chem Soc 127:4182–4183
Xiong YB, Wang H, Wang RM, Yan YF, Zheng B, Wang YP (2010) Chem Commun 46:3399–3401
Peng JJ, Deng Y (2001) New J Chem 25:639–641
Xiao LF, Lv DW, Wu W (2011) Catal Lett 141:1838–1844
Ghazali-Esfahani S, Song HB, Paunescu E, Bobbink FD, Liu HZ, Fei ZF, Laurenczy G, Bagherzadeh M, Yan N, Dyson PJ (2013) Green Chem 15:1584–1589
Choi HJ, Selvaraj M, Park D (2013) Chem Eng Sci 100:242–248
Cheng WG, Chen X, Sun J, Wang JQ, Zhang S (2013) Catal Today 200:117–124
Li FW, Xiao LF, Xia CG, Hu B (2004) Tetrahedron Lett 45:8307–8310
Xiao LF, Li FW, Peng JJ, Xia CG (2006) J Mol Catal A Chem 253:265–269
Dai WL, Jin B, Luo SL, Luo XB, Tu XM, Au CT (2014) Appl Catal A Gen 470:183–188
Agrigento P, Al-Amsyar SM, Sorée B, Taherimehr M, Gruttadauria M, Aprile C, Pescarmona PP (2014) Catal Sci Technol. doi:10.1039/C3CY01000G
Sun JM, Fujita SI, Arai M (2005) J Organomet Chem 690:3490–3497
Handy ST (ed) (2011) Ionic liquids—classes and properties, chap. 12. INTECH, Rijeka, pp 273–310
He Q, O’Brien JW, Kitselman KA, Tompkins LE, Curtisa GCT, Kerton FM (2014) Catal Sci Technol. doi:10.1039/C3CY00998
Sakakura T, Kohno K (2009) Chem Commun 1312–1330
Zhou Y, Hu S, Ma X, Liang S, Jiang T, Han B (2008) J Mol Catal A Chem 284:52–57
Sun J, Zhang SJ, Cheng WG, Ren JY (2008) Tetrahedron Lett 49:3588–3591
Han LN, Choi SJ, Park MS, Lee SM, Kim YJ, Kim MT, Liu BY, Park DW (2012) React Kinet Mech Catal 106:25–35
Srivastava R, Srinivas D, Ratnasamy P (2006) Microporous Mesoporous Mater 90:314–326
Zhang XH, Zhao N, Wei W, Sun YH (2006) Catal Today 115:102–106
Shiels RA, Jones CW (2007) J Mol Catal A Chem 261:160–166
Lu JN, Toy PH (2011) Synlett 2011:659–662
Jagtap SR, Raje VP, Samant SD, Bhanage BM (2007) J Mol Catal A Chem 266:69–74
Dai WL, Jin B, Luo SL, Luo XB, Tu XM, Au CT (2013) J Mol Catal A Chem 378:326–332
Yu KMK, Curcic I, Gabriel J, Morganstewart H, Tsang SC (2010) J Phys Chem A 114:3863–3872
Bates ED, Mayton RD, Ntai I, Davis JH Jr (2002) J Am Chem Soc 124:926–927
Xue ZM, Zhang ZF, Han J, Chen Y, Mu TC (2011) Int J Greenh Gas Control 5:628–633
Chen JJ, Li WW, Li XL, Yu HQ (2012) Phys Chem Chem Phys 14:4589–4596
Peng H, Zhou YL, Liu J, Zhang HB, Xia CL, Zhou XH (2013) RSC Adv 3:6859–6864
Sharma P, Park SD, Park KT, Nam SC, Jeong SK, Yoon Y, Baek H (2012) Chem Eng J 193–194:267–275
Han LN, Choi HJ, Choi SJ, Liu BY, Park DW (2011) Green Chem 13:1023–1028
Gurkan BE, de la Fuente JC, Mindrup EM, Ficke LE, Goodrich BF, Price EA, Schneider WF, Brennecke JF (2010) J Am Chem Soc 132:2116–2117
Han LN, Li HQ, Choi SJ, Park MS, Lee SM, Kim YJ, Park DW (2012) Appl Catal A Gen 429–430:67–72
Yang ZZ, Zhao YN, He LN (2011) RSC Adv 1:545–567
Acknowledgments
We are grateful to the Chinese National Sciences Foundation (21006021) and the Foundation for Youth Science and Technology Innovation Talents of Harbin of China (RC2013LX018002) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yue, C., Su, D., Zhang, X. et al. Amino-Functional Imidazolium Ionic Liquids for CO2 Activation and Conversion to Form Cyclic Carbonate. Catal Lett 144, 1313–1321 (2014). https://doi.org/10.1007/s10562-014-1241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1241-5