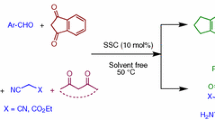
Abstract
Silica-bonded 2-hydroxyethylammonium acetate was synthesized easily by the reaction of 3-chloropropylsilica with ethanolamine followed by ion exchange with acetate. It was used as a heterogeneous, re-usable, efficient and easy to handle catalyst for the one-pot synthesis of a variety of 2-Amino-4H-chromen-4-yl phosphonates and β-phosphonomalonates at room temperature under solvent-free conditions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ionic liquids (ILs) have emerged as promising homogeneous catalysts [1–4] because of their unique physicochemical properties including negligible vapor pressure, wide liquid range, high ionic conductivity and excellent solubility [5]. Although the ability of ILs has been demonstrated successfully in many reactions, their widespread use is still hampered by the following practical drawbacks: (i) product isolation; (ii) catalyst recovery which leads to economical and environmental problems; (iii) difficult in handling and (iv) the use of relatively large amounts of ILs which is costly and may cause toxicological concerns [6]. To overcome these drawbacks, the advantages of ILs with those of heterogeneous support materials have been combined by recent attempts on the supporting ILs on solid materials. It was also claimed that immobilization of ILs can enhance the reactivity and selectivity of ILs when they are involved in catalytic reactions [7]. In this view, a variety of methods has been introduced for covalent immobilization of ILs onto the surface of support matrices such as inorganic silica gel [8].
Among the different types of chromen systems, 2-amino-4H-chromenes are of particular utility for the generation of small molecule ligands with highly pronounced spasmolytic diuretic anticoagulant and antianaphylactic activities [9–12]. For example, the tumor antagonist HA 14-1 (Fig. 1) is a new class of 2-amino-4H-chromene derivatives that exhibit a binding activity for the surface pocket of the cancer-implicated Bcl-2 protein and induce apoptosis or programmed cell death in follicular lymphoma B cells and leukemia HL-60 cells [13, 14].
One-pot reaction of salicylaldehydes, malononitrile and a nucleophile is known as an easy approach for the synthesis of various types of 4-substituted 2-amino-4H-chromenes in order to synthesis analogues of HA 14-1 [15, 16]. In this method, Knoevenagel condensation of salicylaldehyde with malononitrile and subsequent cyclization reaction are occurred to produce an intermediate, which undergoes nucleophilic attack to form 4-substituted 2-amino-4H-chromenes. It is worth mentioning that amongst various 2-amino-4H-chromene derivatives, recently, the synthesis of 2-amino-4H-chromen-4-yl phosphonates has attracted much attention by organic chemists [17–23]. These compounds are found in many natural products and are widely used as cosmetics, pigments and fungicides. They also showed significant biological activities such as antibacterial and antioxidant activities [17]. In the methods that have been reported for the synthesis of these compounds InCl3 [18], β-cyclodextrin [19], K3PO4 [20], Et2NH [21], I2 [17], PEG-400 [22] and ethylendiamine diacetate [23] were used as catalyst for the condensation of salicylaldehyde, malononitrile and phosphite esters. Most of these catalysts are homogeneous catalysts and their use mainly suffers from separation, reusability and less cost effective process. Moreover, the existing methods suffer from requiring a relatively long reaction time or use of a large amount of the catalyst.
In our continuous work on the development of efficient and environmentally benign procedures using heterogeneous catalysts [24–31], herein, we report the synthesis of silica-bonded 2-hydoxyethylammonium acetate (2-HEAA) (Fig. 2). To explore the catalytic activity of silica-bonded 2-HEAA and in continuation of our studies on the synthesis of phosphonate derivatives [24–35], we have then investigated the application of this new catalyst for the one-pot synthesis of 2-amino-4H-chromen-4-yl phosphonates.
2 Experimental
2.1 General
Silica gel (surface area: 550 m2/g, average pore size: 60 Ǻ) and other chemicals were purchased from Merck Chemical Company. 3-Chloropropylsilica 1 was prepared according to a previously reported procedure [36]. Melting points were determined by Buchi 510 apparatus and are uncorrected. FT-IR spectra were recorded on a JASCO FT-IR 460 plus spectrophotometer. NMR spectra were recorded on a Bruker Avance DPX-250 and 400. Mass spectra were recorded on a Shimadzu GCMS-QP5050A. The purity of the products and the progress of the reactions were accomplished by TLC on silica gel polygram SILG/UV254 plates. Thermo gravimetric analysis (TGA) was performed using a Shimadzu thermo gravimetric analyzer (TG-50). Elemental analysis was carried out on a Vario EL III CHNS elemental analyzer. The BET surface area measurements were performed on a BEL-MAX (Japan) instrument at liquid nitrogen temperature.
2.2 Synthesis of Silica-Bonded 2-Hydroxyethylammonium Chloride 2
Chloropropyl silica (5.0 g, 1.89 mmol/g based on elemental analysis and TGA) was placed in a flask containing anhydrous toluene (50.0 mL) and ethanolamine (0.6 g). The mixture was refluxed with stirring for 24 h. The cooled modified silica was washed in turn with toluene, ethanol and methanol and dried at 60 °C for 10 h. The silica-bonded 2-hydroxyethylammonium chloride 2 was obtained as white powder (5.5 g, 1.67 mmol/g based on elemental analysis and TGA).
2.3 Synthesis of Silica-Bonded 2-HEAA 3
NaOAc (10.0 mmol) was added to a mixture of 2 (5.0 g) in water (50 mL) and vigorously stirred at room temperature for 24 h. Then the mixture was filtrated and washed with water until impurities of sodium chloride were removed. Absence of chloride ions was tested by AgNO3 solution. The catalyst was dried under reduced pressure to give the silica-bonded 2-HEAA 3 (5.0 g, 1.52 mmol/g based on TGA and 1.57 mmol/g based on elemental analysis).
2.4 Synthesis of 2-Amino-4H-chromen-4-yl Phosphonates
Silica-bonded 2-HEAA 3 (0.08 g) was added to a stirred mixture of salicylaldehyde (1 mmol), malononitrile or ethylcyano acetate (1 mmol) and trialkyl phosphite (1 mmol). The reaction mixture was stirred for the appropriate time at room temperature (Table 2). EtOH (10 mL) was added to the reaction mixture. The catalyst was filtered and washed three times with warm EtOH (5 mL). Pure product was obtained by column chromatography eluted with n-hexane:EtOAc (1:3) after evaporation of the filtrate. The catalyst was dried in vacuo at 50 °C for 24 h and reused for the same reaction.
2.5 Synthesis of β-Phosphonomalonates
Silica-bonded 2-HEAA 3 (66 mg) was added to a stirred mixture of aldehyde (1 mmol), malononitrile (1 mmol) and trialkyl phosphite (1 mmol). The reaction mixture was stirred for the appropriate time at room temperature (Table 4). EtOH (10 mL) was added to the reaction mixture. The catalyst was filtered and washed three times with warm EtOH (5 mL). Pure product was obtained by column chromatography eluted with n-hexane:EtOAc (1:2) after evaporation of the filtrate.
3 Results and Discussion
3.1 Preparation and Characterization of Silica-Bonded 2-HEAA 3
Silica-bonded 2-HEAA 3 was synthesized by the route outlined in Scheme 1. 3-Chloropropylsilica 1 was prepared according to the previously reported procedure [36] and reacted with ethanolamine in toluene under reflux conditions to afford 2. Silica-bonded 2-HEAA 3 was obtained by ion exchange of chloride in 2 with acetate ions.
The thermal behavior of silica-bonded 2-HEAA 3 is shown in Fig. 3. A significant decrease in the weight percentage of the silica-bonded 2-HEAA 3 at about 150 °C (Fig. 3) is related to desorption of water molecules from the catalyst surface. In addition, the analysis showed 2 other decreasing peaks. First peak appears in the temperature range from 170 to 305 °C due to the decomposition of 2-HEAA. This is followed by a second peak at 305–408 °C, corresponding to the loss of the organic spacer group. According to the TGA, the amount of 2-HEAA functionalized on silica is evaluated to be 1.52 mmol/g. These results are in agreement with those of elemental analysis (1.57 mmol/g, n = 2.198 % and C = 12.516 %).
FT-IR spectra of silica gel, silica-bonded 2-HEAA 3 and recovered silica-bonded 2-HEAA 3 are shown in Fig. 4. In the FT-IR spectra of silica-bonded 2-HEAA 3, characteristic adsorption bands due to the stretching vibration of C = O and C–H groups are observed at 1,683 and 2,972 cm−1, respectively. A broad peak placed in the range of 1,000–1,200 cm−1 is related to the stretching vibration of Si–O bond. This peak is also observed in the FT-IR spectra of silica gel. The strong and broad band at around 3,400 cm−1 corresponds to the hydrogen bonded Si–OH groups and adsorbed water. Another broad band at 1,635 cm−1 is also due to the OH vibration of adsorbed water.
It is worth to note that high similarity between FT-IR spectra of the silica-bonded 2-HEAA 3 and the recovered catalyst (3) is observed. This observation showed that the structure of the catalyst did not change during the reaction and also in the work-up process.
The N2 adsorption–desorption isotherm and pore size distribution of the silica-bonded 2-HEAA 3 are illustrated in Fig. 5. Silica-bonded 2-HEAA 3 showed a lower BET surface area and average pore size (263.12 m2/g and 3 nm, respectively) compared with bare silica gel (550 m2/g and 6 nm, respectively). These results confirmed the immobilization of 2-HEAA on the surface of silica gel. The narrow pore size distribution showed that the immobilization of 2-HEAA occurred uniformly on the surface of silica gel.
3.2 Catalytic Activity of Silica-Bonded 2-HEAA 3
As part of our continued interest on the development of new eco-friendly methods for the synthesis of organic compounds, we have recently synthesized β-phosphonomalonates, 4-substituted 2-amino-4H-chromenes, bis(pyrazolyl)methanes, vinylphosphonates and 2-amino-3,5-dicarbonitrile-6-thio-pyridines in the presence of task-specific ionic liquids [34, 35, 37, 38]. Due to the advantages of using heterogeneous catalysts and also importance of biological activities of 2-amino-4H-chromen-4-yl phosphonates, in this paper, we have studied the application of silica-bonded 2-HEAA 3 for the synthesis of these compounds.
At first, to optimize the reaction conditions, the coupling reaction of salicylaldehyde, malononitrile and triethyl phosphite was chosen as a model reaction. This reaction with different catalytic amounts of silica-bonded 2-HEAA 3 was investigated under solvent-free conditions at room temperature (Table 1, entries 1–4). The best yield of the desired product was obtained in the presence of 12 mol% of the catalyst. The reactions proceeded with lower yields in solvents such as EtOH, CH3CN, n-hexane, toluene and H2O (Table 1, entries 5-9). The desired product was obtained in low yields in the presence of SiO2 or 2-HEAA (Table 1, entries 10 and 11). These observations indicated that the catalytic efficiency of 2-HEAA was increased by bonding onto silica gel. The reaction proceeded in 83 % yield in the presence of silica-bonded 2-hydroxyethylammonium chloride 2. Comparison of these results with those obtained in the presence of silica-bonded 2-HEAA 3 showed that the anion did not have any influence on the progress of the reaction (Table 1, entry 12). We have also found that without the addition of the catalyst, this reaction led to the formation of the desired product 4a in low yield (20 %) after 12 h (Table 1, entry 13).
In order to establish the generality of this methodology, the synthesis of a variety of 2-amino-4H-chromen-4-yl phosphonates in the presence of silica-bonded 2-HEAA 3 under the best reaction conditions (12 mol% of 3, solvent-free conditions) was investigated. The results of these studies are summarized in Table 2.

As shown in Table 2, salicylaldehyde underwent coupling reaction with malononitrile and triethyl/trimethyl/tri-iso-propyl phosphite in the presence of silica-bonded 2-HEAA 3 and produced the corresponding products in good yields (Table 2, entries 1–3). The reaction of malononitrile or ethylcyano acetate and triethyl phosphite with salicylaldehydes substituted with electron-releasing and electron-withdrawing groups proceeded well to give the desired products in 71–87 % yields (Table 2, entries 4–11). The catalyst was compatible with functional groups such as Cl, Br, O-Me and O-Et. No competitive nucleophilic methyl/ethyl ether cleavage was observed for the substrates which possessed aryl-O-Me or aryl-O-Et groups (Table 2, entries 7–10), despite the strong nucleophilicity of phosphites.
The reusability of the catalyst was also examined for the synthesis of 2-amino-4H-chromen-4-yl phosphonate 4a from the reaction of salicylaldehyde, malononitrile and triethyl phosphite. The catalyst was recovered easily by adding warm EtOH to the reaction mixture followed by filtration and drying. It was seen that the catalyst displayed good reusability after five runs (Fig. 6).
A plausible mechanism for the formation of 2-amino-4H-chromen-4-yl phosphonates in the presence of silica-bonded 2-HEAA 3 is shown in Scheme 2. The process represents a typical tandem reaction in which initial condensation of salicylaldehyde and malononitrile occurred to form the Knoevenagel product. The spontaneous cyclization as a result of nucleophilic attack of the hydroxyl group on the cyano group led to 2-imino-2H-chromene-3-carbonitrile which underwent nucleophilic attack by trialkyl phosphites to produce the desired product 4. In this mechanism, we have postulated that the hydroxyl group and ammonium moiety of silica-bonded 2-HEAA 3 may have important influence on the progress of the reaction.
To show the role of silica-bonded 2-HEAA 3 in Knoevenagel condensation and subsequent cyclization reaction, the reaction between salicylaldehyde and malononitrile in the absence of any catalyst and in the presence of silica gel, chloropropyl silica, silica-bonded 2-hydroxyethylammonium chloride 2 and catalyst 3 at room temperature was investigated (Table 3).
These results showed that the reaction could be catalyzed by hydroxyl groups on the surface of silica gel to produce 2-imino-2H-chromene-3-carbonitrile in 84 % yield after 3 h (Table 3, entry 2). Alkylation of hydroxyl groups on the surface of silica gel reduced the catalytic activity of chloropropyl silica (Table 3, entry 3). Similar reactions were occurred immediately in the presence of silica-bonded 2-hydroxyethylammonium chloride 2 and catalyst 3 (Table 3, entries 4 and 5). These results showed the influence of ammonium salt on the progress of the reaction. It was also found that the reaction outcome did not depend on the anion in the catalyst. The reaction in the absence of any catalyst proceeded slowly (12 h) to afford the desired product in 82 % yield (Table 3, entry 1).
Following this initial success in the development of a one-pot protocol for the synthesis of 2-amino-4H-chromen-4-yl phosphonates prompted us to investigate the potential of this method for the synthesis of β-phosphonomalonates. For this purpose, at first, the reaction of benzaldehyde, malononitrile and triethyl phosphite was studied in the presence of 12, 6, 3 and 1 mol% of catalyst 3 under solvent-free conditions at room temperature. It was found that a small amount of catalyst 3 (1 mol%) could promote the reaction and produced the desired product in 80 % yield after 15 min. Having these results in hand, the synthesis of different types of β-phosphonomalonates from the reaction of various aldehydes, malononitrile and trialkyl phosphites was performed in the presence of silica-bonded 2-HEAA 3 (1 mol%). The results of these studies were depicted in Table 4.

The results showed that the reactions of benzaldehyde and malononitrile were equally proceeded with triethyl, trimethyl and tri-iso-propyl phosphites and produced the corresponding β-phosphonomalonates in 75–81 % yields (Table 4, entries 1–3). Arylaldehydes with electron-donating or electron-withdrawing groups on the benzene ring underwent the reaction with malononitrile and triethyl phosphite affording 5d–j in 79–91 % yields (Table 4, entries 4–10). Interestingly, silica-bonded 2-HEAA 3 efficiently promoted the synthesis of β-phosphonomalonates using acid-sensitive aldehydes such as furfural and pyridine-3-carbaldehyde without polymerization or decomposition (Table 4, entries 11 and 12). This method is also applicable for the synthesis of β-phosphonomalonates from the reaction of malononitrile and triethyl phosphite with aliphatic aldehydes (Table 4, entries 13 and 14).
4 Conclusions
In conclusion, we have demonstrated the synthesis of silica-bonded 2-hydroxyethylammonium acetate easily by the reaction of 3-chloropropylsilica with ethanolamine followed by ion exchange with acetate. Silica-bonded 2-hydroxyethylammonium acetate was used as a new heterogeneous, re-usable, efficient and easy to handle catalyst for one-pot synthesis of a variety of 2-amino-4H-chromen-4-yl phosphonates and β-phosphonomalonates at room temperature under solvent-free conditions. This silica-bonded 2-hydroxyethylammonium acetate offered practical convenience in product separation and showed a higher catalytic performance than that of non-supported 2-hydroxyethylammonium acetate.
References
Wasserscheid PJ, Keim W (2000) Angew Chem Int Ed 39:3772
Brown RA, Pollet P, Mckoon E, Eckert CA, Liotta CL, Jessop PG (2001) J Am Chem Soc 123:1254
Leitner W (2003) Nature 423:930
Kumar A, Pawar SS (2004) J Org Chem 69:1419
Welton T (1999) Chem Rev 99:2071
Zhao D, Liao Y, Zhang Z (2007) Clean 35:42
Kim DW, Chi DY (2004) Angew Chem Int Ed 43:483
Gadenne B, Hesemann P, Polshettiwar V, Moreau JJE (2006) Eur J Inorg Chem 2006:3697
Desimone RW, Cirrie KS, Mitchell SA, Darrow JW, Pippon DA (2004) Comb Chem High Throughput Screen 7:473
Bonsignore L, Loy G, Secci D, Calignano A (1993) Eur J Med Chem 28:517
Patchett AA, Nargund RP (2000) Annu Rep Med Chem 35:289
Andreani LL, Lapi E (1960) Boll Chim Farm 99:583
Skommer J, Wlodkowic D, Mättö M, Eray M, Pelkonen J (2006) Leukemia Res 30:322
Wang JL, Liu D, Zhang Z, Shan S, Han X, Srinvasula SM, Croce CM, Alnemeri ES, Huang Z (2000) Proc Natl Acad Sci USA 97:7124
Elinson MN, Dorofeev AS, Miloserdov FM, Ilovaisky AI, Feducovich SK, Belyakov PA, Nikishin GI (2008) Adv Synth Catal 350:591
Moafi L, Ahadi S, Bazgir A (2010) Tetrahedron Lett 51:6270
Rajasekhar M, Rao KUM, Sundar CS, Reddy NB, Nayak SK, Reddy CS (2012) Chem Pharm Bull 60:854
Jayashree P, Shanthi G, Perumal PT (2009) Synlett 6:917
Narayana Murthy S, Madhav B, Prakash Reddy V, Nageswar YVD (2010) Tetrahedron Lett 51:3649
Gaikwad DS, Undale KA, Shaikh TS, Pore DM (2011) C R Chimie 14:865
Kulkarni MA, Pandurangi VR, Desai UV, Wadgaonkar PP (2012) C R Chimie 15:745
Das B, Balasubramanyam P, Reddy GC, Nayaki S (2011) Helv Chim Acta 94:1347
Kolla SR, Lee YR (2012) Tetrahedron 68:226
Sobhani S, Safaei E, Asadi M, Jalili F, Tashrifi Z (2008) J Porphyrins Phthalocyanines 12:849
Sobhani S, Rezazadeh S (2011) J Iran Chem Soc 8:198
Sobhani S, Tashrifi Z (2009) Heteroatom Chem 20:109
Sobhani S, Tashrifi Z (2009) Synth Commun 39:120
Sobhani S, Rezazadeh S (2010) Synlett 2010:1485
Sobhani S, Vafaee A (2009) Tetrahedron 65:7691
Sobhani S, Pakdin Parizi Z, Razavi N (2011) Appl Catal A 162:409–410
Sobhani S, Pakdin Parizi Z, Rezazadeh S (2011) J Organomet Chem 696:813
Sobhani S, Safaei E, Asadi M, Jalili F (2008) J Organomet Chem 693:3313
Sobhani S, Pakdin Parizi Z (2011) Tetrahedron 67:3540
Sobhani S, Honarmand M (2012) J Iran Chem Soc 9:661
Sobhani S, Honarmand M (2013) Synlett 24:236
Bi W, Zhou J, Row KH (2010) Anal Chim Acta 677:162
Sobhani S, Honarmand M (2013) C R Chim. doi:10.1016/j.crci.2012.10.011
Sobhani S, Nasseri R, Honarmand M (2012) Can J Chem 90:1
Acknowledgments
We are thankful to University of Birjand Research Council for their support on this work.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sobhani, S., Honarmand, M. Silica-Bonded 2-Hydroxyethylammonium Acetate as an Efficient and Recyclable Catalyst for the Synthesis of 2-Amino-4H-chromen-4-yl Phosphonates and β-Phosphonomalonates. Catal Lett 143, 476–485 (2013). https://doi.org/10.1007/s10562-013-0968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-0968-8