Abstract
The functionalized Poly(ethylene glycol) (PEG)-stabilized Pd(0) nanoparticles have been utilized to selectively oxidize alcohols to the corresponding aldehydes or ketones in supercritical carbon dioxide (scCO2)/PEG (PEG-2000) biphasic system. It was demonstrated that the Pd(0) nanocatalyst was more active and selective, in comparison with the commercially available Pd/C catalyst, PVP-stabilized Pd nanocatalyst and Pd(0) catalyst without stabilization. The effects of CO2 pressure, reaction time and temperature on activity and selectivity were also further investigated in detail. The scCO2/PEG biphasic system was proved to be not only cheap and clean, but also recyclable for the aerobic oxidation of alcohols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The selective oxidation of alcohols into the corresponding aldehydes or ketones is a class of transformation for the production of a large variety of important intermediates and fine chemical products [1], and numerous approaches have been explored successfully [2, 3]. Generally, oxidation processes based on the use of stoichiometric or excess amounts of oxidants have suffered from the disadvantage of producing much toxic waste such as chromium(VI) and manganese(IV) compounds, thus causing serious environmental problems [1, 4]. The development of clean and efficient oxidation methods for fine chemicals synthesis is a major goal of green chemistry. This has resulted in much attention being recently directed toward the development of new protocols for the aerobic oxidation of alcohols using transition-metal catalysts. Among them, palladium-based catalysts show promising catalytic activity, and different types of palladium-based homogeneous [5–7] and heterogeneous catalysts [8–10] in the form of metal complexes or nanoparticles have been reported.
Poly(ethylene glycol) (PEG) is an inexpensive, non-volatile and environmentally benign solvent, whereas the utilization of PEG as reaction media has attracted much attention [11, 12]. Supercritical carbon dioxide (scCO2, Tc = 31.0 °C, Pc = 7.4 MPa) combines liquid-like solvent properties with typical gas phase properties such as rapid mass transfer and negligible surface tension [13–16]. In recent years, scCO2 has been extensively used to replace conventional and potentially hazardous solvents for catalytic reactions because of its non-toxicity, non-flammability and natural abundance [17]. The properties of scCO2 can be varied over a wide range with small variations of pressure and temperature, and these specific properties have been largely exploited for the improvement of reaction rates and product selectivity of the reactions [18–20]. Additionally, CO2 is much easier to remove from reaction products than organic solvents, which provides an effective approach to separate products from catalyst phase.
More recently, many interests have been focused on biphasic catalysis system (scCO2/PEG) [21, 22] where scCO2 is used as the continuous phase (extracting CO2-soluble products) and PEG as the stationary catalyst phase (immobilized PEG-soluble catalyst). As a result, the biphasic system could offer the possibility of recovering the expensive metal catalyst and running the metal-mediated chemical reactions under continuous flow conditions. This approach has also the potential to solve some of the problems typically associated with homogeneously dispersed nanoparticles, such as deactivation due to formation of Pd-black and difficulties in catalyst separation and recycling. For example, the Pd clusters [Pd561phen60(OA)180] have been stabilized in PEG matrix, and the Pd nano-catalysts show both high activity and good recyclability for the aerobic oxidation of alcohols in scCO2/PEG biphasic system [23]. Moreover, the palladium nanoclusters stabilized and immobilized in PEG-modified silica, in combination with supercritical carbon dioxide (scCO2) as reaction medium have also been employed for oxidation of alcohols under continuous-flow conditions [10, 24]. Herein, we continue on the previous work by designing and preparing bidentate nitrogen ligand (2,2′-dipyridylamine) tethered covalently to the tip of PEG, and then we expect that the functionalized PEG could stabilize palladium nanoparticles. More importantly, we further want to understand how the modification of Pd nanoparticles by the bidentate nitrogen ligand linker affects the activity of alcohol oxidation in scCO2/PEG biphasic media.
2 Experimental Section
2.1 Materials and Methods
PEG-2000 and sodium hydride (95%) were obtained from SCRC (Sinopharm Chemical Reagent Co ltd., Shanghai). Polyvinyl pyrrolidone (PVP), 2, 2′-Dipyridylamine and palladium acetate were purchased from Aldrich. A commercially available 10% Pd on activated carbon (Pd/C) catalyst was purchased from Sinopharm Chemical Reagent Co Ltds. Crotonaldehyde (Aldrich, 99.5%). CO2 (99.99%), O2 (99.9%) and H2 (99.95%) was supplied by Shanghai Shangnong Gas Factory. All manipulations involving air-sensitive materials were carried out using standard Schlenk line techniques under an atmosphere of nitrogen. All NMR spectra were recorded on a Bruker Avance 500 instrument (500 MHz 1H, 125 MHz 13C) using CDCl3 and TMS as solvent and reference, respectively. Chemical shifts (δ) were given in parts per million and coupling constants (J) in hertz. The images of TEM were recorded with JEOL-JEM-2100F HRTEM instrument. The X-ray diffraction (XRD) analyses were performed in D/MAX 2550 VB/PC using a graphite crystal as monochromator. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo ESCALAB 250 spectrometer. Nonmonochro Al Kα radiation was used as a primary excitation. The binding energies were calibrated with the C1s level of adventitious carbon (284.8 eV) as the internal standard reference.
2.2 Catalyst Preparation
The process of preparing the functionalized PEG-stabilized Pd(0) (Cat. 1) nanocatalyst was shown in Fig. 1.
2.2.1 Preparation of the Functionalized PEG
In the typical experiment, Mesylation of PEG was synthesized in high yields (97%) according to reference [25]. Dimesyl PEG was obtained as a white waxy solid. 1H NMR (500 MHz, CDCl3): 4.39 (t, 4H), 3.50–3.73 (m, PEG), 3.09 (s, 6H).
In the next step, 2, 2′-dipyridylamine (0.67 g, 3.91 mmol) was dissolved in anhydr-DMF (5 mL). To this solution was added NaH (0.1 g, 4.17 mmol), and the resulting mixture was stirred for 2 h at 50 °C, after which, dimesyl PEG (4.21 g, 1.95 mmol) dissolved in DMF (10 mL) was added. The solution was stirred at 90 °C for 4 h and then DMF was evaporated under reduced pressure. The brown residue was redissolved in toluene (15 mL) and filtered to remove residue. The volume of organic layer was reduced by 95% under reduced pressure. The remaining solution was cooled to 0 °C, and anhydr-Et2O (100 mL) was slowly added with stirring. The 2, 2′-dipyridylamine functionalized PEG was obtained as a yellow solid, then filtered and washed with Et2O three times. From 1H NMR analysis, more than 92% of PEG-OH groups were functionalized by bis(2, 2′-dipyridylamine) ligand. 1H NMR (500 MHz, CDCl3): 8.25 (dt, 4H), 7.44 (m, 4H), 7.08 (dd, 4H), 6.79 (m, 4H), 4.32 (t, 4H), 3.48–3.70 (m, PEG). 13C NMR (125 MHz, CDCl3): 155.7, 148.4, 137.6, 117.6, 115.5, 70–72, 48.3.
2.2.2 Preparation of Nano-Pd
In the typical experiment, a mixture of the functionalized-PEG (21 mg, 0.009 mmol), palladium acetate (4.1 mg, 0.018 mmol) in acetic acid (3 mL) was stirred vigorously at room temperature for 4 h. Afterwards, hydrogen was introduced to the solution to reduce the Pd(II) complex under vigorous stirring at room temperature. After 20 min, a dark brownish ‘solution’ was obtained, and the black powder was isolated by centrifugation (4,000 rpm, 5 min, 2 times), washing with toluene (5 mL), dried under reduced pressure, the isolated powder was obtained as Cat. 1.
The Pd(0) catalyst (Cat. 2) was isolated in a similar way by stirring palladium acetate in acetic acid and reduction by hydrogen in the absence of the functionalized PEG. The polyvinyl pyrrolidone(PVP)-stabilized palladium nanoparticle (Cat. 3) was prepared according to previous reported procedures [26].
2.3 Representative Procedure for the Oxidation of Alcohols
The oxidation of alcohols was performed with the isolated Pd(0) particles embedded in PEG in a 42 mL high-pressure stainless steel reactor. After a mixture of 2.2 mmol L−1 solution of isolated Pd(0) nanoparticles in acetic acid (1 mL) and PEG (8 g) was stirred at 50 °C for 1 h, evaporated the solvent under vacuum, the reactant was then charged into the reactor under nitrogen. The reactor was pressured with a defined CO2/O2 mixture (molar ratio 94:6) and heated under vigorous stirring to the desired temperature for the set time. After the reaction, CO2 (70 °C, 18 MPa) was flushed slowly through the liquid PEG phase at an outlet flow-rate of approximately 6 L/h for 8 h. The vessel was then cooled to room temperature, bled to 0.1 MPa, and opened under nitrogen. Fresh reactant was introduced to begin the next cycle. The products were collected in two serial cold traps containing isopropanol (20 mL) as absorbent. The components in the collected solution were identified by GC–MS method and analyzed by GC (n-dodecane as internal standard) equipped with a HP-5 column (30 m long, 0.25 mm i.d., 0.25 μm film thickness) with flame ionization detector. After reaction, the leaching amount of Pd species in the absorbent was analyzed by ICP-AES method. In a preliminary study, the selective oxidation of benzyl alcohol to benzyl aldehyde was used as the model reaction (Scheme 1).
After three consecutive recycles of Pd nanocatalyst, a black powder could be isolated from PEG phase by adding toluene and then centrifugating (4,000 rpm, 5 min, 3 times). Washed two times with acetone and dried under reduced pressure, the isolated powder was analyzed by XRD, transmission electron microscopy (TEM), and XPS.
3 Results and Discussion
3.1 Activity and Recycling of the Catalytic Systems
As shown in Table 1, the functionalized PEG-stabilized palladium nanoparticles (Cat. 1) displayed much higher activity and selectivity than that of the unmodified palladium particles (Cat. 2; Table 1, entries 2 and 6). In addition, the commercially available Pd/C catalyst gave lower activity and selectivity under the same reaction conditions (Table 1, entries 8 and 9), compared with Cat. 1. The Pd nanocatalyst stabilized using PVP (Cat. 3) offered high selectivity to corresponding aldehyde (97%), but only moderate conversion (51%; Table 1, entry 7). These results indicated the present functionalized PEG-stabilized palladium nanoparticles were the most active among them. It was visually observed that palladium nanoparticles (Cat. 1) could be stabilized efficiently and were actually “homogeneous” during reaction. In contrast, the bulky palladium particles were observed in the absence of the functionalized PEG (Cat. 2), which indicated that the functionalized PEG seemed to play a crucial role in stabilizing palladium nanoparticles and also influencing the activity of the palladium catalysts considerably in the scCO2/PEG biphasic system. Although PVP could also stabilize the palladium nanoparticles well as did the functionalized PEG, the palladium nanoparticles stabilized by PVP were much less active than those stabilized by the functionalized PEG (Table 1, entries 2 and 7). We suggested that the high activity of palladium nanoparticles depended on not only good stabilization, but also chemical environment around palladium particles. The building up electron density on the palladium nanoparticles coordinated with the functionalized PEG might make the activation of substrate easier and the reaction rate faster. The blank experiment (Table 1, entry 1) without any catalyst was also carried out. However, only 3.7% product was obtained, which demonstrated that the catalysts indeed played a key role in the oxidation of the alcohol. Moreover, Cat. 1 showed good the stability of activity in four runs (Table 1, entries 2–5) with a slight decrease in the selectivity to benzyl aldehyde, which might be caused by accumulation of water in catalytic recycles, leading to the hydration of benzyl aldehyde, followed by dehydrogenation of the corresponding geminal diol to benzoic acid [27].
The catalysts have been characterized by the different methods. Firstly, TEM analysis was performed before and after reaction for the Cat. 1 (Fig. 2a, b). The fresh Cat. 1 was dispersed in ethanol, placed as a thin film in a carbon coated copper grid and characterized by TEM. Analysis of the micrographs displayed nearly spherical Pd(0) nanoparticles and a nearly monomodal distribution with an average diameter of 3–5 nm. No obvious growth of the Pd(0) particles was observed from the third recycling of Cat. 1 (Fig. 2a, b). Figure 2c showed a TEM image of the fresh Cat. 2. The bulky palladium particles were obviously observed in the absence of the functionalized PEG. Further, the Pd black was formed and precipitated completely from PEG phase after reaction. By comparing these three set of TEM images, it was demonstrated that the combination of the functionalized ligand (2, 2′-dipyridylamine functionalized PEG) in the PEG and supercritical carbon dioxide resulted in an interesting synergistic effect on enhancing the activity, preventing the aggregation of Pd nanoparticles. The XRD pattern (Fig. 3) of the isolated Cat. 1 after three catalytic recycles confirmed the presence of crystalline Pd(0). The most representative reflections of Pd(0) were indexed as face-centered cubic (fcc) with unit cell parameter a = 0.39026 nm. The Bragg reflections at 39.6°, 45.5° and 67.3° corresponded to the indexed planes of the crystals of Pd(0) (111), (200), (220).
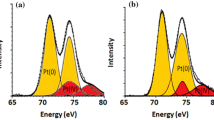
In order to determine the surface characteristics of palladium nanoparticles for the Cat. 1, we characterized the used Cat. 1 using the XPS technique. Figure 4 showed XPS signal of the Pd 3d. The main peak, Pd 3d5/2 was virtually the same as the specimens of the giant clusters of Pd(0) or the bulk metal Pd (335.0 eV), corresponding to palladium in a zero oxidation state. Besides, a very small shoulder with the binding energy E b = 336.8 eV in the spectra could be assigned to the Pd(II) species. The coexistence of spectrum of N 1s with the binding energy E b = 399.5 eV also indicated that the residue of the functionalized PEG was still remained on the surface of the nanoparticles even if the isolation procedure was repeated for two times, which might be the reason that the used catalyst still kept high activity and good selectivity for the oxidation of alcohol.
One of the most striking features of this biphasic catalytic system for the Pd(0)-catalyzed aerobic oxidation of benzyl alcohol into benzyl aldehyde is the facile and clean separation of the oxidized products from the reaction mixture. After reactions, the products were extracted with scCO2 and the PEG catalytic phase was readily reused for several runs without further purification or activation. In addition, we have also examined the possible leaching of the palladium species. The results demonstrated that the concentration of Pd in the absorbent (20 mL isopropanol) was at the level of 0.5 ppm.
3.2 Effect of Reaction Time
Firstly, the changes of the conversion of benzyl alcohol and the selectivity to benzyl aldehyde with the reaction time were studied. As shown in Fig. 5, the conversion of benzyl alcohol increased but the selectivity to benzyl aldehyde decreased slightly with the reaction time. After reaction for 6 h, almost all benzyl alcohol can be converted to benzyl aldehyde, while longer reaction time resulted in the formation of benzoic acid as the main byproduct.
3.3 Effect of Temperature
The influence of reaction temperature on benzyl alcohol conversion and the selectivity to benzyl aldehyde was investigated with a reaction time of 6 h in the range of 50–80 °C. As can be seen from Fig. 6, increasing reaction temperature accelerated the reaction rate significantly but resulted in a slight decrease in the selectivity to benzyl aldehyde.
The dependence of benzyl alcohol conversion and the selectivity to benzyl aldehyde on the reaction temperature in scCO2. Reaction condition: 0.018 mmol nano-Pd, 1.8 mmol benzyl alcohol, 8 g PEG-2000, CO2/O2 mixture (molar ratio 94:6), d(CO2/O2) = 0.44 g/mL, Reaction time: 6 h. It was worth noticing that at each reaction temperature, the experiment was repeated at least three times for good reproducibility
3.4 Effect of CO2 Pressure
Figure 7 shows the effect of reaction pressure on the conversion and selectivity with a reaction time of 5 h. The conversion of benzyl alcohol was slightly decreased from 75 to 65%, and simultaneously the selectivity to benzyl aldehyde was increased from 85 to 98% as the total pressure increased from 6 to 20 MPa. On the basis of above results, the presence of CO2 was proved to be beneficial for improving the selectivity towards benzyl aldehyde, especially in the low-pressure region. A rough explanation is that the solvent power of CO2 increases with increasing pressure [27]. Therefore, less substrate existed in the PEG phase at higher pressure, which favored reduction of the over-oxidation of the substrate. However, as the pressure was further increased, the solvent power of scCO2 became stronger, which was unfavorable for adsorption of the reactants on the surface of the catalyst and reduced the catalytic activity. The similar phenomenon has also been observed by other authors [18, 22, 28].
3.5 Oxidation of Other Alcohols
The scope of the substrates was further explored as shown in Table 2. The various alcohols, including primary, secondary, aliphatic, alicyclic, allylic and aromatic alcohols were chosen to be tested in the biphasic scCO2/PEG system for the aerobic oxidation. Both activated and non-activated alcohols were converted to the corresponding carbonyl compounds. However, activated alcohols such as benzyl alcohol (Table 2, entry 1) afforded a much higher reaction rate than the non-activated alcohols such as 1-hexanol (Table 2, entry 5). Alicyclic alcohols such as cyclohexanol (Table 2, entry 4) were also successfully converted to the corresponding cyclic ketones with excellent selectivity although the reaction rate was slightly lower than that of benzyl alcohol. Oxidation of allylic alcohols such as cinnamyl alcohol (Table 2, entry 2), 3-methyl-2-buten-1-ol (Table 2, entry 6) and geraniol (Table 2, entry 7) also proceeded smoothly, resulting in the formation of the corresponding α, β-unsaturated carbonyl compounds in excellent selectivity. It is noteworthy that in the oxidation of allylic alcohols, C = C double bonds remained intact without an intramolecular hydrogen transfer. For the oxidation of the aliphatic primary alcohol, the corresponding ester was formed as main product possibly through a hemiacetal intermediate, which is unstable under the reaction conditions and is subsequently oxidized to the corresponding ester [29], whereas the corresponding carboxyl acid is produced possibly through a geminal diol intermediate [30].
4 Conclusion
In this work, we prepared a functionalized PEG-stabilized palladium nanocatalyst and as-synthesized nanocatalyst has been utilized for the efficient oxidation of various alcohols, including aromatic alcohols, alicyclic alcohols, aliphatic alcohols and allylic alcohols. The nanocatalysts stabilized in PEG can be combined perfectly with scCO2, and environmentally benign biphasic system is beneficial for the fast and clean separation of products and the dispersion of nanoparticles. Moreover, this environmentally benign biphasic catalytic system can be recycled directly after in situ extraction of the products using scCO2, and it remains satisfactorily active after being reused at least four times.
References
Sheldon RA, Arends IWCE, ten Brink GJ, Dijksman A (2002) Acc Chem Res 35:774
Mallat T, Baiker A (2004) Chem Rev 104:3037
Schultz MJ, Sigman MS (2006) Tetrahedron 62:8227
Cainelli G, Cardillo G (1984) Chromium oxidants in organic chemistry. Springer, Berlin
Iwasawa T, Tokunaga M, Obora Y, Tsuji Y (2004) J Am Chem Soc 126:6554
ten Brink GJ, Arends IWCE, Sheldon RA (2000) Science 287:1636
Hu Y, Yu YY, Hou ZS, Li H, Zhao XG, Feng B (2008) Adv Synth Catal 350:2077
Karimi B, Zamani A, Abedi S, Clark JH (2009) Green Chem 11:109
Pillai UR, Sahle-Demessie E (2004) Green Chem 6:161
Hou ZS, Theyssen N, Leitner W (2007) Green Chem 9:127
Chen J, Spear SK, Huddleston JG, Rogers RD (2005) Green Chem 7:64
Wang L, Zhang YH, Liu LF, Wang YG (2006) J Org Chem 71:1284
Ke J, Han BX, George MW, Yan HK, Poliakoff M (2001) J Am Chem Soc 123:3661
Baiker A (1999) Chem Rev 99:453
Leitner W (2002) Acc Chem Res 35:746
Licence P, Ke J, Sokolova M, Ross SK, Poliakoff M (2003) Green Chem 5:99
Beckman EJ (2004) J Supercrit Fluids 28:121
Wang XG, Kawanami H, Dapurkar SE, Venkataramanan NS, Chatterjee M, Yokoyama T, Ikushima Y (2008) Appl Catal A 349:86
Theyssen N, Hou ZS, Leitner W (2006) Chem Eur J 12:3401
González-Núñez ME, Mello R, Olmos A, Acerete R, Asensio G (2006) J Org Chem 71:1039
Heldebrant DJ, Jessop PG (2003) J Am Chem Soc 125:5600
Wang JQ, Cai F, Wang E, He LN (2007) Green Chem 9:882
Hou ZS, Theyssen N, Brinkmann A, Leitner W (2005) Angew Chem Int Ed 44:1346
Hou ZS, Theyssen N, Brinkmann A, Klementiev KV, Grünert W, Bühl M, Schmidt W, Spliethoff B, Tesche B, Weidenthaler C, Leitner W (2008) J Catal 258:315
Mai W, Gao L (2006) Synlett 2553
Hou WB, Dehm NA, Scott RWJ (2008) J Catal 253:22
Caravati M, Grunwaldt J-D, Baiker A (2005) Phys Chem Chem Phys 7:278
Xie Y, Zhang ZF, Hu SQ, Song JL, Li WJ, Han BX (2008) Green Chem 10:278
Jenzer G, Schneider MS, Wandeler R, Mallat T, Baiker A (2001) J Catal 199:141
Gunanathan C, Shimon LJW, Molstein D (2009) J Am Chem Soc 131:3146
Acknowledgments
The authors are grateful for the support from the National Natural Science Foundation of China (no. 20773037), ECUST (no. YJ0142136), the Commission of Science and Technology of Shanghai Municipality (no. 07PJ14023), China, and Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission & Ministry of Education, Hubei Province, South-Central University for Nationalities (no. CHCL08001), PR China. The authors would also like to express their thanks to Prof Walter Leitner’s helpful suggestion and Dr. Nils Theyssen’s assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Yang, H., Feng, B. et al. Functionalized Poly(ethylene glycol)-Stabilized Palladium Nanoparticles as an Efficient Catalyst for Aerobic Oxidation of Alcohols in Supercritical Carbon Dioxide/Poly(ethylene glycol) Biphasic Solvent System. Catal Lett 132, 34–40 (2009). https://doi.org/10.1007/s10562-009-0038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0038-4