Abstract
The Keggin phosphotungstic acid, H3PW12O40 (HPW), was successfully immobilized on the surface of mesostructured LaSBA-15 by means of chemical bonding to aminosilane groups. The catalysts were characterized by elemental analysis, N2 adsoption, TEM, DRS-UV, and FTIR spectroscopy. Characterization results suggest that the surface area decreased after grafting amino groups to silica and the structures of heteropolyanions on amine-modified LaSBA-15 was maintained. Their catalytic behaviors were investigated in the alkylation of o-xylene with styrene. Among the functionalized catalysts, when the content of amino-groups was suitable, it had the best catalytic performances in terms of yield and stability. It is worth mentioning that the catalysts could be used repeatedly without loss of the activity and selectivity during several catalytic cycles. The good stability can be attributed to the strong interaction between the amino groups on the surface of LaSBA-15 and HPW anions.
Graphical Abstract
The Keggin phosphotungstic acid, H3PW12O40 (HPW), was successfully immobilized on the surface of mesostructured LaSBA-15 by means of chemical bonding to aminosilane groups. The obtained catalysts were highly effective for alkylation of o-xylene with styrene in terms of yield and stability. Compared with the HPW/LaSBA-15 catalyst, both the catalytic stability and reusability were greatly enhanced.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The alkylation of o-xylene with styrene to give 1-phenyl-l-arylethane is an industrially important reaction [1]. Phenylxylylethane (PXE) is widely used as a high-energy fuel for turbojets, jets, rockets, missile engines; as a high-stability lubricant and as an important additive in the corrosion protective coatings of chlorinated rubber and synthetic resins [1]. One of the main problems in this reaction is the formation of styrene oligomers generated as byproducts, especially when homogeneous Brønsted or Lewis acid catalysts are used [1]. Traditionally, various homogeneous catalysts have been used in this reaction, for instance, H2SO4, BF3, HF, AlCl3 or FeCl3 but their environmental non-friendliness, troublesome product recovery and purification, and impossibility of catalyst recycling make them unsuitable for the purpose. Many efforts have been made to exploit the environmentally friendly catalytic technology for the target reaction. In recent years, use of heterogeneous catalysts in liquid phase reactions has received great attention due to their advantages such as high activity and selectivity, reusability, ease of separation, no corrosion or disposal of effluent problems, etc. For PXE synthesis, solid acids such as silica-alumina [2], cation-exchange resins [3], sulfated zirconia/titania [4, 5] and Al-MCM-41 [6] have been used as catalysts.
Heteropolyacids, particularly the Keggin-type phosphotungstic acid (hereafter HPW), exhibit high acidic strengths and have performed admirably in a wide variety of acid-catalyzed reactions [7, 8]. The major disadvantages of HPW lie in extremely low surface area and water-solubility, which always limit its practical applications [9]. Impregnation is an easy way to increase its surface area by supporting HPW onto various carriers, such as silica gel, zeolite Y, ordered mesoporous silica (e.g. SBA-15 or MCM-41), cellular foam silica and so on [10]. However, HPW has the tendency to leach from carriers into polar reaction media, which leads to the poor catalytic reusability.
It is desired to develop a support with controlled strength and concentration of surface basic sites can prevent HPW leaching without destroying its acidity. Grafting method, mainly involving the pre-functionalization of carrier with organic components and chemical linkage of HPW, has been chosen to anchor HPW on carriers for avoiding leaching. Based on this premise, Pizzio et al. [11] revealed that HPW supported on amine-functionalized silica and SiMCM-41 exhibited negligible solubility and high activity in the synthesis of isoamylacetate. Kim et al. [13] has also reported that H3PMo12O40 was chemically immobilized on a kind of porous carbon by forming a positive charge on the support via surface modification. Jin et al. [12] anchored Keggin-structured tungstovanadogermanic HPA (H5GeW11VO40) on the aminesilane functionalized SBA-15, through which HPA clusters were attached firmly to the surface of SBA-15 evidenced by spectroscopic characterizations. SBA-15, a well-ordered hexagonal mesoporous silica with an uniform pore size up to ~30 nm, is an ideal support for acid catalyst for its thicker pore walls and higher thermal and hydrothermal stability [14, 15]. Very recently, our research group successfully incorporated rare-earth element La into SBA-15 silicate framework by direct synthesis method [16]. Compared with the SBA-15, Lanthanum doped SBA-15 (LaSBA-15) possesses some weak Lewis acid and higher thermal stability, besides a tunable pore and large surface area, which is ideal for the dispersion of catalytically active entities, thus being widely used as a catalytic support [17] or catalyst [18, 19]. However, to the best of our knowledge, no attempt has been made to immobilize Keggin-structured phosphotungstic acid (HPW) on the aminopropyl-functionalized mesostructured LaSBA-15 materials. Furthermore, no systematic investigation on the amount of amino-groups used for the surface functionalization of mesoporous materials has been conducted yet.
In this work, mesostructured LaSBA-15 was prepared via a surfactant templating method. The as-obtained LaSBA-15 silica was then modified by grafting 3-aminopropyltriethoxysilane (APTES) to provide sites for the immobilization of HPW. By taking advantage of the overall negative charge of [PW12O40]3−, the HPW catalyst was chemically immobilized on the surface of modified LaSBA-15 (NH2-LaSBA-15) materials as a charge matching component. The characteristics of HPW catalyst immobilized on the NH2-LaSBA-15 materials were characterized in terms of various physicochemical techniques. The catalytic properties of the catalysts were assessed in the alkylation of o-xylene with styrene. Special attention was paid to catalyst stability and reusability.
2 Experimental
2.1 Mesoporous Materials Preparation
Lanthanum-substituted mesoporous SBA-15 materials has been prepared by direct synthesis. 9.4 g of tetraethyl-orthosilicate and 0.97 g of Lanthanum nitrate (Si/La = 20 molar ratio) were added to 10 mL of HCl aqueous solution at pH 1.5. This solution was stirred for over 3 h and then added to a second solution containing 4 g of amphiphilic triblock copolymer poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (EO20PO70EO20) (Pluronic 123 from Aldrich) in 120 mL of HCl aqueous solution at pH 1.5 at 40 °C. The mixture was stirred vigorously for 24 h and then transferred into a Teflon-lined autoclave and aged for 48 h at 120 °C. The resulting solid was filtered, washed and dried at 120 °C for 24 h. The final mesoporous LaSBA-15 products were obtained after calcination in air at 540 °C for 6 h (heating rate: 2 °C min−1).
The adsorption of HPW on the surface of LaSBA-15 was carried out by the following procedure: 1 g of LaSBA-15 was added to an aqueous solution containing HPW (0.60 g) with vigorous stirring at 80 °C. After being stirred for 8 h, the solid was filtered, washed with distilled water and then it was dried overnight at 80 °C. The obtained sample is designated as HPW/LaSBA-15 catalyst.
2.2 Surface Modification of LaSBA-15 and Immobilization of HPW
Figure 1 shows the schematic procedures for the surface modification of LaSBA-15 materials and the subsequent immobilization of HPW on the surface of modified LaSBA-15 (NH2-LaSBA-15) materials. The surface modification of the LaSBA-15 materials was achieved by reacting the silanol group of the LaSBA-15 materials with APTES under a nitrogen atmosphere. A known amount of APTES was slowly added to a dry toluene solution containing 1 g of LaSBA-15 materials with constant stirring at room temperature. After the solid product was filtered and dried, it was calcined at 180 °C for 2 h to yield the NH2-LaSBA-15 samples. A series of NH2-LaSBA-15 samples (NH2-LaSBA-15-1.0, NH2-LaSBA-15-1.5, NH2-LaSBA-15-2.0, NH2-LaSBA-15-2.5, and NH2-LaSBA-15-3.0) were prepared by adjusting the amount of APTES added to 1 g of NH2-LaSBA-15 materials. For example, NH2-LaSBA-15-1.0 denotes the NH2-LaSBA-15 materials prepared by the addition of 1.0 mmol of APTES to 1 g of NH2-LaSBA-15 materials.
The immobilization of HPW on the NH2-LaSBA-15 support was carried out as follows (shown in Fig. 1). 1 g of NH2-LaSBA-15 was added to an aqueous solution containing HPW (0.60 g) with vigorous stirring at 80 °C. After being stirred for 8 h, the solid was filtered, washed with distilled water and then it was dried overnight at 80 °C to yield the HPW/NH2-LaSBA-15 catalysts (HPW/NH2-LaSBA-15-1.0, HPW/NH2-LaSBA-15-1.5, HPW/NH2-LaSBA-15-2.0, HPW/NH2-LaSBA-15-2.5, and HPW/NH2-LaSBA-15-3.0).
2.3 Characterization
The nitrogen adsorption–desorption isotherms were measured at −196 °C on a Micromeritics ASAP 2020 apparatus. The surface areas and pore volumes of the prepared samples were calculated using the BET equation and the BJH model, respectively. Nitrogen contents were determined by CHN elemental analyses (EC Instrument, EA1110). The HPW contents were obtained by X-ray fluorescenece (XRF) measurements on a SWITZERLAND ARL9800 XRF. Pore structures of the samples were examined by TEM (Jeol, JEM-2000EXII).
Infrared spectra were recorded on a Bruker Tensor 27 using DRIFT techniques, scanned from 4,000 to 400 cm−1. The sample was ground with KBr and pressed into a thin wafer.
The diffuse reflectance UV–Vis spectra were collected using a SHIMADZU UV3600 scanning spectrophotometer. The powder sample was loaded into a quartz cell, and the spectra were collected over the range of 200–800 nm reference to BaSO4.
2.4 Catalytic Tests
The alkylation reactions were carried out in a continuously stirred batch reactor under reflux conditions using a three-neck 100-ml round-bottom flask equipped with a condenser. Preliminary runs were conducted with 7.50 g (0.0721 mol) of styrene, 57.35 g (0.5402 mol) of o-xylene (mole ratio of o-xylene to styrene, 7.5:1) and 1.50 g of catalyst (20% w/w of styrene) at 120 °C for 180 min. The required amount of o-xylene was initially added to the reactor at the reaction temperature, followed by the desired amount of catalyst, a known amount of styrene was then added to the reaction mixture at the same temperature. After the reaction, unreacted o-xylene was distilled out under atmospheric pressure and then a collected part was called as crude product. The crude product was analyzed with GC-9890A gas chromatograph equipped with OV-1 capillary column and a flame ionization detector (FID). The yield of PXE was defined as follows:
3 Results and Discussion
3.1 Characterization of the HPW/NH2-LaSBA-15 Catalysts
The elemental analysis data and textural properties of the initial supports and supported HPW samples are given in Tables 1 and 2, respectively. The amount of aminopropyl functional group in the NH2-LaSBA-15 materials was indirectly measured by CHN elemental analysis. As can be seen, the surface area of the NH2-LaSBA-15 and HPW/NH2-LaSBA-15 materials decrease with increasing amount of APTES used, while the nitrogen content in the NH2-LaSBA-15 materials increase roughly with increasing amount of APTES used. As expected, no nitrogen has been detected in the bare LaSBA-15 sample. These results strongly suggest that aminopropyl functional group has been successfully grafted on the LaSBA-15 materials via the surface modification step. It could be also found that the nitrogen content increase linearly with increasing amount of APTES used up to 2.0 mmol. However, no additional increase of nitrogen content can be observed when the amount of APTES is greater than 2.0 mmol. Furthermore, the HPW/NH2-LaSBA-15 catalysts show a lower surface area than the corresponding NH2-LaSBA-15 supports, due to the loading of the HPW species (Tables 1 and 2). The HPW loading depends on the surface concentration of NH2 groups on the support. Although the nitrogen content in the NH2-LaSBA-15 supports is the highest when 2.0 mmol of APTES is used (Table 1), no substantial difference in HPW loading is found in the HPW/NH2-LaSBA-15 catalysts when the amount of APTES exceeded 2.0 mmol (Table 2). The above results indicate that 2.0 mmol of APTES is sufficient to modify the surface of the NH2-LaSBA-15 materials for the maximum loading of HPW species. In other words, the surface modification of LaSBA-15 supports to provide anchoring sites for HPW species is essential for the successful immobilization of HPW species on LaSBA-15 supports.
Figure 2 shows the N2 adsorption–desorption isotherms and pore size distributions of LaSBA-15, NH2-LaSBA-15-2.0, and HPW/NH2-LaSBA-15-2.0. All the samples exhibit typical IV type isotherms and H1 type hysteresis loops at high relative pressures. This indicates that LaSBA-15 with a fairly uniform pore size distribution was successfully prepared. Interestingly, the NH2-LaSBA-15-2.0 and HPW/NH2-LaSBA-15-2.0 show very similar isotherm patterns (inset) and pore size distributions compared to those of LaSBA-15, indicating that the mesopore structure of LaSBA-15 was still maintained even after the surface modification step and the subsequent immobilization step of HPW.
Figure 3 shows the TEM images of LaSBA-15, NH2-LaSBA-15-2.0, and HPW/NH2-LaSBA-15-2.0. The well-ordered hexagonal arrays of mesopore of LaSBA-15 can be clearly seen in all samples. The pore diameters of all samples determined from TEM images are ca.7 nm with no great difference, in good agreement with the pore size distribution calculated from the BJH isotherm model (Fig. 2). This result clearly indicates that the samples keep the pore structure of the support very well.
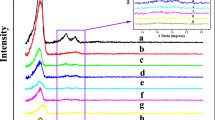
The successful immobilization of the HPW catalyst on the aminopropyl-functionalized LaSBA-15 materials can also be confirmed by FT-IR analyses as shown in Fig. 4. In the case of LaSBA-15, NH2-LaSBA-15-2.0, and HPW/NH2-LaSBA-15-2.0, the asymmetric and symmetric stretching vibrations of the Si–O–Si framework bands originated from LaSBA-15 materials were observed at around 465, 800 and 1,080 cm−1. A broad band at 3,000–3,400 cm−1 and a weak band at around 2,930 cm−1 observed in the NH2-LaSBA-15-2.0 and HPW/NH2-LaSBA-15-2.0 are attributed to the –CH2– stretching Vibration, indicating the presence of aminopropyl functional group in the NH2-LaSBA-15-2.0 and HPW/NH2-LaSBA-15-2.0 [20]. The IR spectra of the pure HPW, NH2-LaSBA-15-2.0, and HPW/NH2-LaSBA-15-2.0 are shown in Fig. 4b. Pure HPW shows IR bands approximately at 1,080 (P–O in the central tetrahedron), 980 (terminal W = O) and 890 and 800 (W–O–W) cm−1 corresponding to asymmetric vibration associated with Keggin ion. From the FTIR analysis, it is found that no structural collapse of pore structures occurred by the incorporation of HPW for Si–O bonds. The characteristic IR bands of [PW12O40]3− in the HPW/NH2-LaSBA-15-2.0 are different from those of the unsupported one. The P–O band in the HPW/NH2-LaSBA-15-2.0 sample is not clearly identified due to the overlapping by the broad Si–O–Si band. However, W–O and W–O–W bands of [PW12O40]3− in the HPW/NH2-LaSBA-15-2.0 sample appear at slightly red-shift positions (944 cm−1) compared to those of the unsupported one, indicating the presence of a strong interaction between [PW12O40]3− and NH2-LaSBA-15-2.0 supports [21].
3.2 Catalytic Activity
The catalytic activity of different catalysts is showed in Table 3. The detailed reaction scheme is shown in Scheme 1, reaction 1 is the PXE formation reaction whereas reaction 2 and 3 represent the formation of styrene oligomers and more substitutes, respectively.
As listed in Table 3, the homogeneous HPW show very high catalytic performances for the reaction, however, it is difficult to separate the HPW from the product mixture. Furthermore, the NH2-LaSBA-15 support itself shows no activity performances. It can be seen that the PXE yield decreases in the following order: HPW/NH2-LaSBA-15-3.0 (93.2%) ≈ HPW/NH2-LaSBA-15-2.5 (93.0%) ≈ HPW/NH2-LaSBA-15-2.0 (92.9%)> HPW/NH2-LaSBA-15-1.5 (73.3%) > HPW/NH2-LaSBA-15-1.0 (56.2%). It is intriguing that much higher conversion (100%) and excellent selectivity (~90%) were also obtained when HPW content is higher than 30% (see Table 2). The differences in catalytic performances can be attributed to the fact of the diverse active HPW species amounts. The HPW content of the HPW/NH2-LaSBA-15 catalysts increases with the increasing amount of APTES used. However, no substantial difference in HPW loading is found in the HPW/NH2-LaSBA-15 catalysts when the amount of APTES exceeded 2.0 mmol. In this case a significant difference is found among these catalysts as a function of the HPW loading. Due to the less amounts of active site in the sample, the catalysts of HPW/NH2-LaSBA-15-1.0 and HPW/NH2-LaSBA-15-1.5 show less product yield than that of the 2.0, 2.5 and 3.0 HPW/NH2-LaSBA-15 catalysts. This result suggests that 2.0 mmol of APTES is sufficient to modify the surface of the NH2-LaSBA-15 materials for the maximum loading of HPW species. In addition, it is worth of noting that the HPW/LaSBA-15 shows higher catalytic performances, because it has higher surface and HPW content (see Table 2).
The important questions that must be addressed while studying alkylation processes over a solid catalyst relate to the stability of the catalyst to leaching of the active component and the possibility of catalyst recycling. The catalytic reusability of the HPW/NH2-LaSBA-15-2.0 and HPW/LaSBA-15 catalysts was evaluated by carrying out the reaction with used catalyst under the optimized conditions. After each run, the catalyst was recovered by filtration, then washed with ethanol, dried and used again. The data obtained are shown in Fig. 5. It can be seen that only 5% reduction in the activity is observed after 6 runs on the HPW/NH2-LaSBA-15-2.0 catalyst. In contrast, the deactivation of the HPW/LaSBA-15 catalyst is much faster and the PXE yield drops to a very low level of 66.0% after the sixth reaction cycle. The poor catalytic stability of HPW/LaSBA-15 may be due to the possibility that HPW leaching from the catalyst support into the liquid solvent may result in the low conversion. On the other hand, the decrease of yield arising from catalysts lost during separation and transfer of catalysts into the next reaction cycle cannot be excluded. This observation reveals satisfied reusability for HPW/NH2-LaSBA-15-2.0, which means that HPW have only a slight tendency to leach from the functionalized LaSBA-15 carrier in reaction.
Figure 6 compares FIIR spectra of HPW/NH2-LaSBA-15-2.0 catalyst before and after reaction. It can be seen that the bands assigned to Keggin structure at 980 cm−1 (terminal W = O), 890 and 800 cm−1 (W–O–W) of used HPW/NH2-LaSBA-15-2.0 are still observed, demonstrating that Keggin HPW still stayed on the surface of amine-modified LaSBA-15 and kept its own structure.
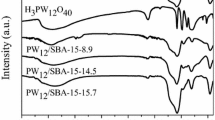
The leaching of the HPW from the various supports was also studied by UV–Visible spectroscopy. Figure 7 shows the UV–Vis diffuse reflectance spectra of HPW/LaSBA-15 and HPW/NH2-LaSBA-15-2.0 catalysts after six catalytic cycles. As can be seen, a strong signal in the UV–Visible spectra at λ = 262 nm is observed for the HPW/NH2-LaSBA-15-2.0 catalyst while no obvious absorption in this region is detected in the case of HPW/LaSBA-15 catalyst. According to the literature [22], this characteristic band can be assigned to the oxygen-metal charge transfer of tungstophosphate anion PW12O40 3−. This evidence indicates little leaching of HPW from the catalyst and confirms strong immobilization of HPW on the support.
In conclusion, we can thus be certain that grafting the silica surface with amine group results in efficient immobilization of HPW, which maintains its high activity in acid-catalyzed reactions. The well stability of HPW on functionalized LaSBA-15 may be attributed to the strong chemical interaction of ≡ Si(CH2)3NH3 + and HPW, where modified LaSBA-15 with a positive charge provided sites for HPW as a charge compensating component [23, 24]. The above results imply that the amine groups in the LaSBA-15materials played a key role for the immobilization of HPW, which can prevent HPW leaching from the catalyst support.
4 Conclusions
In this work, the heteropoly phosphotungstic acid, H3PW12O40, has been successfully immobilized on the surface of mesoporous LaSBA-15 by means of chemical bonding to aminosilane groups. Characterization results from elemental analysis and N2 sorption indicate that the surface area decreased after grafting organic amine to LaSBA-15 materials. The aminopropyl functional groups were successfully grafted on the LaSBA-15 from FT-IR results. The strong interaction between the NH2 groups in the surface of LaSBA-15 and HPW molecules is shown by FTIR and DRS-UV spectroscopy. The HPW/NH2-LaSBA-15 is highly efficient in the alkylation of o-xylene with styrene. When the content of amino-groups was 2.0 mmol, it had the best catalytic performances with styrene conversion up to 100% and PXE yield up to 93%. The HPW/NH2-LaSBA-15-2.0 catalyst could be used for more than six times without any significant loss of activity and leaching of tungsten species in the reaction mixture. The good stability can be attributed to the strong interaction between the NH2 groups in the surface of LaSBA-15 and HPW molecules.
References
Dixit AB, Yadav GD (1995) React Funct Polym 31:237
Sato A, Shimizu I (1979) US, 4144279
Sato A, Shimizu I, Mesalito G (1981) US, 4289918
Lunsford JH, Sang H, Campball SM, Liang CH, Anthony RG (1994) Catal Lett 27:305
Wang YP, Qu RJ, Wang CH (2009) Appl Chem Ind 38:822
Dang FM, Zhen XP, Niu CG, Sun C, Wang JD, Su XT (2009) Yingyong Huaxue 26(12):1404
Heydari A, Khaksar S, Sheykhan M, Tajbaksh M (2008) J Mol Catal A chem 287:5
Kumar A, Singh P, Kumar S, Chandra R, Mozumdar S (2007) J Mol Catal A chem 276:95
Okuhara T, Kimura M, Kawai T, Xu Z, Nakato T (1998) Catal Today 45:73
Rao PM, Wolfson A, Kababya S, Vega S, Landau MV (2005) J Catal 232:210
Pizzio LR, Vázquez PG, Cáceres CV, Blanco MN (2003) Appl Catal A: Gen 256:125
Jin H, Wu Q, Zhang P, Pang W (2005) Solid State Sci 7:333
Kim H, Kim P, Lee KY, Yeom SH, Yi J, Song IK (2006) Catal Today 111:361
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Science 279:548
Lapkin A, Bozkaya B, Mays T, Borello L (2003) Catal Today 81:611
Sheng XL, Zhou YM, Duan YZ, Zhang YW, Xue MW (2011) J Porous Mater 18:677–683
Bendahou K, Cherif L, Siffert S, Tidahy HL, Benaissa H, Aboukais A (2008) Appl Catal A Gen 351:82
Min J, Jae KP, Eun WS (2004) Microporous Mesoporous Mater 75:159
Geraldo E, Luz Jr, Stevie H (2010) J Mater Sci 45:1117
Wang ZQ, Zhou YM, Yao QZ (2009) Appl Surf sci 256:1404
Wu SS, Wang J, Zhang WH, Ren XQ (2008) Catal Lett 125:308
Hu JC, Wang YD, Chen LF, Richards R, Yang WM, Liu ZC, Xu W (2006) Microporous Mesoporous Mater 93:158
Li W, Li L, Wang Z, Cui A, Sun C, Zhao J (2001) Mater Lett 49:228
Kim H, Jung JC, Yeom SH, Lee K, Yi J, Song IK (2007) Mater Res Bull 42:2132
Acknowledgments
The authors are grateful to National Natural Science Foundation of China (50873026, 21106017), Specialized Research Fund for the Doctoral Program of Higher Education of China (20100092120047) and Production and Research Prospective Joint Research Project of Jiangsu Province of China (BY2009153) for financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, X., Zhou, Y., Zhang, Y. et al. Highly Active and Green Aminopropyl-Immobilized Phosphotungstic Acid on Mesoporous LaSBA-15 for Alkylation of O-xylene with Styrene. Catal Lett 142, 360–367 (2012). https://doi.org/10.1007/s10562-012-0769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0769-5