Abstract
An environmentally friendly catalytic system for trimethylsilylation of alcohols and phenols with hexamethyldisilazane can be successfully carried out for the first time over sulfonated mesoporous carbon catalyst (CMK-5-SO3H) in dichloromethane at ambient temperature and excellent conversions were obtained. Furthermore, the catalyst displays high activity and thermal stability (to 200 °C) and it can be reused repeatedly for at least 25 cycles without any evidence of loss of activity, confirming the stability of covalent bonding of acidic centers.
Graphical Abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among the routinely employed methodologies for the protection of hydroxyl groups in organic chemistry, silylation undoubtedly plays a major role, from both analytical and synthetic point of views [1, 2]. As it is widely known, silyl ethers are resistant to oxidation, have a good stability for most non-acidic reagents and are easily deprotected to provide the free alcohols [3]. Generally, the formation of silyl ethers carried out by treatment of parent alcohols with silyl halides or silyl triflates in the presence of stoichiometric amounts of a base [4–6], Li2S [7], and occasionally with a nonionic super base catalyst [8–11]. However, these base-catalyzed silylation methods have serious disadvantages, since careful extraction and filtration are required to remove ammonium salts derived from the reaction of by-produced acids and co-bases during the silylation reaction.
On the other hand, 1,1,1,3,3,3-hexamethyldisilazane (HMDS) as an inexpensive, commercially available and stable reagent, is frequently used for the trimethylsilylation of hydroxyl groups, giving ammonia as the only byproduct. Even though the handling of this reagent is easy, its main drawback is its poor silylating power which needs forceful conditions and long reaction times in many instances [12]. Therefore, a variety of catalysts have been developed for activation of this reagent, such as sulfuric acid [1, 2], (CH3)3SiCl [13], sulfonic acids [14], K-10 montmorilonite [15, 16], iodine [17], tungstophosphoric acid (H3PW12O40) [18], LiClO4 [19], Mg(OTf)2 [20], CuSO4·5H2O [21], TBBDA, and PBBS [22], [PdCl(η3-C3H5)]2-PPh3 [23], MgBr2·OEt2 [24], InBr3 [25], LaCl3 [26], HReO4 [27], silica supported perchloric acid [28], trichloroisocyanuric acid [29], Fe(F3CCO2)3 [30], Zr(OTf)2 [31], Bi(OTf)3 [32], and TiCl2(OTf)–SiO2 [33]. Although several of these procedures are useful, some of them suffer from the use of homogenous and often corrosive catalyst, tedious workup and long reaction times. Besides, many of these reagents are expensive and non-recoverable leading to the generation of large amount of toxic waste particularly when large scale applications are considered. On these bases, developments of milder conditions were tried and investigations on green, simple and highly efficient processes over solid acid catalysts became the chemists interesting undertaking.
Solid acids are conventional materials that have wide applications in chemical production, separation, and purification and the chemical industry is currently searching for highly active and stable solid acids to improve the environmental safety of the production of chemicals [34–40]. In other word, the green approach to chemical processes has stimulated the use of recyclable strong solid acids as replacement for unrecyclable homogenous acid catalysts. In this regard, Karimi et al. have demonstrated the application of recoverable sulfonic acid functionalized ordered mesoporous silica (MCM-41-PrSO3H) for efficient silylation of alcohols and phenols [41].
It is notable that, the availability of ordered mesoporous silica also entails great opportunities for the templating synthesis of highly ordered mesoporous carbon [42–49] for reasons of both practical and fundamental interest. Carbon material is inert and has good conductivity and resistance to environmental and chemical attack. The carbon surface has been exclusively used as a support substrate, especially as electrodes with a wide potential window in electrochemistry for catalytic, analytical and biotechnological applications [46–48]. Moreover, carbonaceous solid acids can be maintained strong acidity even in water, participating in many industrially important acid-catalyzed reactions.
2 Experimental Section
2.1 General Procedure for the Trimethylsilylation of Alcohols
To a solution of alcohol (1 mmol) and hexamethyldisilazane (0.7 mmol) in dichloromethane (3 mL) the catalyst CMK-5-SO3H (23–35 mg, 2–3 mol%) was added. The mixture was stirred at room temperature for the period of time indicated in Table 1. Reaction progress was monitored by thin layer and gas chromatography. After completion of the reaction, the product was isolated by filtration. Evaporation of the solvent under reduced pressure gave the corresponding silyl ether in good to excellent yields (Table 1).
2.2 Preparation of SBA-15
The synthesis of SBA-15 has been achieved using known procedure described by Yu et al. [50] including a mixture of P123 (0.02 mol), TEOS (1 mol), KCl (1.5 mol), HCl (6 mol), and H2O (166 mol). Briefly, 12 g of P123 was dissolved in a mixture of 74.4 g of concentrated HCl and 375.6 g of distilled water at 38 °C, followed by the addition of 16.5 g of KCl. To the solution, 31.5 g of TEOS was added with vigorous stirring for 8 min. The mixture was kept statically at the same temperature for 24 h, and the mixture was transferred to Teflon lined autoclaves and put in an oven at 130 °C for another 24 h. The solid was recovered by filtration, washed by water. The surfactant was then extracted by refluxing with a solution of EtOH using a Soxhelet apparatus for 36 h and the obtained SBA-15 was then dried overnight at 100 °C.
2.3 Preparation of CMK-5
Templated synthesis of CMK-5 has been achieved using known procedure described by Ryoo et al. [49, 51]. Initially, Al was incorporated into SBA-15 (molar ratio Si/Al = 20) by well dispersion of calcined SBA-15 into an aqueous solution of AlCl3, followed by removal of H2O by rotaevaporator and calcination in air. Impregnation of furfuryl alcohol (FA) into Al-SBA-15 was achieved by incipient wetness infiltration at room temperature. The mixture was then heated up at 80 °C oven for 16 h for Al-catalyzed polymerization of FA. The obtained composite was recovered by filtration to remove excess and unpolymerized FA, and washed by EtOH and acetone. The composite was heated to 850 °C under vacuum at a ramp of 10 °C/min, and the carbonization was carried out at the same temperature for 3 h under vacuum. Ordered mesoporous carbon (CMK-5) was obtained by removal of silica template by HF (10% in 1: 1 EtOH–H2O), washed with copious water and EtOH, and finally dried at 100 °C.
2.4 Preparation of 4-Benzenediazoniumsulfonate
4-Benzenediazoniumsulfonate was synthesized by diazotization of p-sulfonilic acid [52]. In a three-necked ground flask, 12.99 g (0.075 mol) of p-sulfonilic acid was dispersed in 1 M HCl, resulting a 0.1 M suspension. To the well-stirred suspension in an ice-water bath (3–5 °C), was dropwise added a 10% excess of 1 M aqueous solution of NaNO2 (82.5 mL). The solid p-sulfanilic acid was slowly dissolved during of the addition of NaNO2 and a clear solution was obtained after all of NaNO2 solution was added. The mixture was stirred for another 45 min at the same temperature. The white precipitate formed was filtered off, washed by small amount of cold water, and dried under reduced pressure.
2.5 Preparation of Sulfonated Ordered Mesoporous Carbon (CMK-5-SO3H)
In a typical modification, 1.2 g of CMK-5 was added in a three-necked ground flask containing 12.0 g of 4-benzene-diazoniumsulfonate in 200 mL of distilled water and 200 mL of ethanol. Subsequently, the mixture was cooled down to 5 °C and 200 mL of 50 wt% H3PO2 aqueous solution was added. After stirring for 30 min, another 200 mL of H3PO2 aqueous solution was added. The mixture was stirred at 5 °C for another 30 min. The sulfonic acid functionalized carbon material (denoted as CMK-5-SO3H) was recovered by filtration, washed thoroughly with distilled water and finally acetone, and dried in an oven at 80 °C.
3 Results and Discussion
Covalent attachment of aryl sulfonic acid on ordered mesoporous carbon with mesoporosity both inside nanopipes and between nanopipes can be produced by homogenous reduction of 4-benzene-diazoniumsulfonate by hypophosphorous acid, which has been reported very most recently by Feng et al. [53], resulted in preparation of sulfonated ordered mesoporous carbon (CMK-5-SO3H). This catalyst was prepared in our laboratory following described procedure (Fig. 1) and was characterized by TGA (Fig. 2), BET surface area measurement, BJH, and N2 adsorption–desorption analysis (supporting information).
The organic composition of the solid sulfonic acid was quantitatively determined by potentiometric titration. Typically a loading of ca. 0.87 ± 0.03 mmol/g was obtained. It is worthwhile to mention that the weight loss of CMK-5-SO3H below 100 °C (~7 wt%) can be attributed to the physically adsorbed water. Further weight loss of CMK-5-SO3H at higher temperature (about 200 °C) accounts for loss of the SO3H segment. Accordingly, the total weight loss reaches 22.4%.
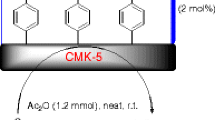
Due to the attributes described above for the use of sulfonated ordered nanoporous carbon in catalysis and our current interest in developing environmentally benign synthetic protocols for different organic transformations [41, 54–56], we reveal herein for the first time, highly reusable CMK-5-SO3H catalyzed trimethylsilylation of a wide variety of alcohols and phenols at ambient temperature (Scheme 1).
At the outset, efforts were made towards the catalytic evaluation of CMK-5 towards the synthesis of silyl ethers using 1 equiv. of benzyl alcohol, 1 equiv. of HMDS and 0.02 g of CMK-5. These were stirred at room temperature in CH2Cl2. After 2 and 24 h, only 30 and 48% of benzyloxytrimethyl silane were obtained, respectively. It should be noted that, when no catalyst was used, the reaction yielded only 20% of expected product after 2 h. The same reaction was then carried out using 23 mg (2 mol%) of CMK-5-SO3H under similar reaction conditions. Interestingly, a significant improvement was observed and the yield of silylated product was increased to 99% gas chromatography yield after stirring the mixture for only 25 min. For establishing the best reaction conditions, an optimization study was performed using the reaction of benzyl alcohol in the presence of varying amounts of HMDS and CMK-5-SO3H. Gratifyingly, 98% of corresponding product was obtained in the presence of 0.7 mmol HMDS and 2 mol% of sulfonated catalyst CMK-5-SO3H after only 30 min. After achieving this favorable result, in order to ascertain the scope and generality of this catalyst, we screened a range of alcohols and phenols. The experiment is shown in Scheme 1 and the results are collected in Table 1.
Compounds that were silylated in this way are benzylic, primary, hindered and unhindered secondary, and unhindered tertiary alcohols (Table 1). Generally, in the case of primary alcohols, the reactions were completed within less than 2 h in CH2Cl2 at room temperature accompanied by a fast evolution of NH3 gas from the reaction mixture (Table 1, entries 1–11). This method is also efficient for the silylation of secondary alcohols (Table 1, entries 12–17). Meanwhile, primary and secondary aliphatic alcohols were readily transformed to their corresponding silyl ethers in high yields (Table 1, entries 10–16). Phenol, 4-chlorophenol, 3-nitrophenol, 3,5-dimethylphenol and 2-hydroxy benzaldehyde were also silylated in an efficient manner in excellent yields (Table 1, entries 18–22). Our successful synthesis of primary and secondary silyl ethers, led us to embark upon a study of silylation of hindered tertiary alcohols. 1-Adamantanol was converted to corresponding silylated product in good yield (Table 1, entry 23). Nevertheless, triphenylmethanol gave related silyl ether in only 22% yield under standard reaction condition after 24 h, which can be improved to 50% when 3 mol% of CMK-5-SO3H was used after 24 h (Table 1, entry 24). It should be noted that, in the case of hindered secondary and tertiary alcohols no elimination by-products were observed at all.
To demonstrate whether the protection of alcohols catalyzed by CMK-5-SO3H is actually proceeding in a heterogeneous pathway, the silylation of benzyl alcohol was carried out in dichloromethane in which the catalyst was filtered off at approximately 50% conversion and the resulting clear solution stirred for additional time in the absence of the solid. No significant increase in the yield occurs after removal of the catalyst, thus indicating the solution does not contain any catalytically active species that could have leached from the solid to solution and confirm the covalent stability of acidic centers attached to mesoporous carbon.
Compared with traditional liquid acid catalysts, the catalyst CMK-5-SO3H has the attractive property in that it can be easy recycled. Consequently, after the completion of the first run silylation of benzyl alcohol under the conditions described in Table 1, which afford the corresponding benzyloxytrimethyl silane in 98% yield, a new silylation reaction was then accomplished by removal of solution using syringe and then addition of fresh reactants to the reaction mixture. Gratifyingly, CMK-5-SO3H successfully reused in more than twenty-five successive runs and exhibited consistent activity to afford an average yield of 97.5% with virtually no significant loss of performance, which clearly demonstrates the practical recyclability of this catalyst. This reusability demonstrates the high stability and turnover of CMK-5-SO3H under the conditions employed. Noticeably, the recyclability test was stopped after twenty-five runs, although it can be done continuously.
4 Conclusions
In summary, sulfonated ordered nanoporous carbon (CMK-5-SO3H), is an efficient, thermally stable (to 200 °C), and recoverable catalyst for the silylation of alcohols in dichloromethane at ambient temperature. In view of excellent catalytic capacity, outstanding stability, the exceedingly simple workup and uncomplicated recovery, CMK-5-SO3H was proved the best catalyst for this reaction. Most importantly, this procedure is significant from the viewpoint of avoiding pollution. As a consequence, this system represents a substantial improvement over previous methods, wherein product separation would produces a large amount of wastes. Additionally, by this method, primary, bulky secondary, tertiary and phenolic hydroxyl functional groups were protected in good to excellent yields. To the best of our knowledge, there has not been reported silylation of alcohols using sulfonated carbon in which the catalyst can be recovered and reused over several reaction cycles without considerable loss of reactivity. Further studies will be focusing on exploring of this solid sulfonic acid for other types of functional group transformations in our laboratories.
5 Supplementary Data
Experimental procedure and characterization data (TGA, BET, BJH, and N2 adsorption–desorption isotherm) for CMK-5 and CMK-5-SO3H, and general procedure for the trimethylsilylation of alcohols is available.
References
Greene TW, Wuts PGM (1991) Protective groups in organic synthesis, 2nd edn. Wiley, New York
Kocienski PJ, Enders R, Noyori R, Trost BM (eds) (1994) In protective groups. Thieme, Stuttgart
Langer SH, Connell S, Wender I (1958) J Org Chem 23:50
Corey EJ, Venkateswarlu A (1972) J Am Chem Soc 94:6190
Chaudhary SK, Hernandez O (1979) Tetrahedron Lett 20:99
Lombardo L (1984) Tetrahedron Lett 25:227
Olah GA, Gupta BGB, Narang SC, Malhotra R (1979) J Org Chem 44:4272
D’Sa BA, McLeod D, Verkade JG (1997) J Org Chem 62:5057
D’Sa BA, Verkade JG (1996) J Am Chem Soc 118:12832
Martinez GR, Grieco PA, Williams E, Kanai K, Srinivasan CV (1982) J Am Chem Soc 104:1436
Aizpurua JM, Palomo C (1985) Tetrahedron Lett 26:475
Bruynes CA, Jurriens TK (1982) J Org Chem 47:3966
Gauttret P, El-Ghamarti S, Legrand A, Coutrier D, Rigo B (1996) Synth Commun 26:707
Goldschmidt AGT (1979) German Patent 2 758884. Chem Abstr 90:6530c
Zhang ZH, Li TS, Yang F, Fu CG (1998) Synth Commun 28:3105
Mojtahedi MM, Saidi MR, Bolourchian M, Heravi MM (2002) Phosphorus Sulfur Silicon Relat Elem 177:289
Karimi B, Golshani B (2000) J Org Chem 65:7228
Firouzabadi H, Iranpoor N, Amani K, Nowrouzi F (2002) J Chem Soc Perkin Trans 1:2601
Saidi MR, Azizi N (2004) Organometallics 23:1457
Firouzabadi H, Iranpoor N, Sobhani S, Ghassamipour S (2004) J Organomet Chem 689:3197
Akhlaghinia B, Tavakoli S (2005) Synthesis 11:1775
Ghorbani-Vaghei R, Zolfigol MA, Chegeny M, Veisi H (2006) Tetrahedron Lett 47:4505
Shurakawa E, Hironaka K, Otsuka H, Hayashi T (2006) Chem Commun 37:3927
Mojtahedi MM, Abbasi H, Abaee MS (2006) J Mol Catal A 250:6
Yadav JS, Reddy BVS, Basak AK, Baishya G, Vankat Narsaiah A (2006) Synthesis 22:3881
Narsaiah A (2007) J Organomet Chem 692:3614
Reis PM, Royo B (2007) Catal Commun 8:1057
Shaterian HR, Shahrekipoor F, Ghashang M (2007) J Mol Catal A 272:142
Khazaei A, Zolfigol MA, Rostami A, Ghorbani-Choghamarani A (2007) Catal Commun 8:543
Firouzabadi H, Iranpoor N, Jafari AA, Jafari MR (2008) J Organomet Chem 693:2711
Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpour-Baltork I, Chahardahcheric S, Tavakoli Z (2008) J Organomet Chem 693:2041
Kadam ST, Kim SS (2009) J Organomet Chem 694:2562
Firouzabadi H, Iranpoor N, Farahi S (2009) J Organomet Chem 694:3923
Olah GA, Pradeep SI, Prakash GKS (1986) Synthesis 7:513
Corma A (1995) Chem Rev 95:559
Herron N, Farneth WE (1996) Adv Mater 8:959
Harton B (1999) Nature 400:797
Anastas PT, Kirchhooff MM (2002) Acc Chem Res 35:686
Clark JH, Macquarrie DJ (1998) Chem Commun 8:853
Okuhara T (2002) Chem Rev 102:3641
Zareyee D, Karimi B (2007) Tetrahedron Lett 48:1277
Tamai H, Kakii H, Hirota Y, Kumamoto T, Yasuda H (1996) Chem Mater 8:454
Kawashima D, Aihara T, Kobayashi Y, Kyotani T, Tomita A (2000) Chem Mater 12:3397
Lee J, Sohn K, Hyeon T (2001) J Am Chem Soc 123:5146
Li Z, Jaroniec M (2001) J Am Chem Soc 123:9208
Besenhard JO, Friz HP (1983) Angew Chem Int Ed Engl 22:950
Rice RJ, Pontikos NM, McCreery RL (1990) J Am Chem Soc 112:4617
Tanaka H, Aramata A (1997) J Electroanal Chem 437:29
Joo SH, Choi SJ, Oh I, Kwak J, Liu Z, Terasaki O, Ryoo R (2001) Nature 412:169
Yu C, Fan J, Tian B, Zhao D, Stucky GD (2002) Adv Mater 14:1742
Kurk M, Jaroniec M, Kim T-W, Ryoo R (2003) Chem Mater 15:2815
Bouchet MJ, Rendon A, Wermuth CG, Goeldner M, Hirth C (1987) J Med Chem 30:2222
Wang X, Liu R, Waje MM, Chen Z, Yan Y, Bozhilov KN, Feng P (2007) Chem Mater 19:2395
Karimi B, Zareyee D (2005) Tetrahedron Lett 46:4661
Karimi B, Zareyee D (2008) Org Lett 10:3989
Karimi B, Zareyee D (2009) J Mater Chem 19:8665
Acknowledgments
The authors acknowledge the Islamic Azad University of Qaemshahr Research Councils for support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zareyee, D., Ghandali, M.S. & Khalilzadeh, M.A. Sulfonated Ordered Nanoporous Carbon (CMK-5-SO3H) as an Efficient and Highly Recyclable Catalyst for the Silylation of Alcohols and Phenols with Hexamethyldisilazane (HMDS). Catal Lett 141, 1521–1525 (2011). https://doi.org/10.1007/s10562-011-0621-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0621-3