Abstract
In this paper we demonstrate a way to prepare Pt/CeO2 catalysts and test their catalytic activity in WGSR. The catalysts have been synthesized by a simple and low cost extractive-pyrolytic method. The temperature programmed reduction analysis revealed that the presence of platinum particles enhances the reduction degree of ceria. The morphology of the surface, the particle size and specific surface area were determined by TEM, XRD and BET analysis. EPR studies gave evidence of metal–support interaction, which increases the mobility of oxygen anions in crystal lattice. The effects of various operating parameters including the catalyst bed temperature, space velocity and feed H2O/CO ratio were also investigated. Subjecting the samples to pretreatment with H2 or air had no strong effect on the catalytic activity. By varying the Pt loading, it was also found out that the maximum catalytic activity was achieved with the sample containing 1.2 wt% Pt, reaching up to 98 % CO conversion degree.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ceria (CeO2) supported metal catalysts are of great interest in the modern catalytic industry because of their important applications, particularly in CO oxidation [1] and water-gas shift (WGS) reaction, which recently seem to be of greatest importance. The H2 produced from reformed gas, obtained through steam reforming of hydrocarbons contains up to 12 % CO [2], which is a strong poison for the current generation of PEM fuel cell anode catalysts. Therefore, WGS is a preferred reaction for the first step of CO removal and increasing the concentration of hydrogen in the synthesis gas. Since the CO conversion degree is equilibrium limited, two types of WGS catalysts are commercially available: high-temperature catalysts (400–500 °C), which reduce the CO concentration to about 2–5 vol% and low temperature (LT) catalysts (200–400 °C), over which the CO concentration could be lowered further to about 0.5–1 vol%.
All catalysts used for pure hydrogen production and purification of automotive exhaust gases contain precious metals such as Pt, Pd, Au or Rh, because of their intrinsic high catalytic activity [3–5]. The WGS reaction has been known for a long time and commercial catalysts, based on iron, gold, copper and other transition metals, are now available [6]. From kinetic point of view the WGS chemistry on Pt for instance is complicated, and it depends critically on the oxidation of CO by adsorbed OH and H2O, mainly via the carboxyl intermediate and it can be represented by a simple one-step reaction describing the formation of carboxyl \(({\text{CO}}^{ *} \;{ + }\;{\text{H}}_{2} {\text{O}}^{ *} \to {\text{COOH}}^{ *} \;{ + }\;{\text{H}}^{ *} ) ,\) being the rate-determining step. Then following steps occur [7]:
Because of the high cost of these metals, it is important to reduce their content by keeping the active metals at a high degree of dispersion. This can be achieved using nano-sized metal particles loaded on different supporters.
Ceria is an appropriate support in case in redox reactions, because of its ability to store and release oxygen. This property results from the fact that both Ce3+ and Ce4+ ionic states are stable, allowing the oxide to pass over from CeO2 to CeO2−x and vice versa [8]. For more than 20 years it is well known in the literature that the addition of Pt metals on ceria dramatically enhances the reducibility of the surface oxygen atoms of ceria [9, 10], which opens an additional pathway for pure H2 production. On the other hand, ceria promotes the precious metal dispersion and enhances the catalytic activity at the interphase metal-support sites [11]. Two main hypotheses have been put forward to explain how the WGS reaction takes place on noble metal ceria-supported catalysts. Bunluesin et al. [12] have suggested a redox mechanism that includes CO adsorption on the metal, reduction of the ceria near the metal oxide interphase and oxidation of the reduced ceria by H2O. The process can be represented as follows:
where σ represents adsorption sites on the metal.
According to the second postulated mechanism, referred to as “associative”, the WGS reaction passes through formation of a formate intermediate. It is supposed that the CO and H2O are adsorbed on the catalyst active site followed by the carbonate intermediate formation. This can be written as a sequence of the following steps:
It is important to note that the CO conversion degree depends on the active metal particle size. As it was reported in [13], the specific rate of WGS reaction, expressed per single site located at the periphery of Pt–CeO2 interphase, at 250–350 °C, correlates linearly with the length of the perimeter of Pt–ceria interphase for particles in range 1–8 nm. The authors have found out a small difference in the Eapp (apparent activation energy) of the reaction by 1–2 kcal/mol for 1.3 and 8.0 nm Pt particle size, which leads to increasing of the specific rate of WGS reaction in the case of 8.0 nm Pt size. It is also considered that the size of the Pt nanoparticle influences the oxygen atoms reactivity in the support lattice.
From thermodynamic point of view the WGS reaction is an exothermal one and therefore lower temperatures would be preferable, but in order to overcome the kinetic limitations higher temperatures are usually applied.
Knowing that ceria supported platinum catalysts are suitable for catalyzing the WGS reaction at temperatures in the range of 200–400 °C, it is important to find out a simple and a low cost method to obtain Pt/CeO2 catalysts that answer these requirements. Nano-scaled Pt/CeO2 catalyst synthesized by the micro-emulsion (ME) technique, for example, having different Pt loading, ensures CO conversion degree up to 60–65 % at 400 °C in WGS [14], so we have focused our efforts to find out a new method for synthesis of a catalyst, which can increases the CO conversion degree in WGSR to higher values at lower temperatures.
The aim of the present study was to identify the key physicochemical parameters, which determine the WGSR activity of nanosized platinum particle catalysts supported on ceria, prepared by extractive-pyrolytic method (EPM).
2 Experimental
2.1 Catalyst Preparation
The EPM was used for the catalysts samples preparation. Its simplicity and low cost, characterize the EPM as suitable for nanosized particle catalysts synthesis. As it is shown below, this method allows small amounts of noble metals (1–5 %) with particle sizes ranging from several nanometers to tens of nanometers to be attached onto the surface of the support.
The materials used were: Platinum powder (≥99.9 %; Aldrich), HCI (35 %) and HNO3 (65 %) (Lachema), n-tri-octylamine (C8H17)3N (≥95 %; Fluka) and toluene (analytical grade; Stanchem) were applied to produce the precursors. Ceria powder was purchased from Aldrich.
The method can be described as follows: first, a solution of platinum acid hexachloride was prepared. An amount of the platinum powder was dissolved in aqua regia and then the solution was evaporated adding concentrated HCl to obtain wet salt that was then diluted with 2 M HCl to the necessary concentration (0.5 mol L−1). The organic precursor (the extract) was prepared by extracting the platinum from the HCI solution using (1 M) n-trioctylamine (C8H17)3N solution in toluene. The following chemical interaction occurred during the extraction:
where the subscripts “w” and “o” denote the water phase and organic the phase, respectively. As a result, a solution of tri-n-octylammonium hexachloroplatinate in toluene was obtained.
The results of the analysis of the aqueous solution after extraction using a HITACHI 180-50 atomic absorption spectrometer proved that the platinum had been completely extracted into the organic phase. Different volumes of the extract were used to impregnate the ceria in order to obtain catalysts with different platinum contents. After impregnation, the toluene was evaporated by drying at 90–110 °C. Finally, the samples were heated up to 300 °C at a rate of 10 °C min−1. Thus three Pt nanosized catalyst samples with loadings: 1.2, 2.4 and 4.8 wt% were synthesized, denoted as 1.2Pt/CeO2, 2.4Pt/CeO2 and 4.8Pt/CeO2.
2.2 Catalyst Characterization
X-ray powder diffraction patterns were recorded on a Bruker D8 Advance powder diffractometer with Cu Kα radiation source and SolX detector. The samples were scanned at 2θ angles from 10° to 80° at a rate of 0.04° s−1. The X-ray intensity was operated with a current of 40 mA and a voltage of 45 kV.
The temperature programmed reduction (TPR) measurements were carried out using an apparatus previously described elsewhere [15]. A cooling trap (−40 °C) for removing water formed during reduction was mounted in the gas line before the thermal conductivity detector. A hydrogen-argon mixture (10 % H2), dried over a molecular sieve 5Å (−40 °C), was used to reduce the samples at a flow rate of 24 mL min−1. The temperature was linearly raised at a rate of 15 °C min−1. The amount of charged sample was about 0.05 g. Such amount was selected based on the criterion proposed by Monti and Baiker [16].
The microstructure of the catalyst was observed using a high-resolution transmission electron microscope (HRTEM JEOL 2100).
The EPR spectra were recorded within the temperature range 123–323 K with JES-FA 100 EPR spectrometer operating in the X-band with standard TE011 cylindrical resonator. The samples were placed in quartz cuvettes and were fixed in the cavity center. The Varied Temperature Controller ES-DVT4 was used to permit observation of the EPR spectra at various temperatures. The desired temperature can be easily obtained by feeding cold gas (liquid nitrogen) to the sample area at a temperature, which is controlled by the EPR spectrometer data system computer. The EPR spectra were recorded under following conditions: modulation frequency 100 kHz, microwave power 1 mW, modulation amplitude 0.2 mT, sweep 500 mT, time constant 0.3 s and sweeping time 4 min.
The specific surface area of the catalysts was measured by N2 adsorption–desorption isotherms at 77 K using single point BET method in a FlowSorb 2300 instrument (Micromeritics Instrument Corporation).
2.3 Catalytic Activity Measurements
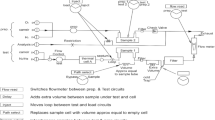
The WGSR activity measurements were conducted in a fixed bed continuous flow reactor used to evaluate the degree of CO conversion within wide range of temperatures (140–350 °C). The activity was measured under steady state conditions at atmospheric pressure. A gas mixture of feed composition 4.44 vol% CO in argon was used. The following experimental conditions were applied: catalyst bed volume −0.5 cm3 (0.63–0.80 mm sieve fraction), space velocity of the dry gas −4000 h−1, partial pressure of water vapor −31.1 kPa. The CO and CO2 concentrations at the reactor outlet were determined by means of “Uras 3G”, Hartmann & Braun AG gas analyzer. The stability of the catalysts was tested under different space velocities (2000, 4000 and 8000 h−1) and different water vapor partial pressures (5–50 kPa).
3 Results and Discussion
3.1 Effect of Platinum Loading on the Catalytic Activity
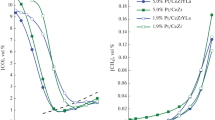
The catalytic activities of the samples with different platinum loading, measured in the temperature range 140–350 °C are represented Fig. 1.
It is seen that the catalyst sample with the highest platinum loading, shows the lowest catalytic activity, while the one with the lowest content of platinum exhibits the highest activity. Such behavior may be attributed to the effect of encapsulation—platinum particles become trapped into the supporting material. The increase of metal loading may lead to this effect [17, 18]. Taking into consideration testing results on sample activity represented in Fig. 1, it can be assumed that, for the catalyst with highest platinum content, encapsulation of metal particles into support is taking place. The CO conversion degrees indicate that at relatively low temperature of 250 ◦C, the catalyst with Pt loading of 1.2 wt% reached the equilibrium conversion of 98 %. The conversion achieved by the catalysts with 1.2 and 2.4 wt% platinum is comparable at temperatures in the range of 150–200 °C. In order to explain this behavior and to find out the most optimal platinum loading, we have tested these samples under various conditions such as different contact times, H2O/CO ratios and temperatures for more than a week. During that period high and stable catalytic activity was observed.
In the next sections we show the results obtained under different conditions and we attempt to find a correlation between the Pt loading of the samples and the experimental data.
3.2 Sample Characterization
3.2.1 Structural Studies
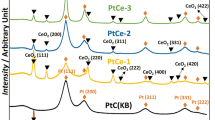
The X-ray phase analysis has revealed (Fig. 2) that the diffraction peak of platinum (111) in X-ray patterns of the composites has a low intensity that makes it impossible to determine the mean size of the metal crystallites on the given carrier with the Pt content in the composite below 2.4 wt% (Fig. 2 curves 1, 2). The mean size of the platinum crystallites (7 nm) was determined only for the catalyst sample with platinum content 4.8 wt% (Fig. 2 curve 3).
The ability of the catalyst to utilize oxygen storage capacity of ceria, depends critically on the nobble metal, and it must be sufficiently well dispersed in order to influence all the oxygen that is available for extraction. The morphology and size of the platinum particles on the ceria support were studied by transmission electron microscopy (Fig. 3).
It can be seen that the platinum particles are uniformly dispersed on aggregate clusters of ceria nanocrystals. The particle size distributions have been estimated by means of the ImageJ software, which is able to measure the diameter of the particles on the images as well as to assess the distribution of particle size. In order to calculate the mean particle diameter of the catalyst with 1.2 wt% platinum content, extra images were taken covering several sections of the crystallites. For statistical processing, we measured 99 platinum particles of the sample1.2Pt/CeO2, and 173 particles of the sample 4.8Pt/CeO2. The statistics yields value of about 4.3 nm mean size for the 1.2Pt/CeO2 catalyst and 6.7 nm for the 4.8Pt/CeO2. The distribution of the particles size is represented in Fig. 4. On the basis of the results from TEM images for 1.2 wt% Pt and 4.8 wt% Pt we could suppose that the mean particle size of the sample with 2.4 wt% Pt loading must be in the same range.
The graphs show that the distribution of particle size for both catalysts is similar, but for the catalyst 1.2Pt/CeO2 there is a distribution shift towards smaller sizes compared to the 4.8Pt/CeO2, which could affect their catalytic properties.
The BET surface areas of the prepared samples were as follows: 1.2Pt/CeO2: 15 m2 g−1, 2.4Pt/CeO2: 18 m2 g−1 and 4.8Pt/CeO2: 24 m2 g−1.
3.2.2 EPR Investigation
The EPR spectra of 2.4Pt/CeO2 catalyst recorded within the temperature range 50–150 °C before and after WGS reaction are represented in Fig. 5a and b respectively.
The EPR spectra of the fresh sample (Fig. 5a) show three lines with g = 2.0124, 2.4695 and 2.8184. An additional signal with g = 4.2384 appears at low temperatures (−100, −150 °C). The signal with g = 2.0124 is assigned to defect in which electrons are trapped at oxygen vacancies next to cerium ions [19, 20]. These defects are probably stabilized by the presence of cationic impurities in the immediate environment as it is indicated by the absence of this signal when pure cerium oxide samples are used [21]. This signal is stable and its position and intensity do not change at various temperatures. Besides this signal a wide signal with g = 2.4695 is observed. This signal disappears upon blowing oxygen trough the sample and at temperatures below 0 °C. This suggests that the sites responsible for the signal are easily oxidizable and located on the outer surface. The absence of this signal in the case of pure CeO2 sample suggests that platinum species are involved in its nature. Most probably these are Pt oxide clusters located on ceria [22]. The modifications observed upon water gas shift (Fig. 5b) are certainly due to changes in the environment of the platinum ions, which could be subjected to stronger spin exchange interactions within the platinum oxide clusters due to a higher reduction degree as a consequence of the higher temperature treatment under WGSR conditions. The absence of the EPR signal with g = 2.4695 indicates that the platinum in these clusters is reduced to a lower oxidation state or that a decrease in their interaction with cerium ions occurs after water gas shift. The EPR line at g = 2.8184 is most likely due to of Pt3+ ions. This g value is different from the values published in the literature (g = 2.64 [23], g = 2.33 [24]), but in those studies other preparation methods have been used. Upon decreasing the temperature this line expands and moves to lower magnetic field and at −100 °C it becomes g = 3.1824. The additional line with g = 4.2384 recorded at temperatures −100 and −150 °C is attributed to traces of Fe3+ probably contained in the starting substances. After water gas shift reaction the Pt3+ signal disappeared for the spend 2.4Pt/CeO2 catalyst and a new EPR signal with g = 2.05 is observed (Fig. 5b). Because of the occurrence of the signal both in case of Pt/TiO2 and Pt/Al2O3 [21] and its absence on the pure ceria after the same treatments, the signal was attributed to Pt+ (d9, S = 1/2).
3.2.3 Temperature Programmed Reduction
The oxygen storage capacity of ceria was widely investigated by many authors using TPR. As it is reported in the literature, ceria should be capable of being reduced in hydrogen media and reoxidised readily. B. Harrison et al. [25] have recorded two reduction peaks: a low temperature peak (500 °C), assigned to the reduction of surface species and a higher temperature peak (800 °C), corresponding to the reduction of bulk oxygen leading to formation of lower oxides of cerium. The presence of platinum nanoparticles on ceria causes a dramatic reducibility enhancement of the surface oxygen of ceria lowering thr temperatures below 200 °C. Abbasi et al. [26] have observed three zone TPR spectra. Such a behavior is also typical for our samples. The TPR profiles of platinum-promoted ceria are represented in Fig. 6. As it can be seen, the presence of platinum particles shifts reduction reaction to lower temperatures in comparison with those of pure CeO2. Analyzing the EPR data such behavior is an indication of the existence of metal–support interactions and it increases the mobility of oxygen inside the ceria crystal lattice, as a result of weakening of the surface O–Ce bond, which improves the redox properties of the catalyst. The first reduction peak of the platinum-promoted ceria appears at 158 °C and it can be assigned to the reduction of oxygen species on the platinum particles [27–30]. The peaks, registered for samples with higher platinum loading of 2.4 and 4.8 %, related to the PtOx species are shifted towards higher temperatures, and appear at 240 and 250 °C respectively. He et al. [31] also reported that the PtOx nanoparticle reduction can take place at a higher temperature of 241 °C on CeO2. It is clearly shown that the next peaks with different shapes have been recorded for the catalyst samples with different platinum loading. The catalyst containing 1.2 % platinum is characterized by sharp peak at 353 °C, which can be related to reduction of oxygen species on the ceria surface. The flattening and the shift of the reduction curve to 371 °C for the sample containing platinum 2.4 and 4.8 wt% as well as the overlapping with the peaks registered at 632 °C, respectively at 657 °C, could indicate that the higher Pt amount causes not only surface ceria reduction but a deeper reduction in the bulk as well. These features of the TPR profile can explain the data obtained for the activity of the catalyst, according to which the sample with the lowest platinum content has the highest activity. In this case, the sample with the lowest 1.2 % platinum content facilitates to the greatest extent the reduction of the ceria surface, as its reduction temperature is lowest −353 °C, in regard to the samples with higher Pt loading, and hence this increases the activity because the catalysis itself, is carried out on the surface of the catalysts, but not in the bulk phase. The small areas of the peaks at the highest temperatures, typical for catalysts with high platinum content, indicate that platinum influences the reduction of oxygen species within the bulk of ceria, thereupon the peak shifts from 800 °C to lower temperatures.
3.3 Effect of Contact Time on the Catalytic Activity
Fig. 7 shows the effect of the contact time on the degree of CO conversion at 225 °C, over the studied platinum–ceria catalysts. The tests were performed at space velocities of 2000, 4000 and 8000 h−1. It is clearly noticeable that the conversion of CO is strictly dependent on the contact time and at a higher space velocity it is lower, and sample of 4.8 % platinum content remains with a significantly lower activity. In order to yield a high value of CO conversion degree, a high adsorption of feed gas mixture, containing CO and H2O on the catalytic surface is needed. As the ceria surface adsorbs CO slightly [32], the metal particles are involved in this process, so it can be expected that the sample with highest platinum content should exhibit highest CO conversion. The possible explanation of the contradiction between the expected and the obtained results could be the Pt encapsulation into the support, leading to lower sorption capacity and hence to much lower CO conversion degree in comparison to the sample with 1.2 wt% platinum.
3.4 Effect of H2O/CO Ratio
The data on the catalytic activity of the samples at four different H2O/CO ratios at the above chosen temperature are represented in Fig. 8. As the feed water promotes CO oxidation on Pt catalysts [33], it was expected that the catalytic activity of the samples would strongly depend on the H2O/CO ratio. From the results obtained under these conditions, it is seen that the degree of conversion is a function of this ratio.
3.5 Catalyst Stability
The samples were subjected to different treatment conditions in order to estimate their stability during the tests. Fig. 9 illustrates the performance of the Pt/CeO2 samples under conditions described above after re-oxidation pretreatment, reduction pretreatment, different H2O/CO ratios as well as that of a fresh sample. Fig. 9a shows the catalytic activity of the 1.2Pt/CeO2 sample: curve 1—after re-oxidation, after the feed H2O/CO ratio tests—curve 2 and after reduction—curve 3. Fig. 9b and 9c data correspond to the samples 2.4Pt/CeO2 and 4.8Pt/CeO2, respectively. It can be noted that the 1.2Pt/CeO2 sample remains as active as it was at the beginning of the experiments during the tests, but the reduction pretreatment significantly decreases its activity, and deteriorates its performance. The 2.4Pt/CeO2 catalyst exhibits different behavior after reduction with hydrogen, and its activity reaches the highest level of conversion at 250 °C. This could be due to the reduced platinum as well as to the reduced ceria surface possessing more oxygen vacancies as a prerequisite for water adsorptive dissociation and higher WGS activity of the catalyst.
In summary, all studied catalysts samples showed stable activity during the long term testing period of WGS reaction. This is in good agreement with the results obtained by TPR experiments.
4 Conclusions
A comparative study of the WGS reaction has been conducted over three catalytic Pt/CeO2 samples having different Pt loading, over a wide temperature range, different space velocities and feed H2O/CO ratios. The presence of platinum nanoparticles lowers WGS reaction temperatures and facilitates reduction process of ceria. The new platinum catalyst has the optimal ratio between the surface platinum active sites and the free ceria surface making it capable to donate oxygen.
Our results have shown that 1.2Pt/CeO2 catalyst samples exhibited the highest WGSR activity. However, the higher amount of platinum does not contribute to the higher activity, probably due to the encapsulation of the metal particles inside the support.
Generally, all samples demonstrate stable reaction capability at low temperature, at different space velocities and feed H2O/CO ratios.
Our study has demonstrated that the activity is high both in the cases of hydrogen or air gas used to pre-treat the catalysts.
References
Bunluesin T, Cordatos H, Gorte RJ (1995) J Catal 157:222
Farrauto R, Hwang S, Shore L, Ruettinger W, Lampert J, Giroux T, Liu Y, Ilinich O (2003) Annu Rev Mater Res 33:1
Yao, Yung-Fang Yu (1984) J Catal 87:152
Andreeva D, Idakiev V, Tabakova T, Ilieva L, Falaras P, Bourlinos A (2002) Travlos A Catal Today 72:51
Robbie B (2006) Phys Chem Chem Phys 8:5483
Fu Q, Weber A, Flytzani-Stephanopoulos M (2001) Catal Lett 77:87
Mhadeshwar AB, Vlachos DG (2005) Catal Today 105:162
Cordatos H, Bunluesin T, Stubenrauch J, Vohs JM, Gorte RJ (1996) J Phys Chem 100:785
Andreeva D, Ivanov I, Ilieva L, Abrashev MV, Zanella R, Sobczak JW, Lisowski W, Kantcheva M, Avdeev G, Petrov K (2009) Appl Catal A 357:159
Yao HC, Yu Yao FY (1984) J Catal 86:254
Trovarelli A (1996) Catal Rev Sci Eng 38:439
Bunluesin T, GoTtea RJ, Grahamb GW (1998) Appl Catal B 15:107
Christos M, Kalamaras SA, Angelos ME (2011) J Catal 279:287
Yeung CMY, Yu KMK, Fu QJ, Thompsett D, Petch MI, Tsang SC (2005) J Am Chem Soc 127:18010
Kotsev N, Shopov D (1971) J Catal 22:297
Monti DAM, Baiker A (1983) J Catal 83:323
Graham GW, Jen HW, Chun W, McCabe RW (1997) Catal Lett 44:185
Jiang JC, Pan XQ, Graham GW, McCabe RW, Schwank J (1998) Catal Lett 53:37
Soria J, Martinez-Arias A, Conesa JC (1995) J Chem Soc, Faraday Trans 91:11
Che M, Kibblewhite JFJ, Tench AJ, Dufax, Naccache C (1973) J Chem Soc, Faraday Trans 1:857
Zhang X, Klabunde KJ (1992) Inorg Chem 31:1706
Soria J, Martinez-Arias A, Coronado JM, Conesa JC (1996) Colloids Surf A 115:215
Hulzinga T, Prins R (1993) J Phys Chem 87:173
Chandrashekhar GV, Title RS (1976) J Electrochem Soc 123:392
Harrison B, Diwell AF, Hallett C (1988) Platinum Metals Rev 32:73
Abbasi et al (2011) J Hazard Mater 186:1445
Pierre D, Deng W, Flytzani-Stephanopoulos M (2007) Top Catal 46:363
Salazar et al (2006) Appl Catal A 310:54
Rogemond et al (1997) Appl Catal A 156:253
Liotta et al (2004) Appl Catal B 48:253
He et al (2012) Int J Hyd Ener 37:11132
Olivier Thinon, Karim Rachedi, Fabrice Diehl, Priscilla Avenier, Yves Schuurman (2009) Top Catal 52:1940
[33] Xue-Qing Gong, P. Hu, R. Raval (2003) The Journal of Chemical Physics 119: 6324
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by ESF (Grant BG051PO001-3.3.06-0050).
Rights and permissions
About this article
Cite this article
Ivanov, I., Petrova, P., Georgiev, V. et al. Comparative Study of Ceria Supported Nano-sized Platinum Catalysts Synthesized by Extractive-Pyrolytic Method for Low-Temperature WGS Reaction. Catal Lett 143, 942–949 (2013). https://doi.org/10.1007/s10562-013-1078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1078-3