Abstract
The production of 1,3-butadiene from acetylene includes the dimerization of acetylene and hydrogenation of the dimer. This work investigated the hydrogenation process catalyzed by ionic liquid stabilized Pd nanoparticles. The Pd nanoparticles were dispersed uniformly the in ionic liquid phase by the N-heterocyclic carbene species adsorbed on the surface of the nanoparticles. However, during the reaction, the N-heterocyclic carbene layer was replaced by a hydrophobic byproduct, which caused the Pd nanoparticles to separate from the [BMIm][BF4] phase and the loss of the catalyst from the reaction phase. To solve this, n-heptane was introduced into the reaction system to re-disperse the Pd nanoparticles in n-heptane, and due to this, the Pd nanoparticles maintained a high catalytic activity for a long term.
Graphical Abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
1,3-Butadiene (BD) is an important chemical intermediate material, which is used as the monomer for the manufacture of rubbers and polymers such as polybutadiene, styrene–butadiene rubber, acrylonitrile–butadiene–styrene polymer and nitrile rubber [1]. The BD requirement is growing quickly due to the rapid economic growth of developing countries such as China and India. In 2011, the global consumption of BD was 10.5 million tons and it has been predicted to reach 13 million tons by 2015, with 3–4 % annual increment [2]. BD is mainly obtained from the petroleum industry from cracking of naphtha or by the dehydrogenation of C4 hydrocarbons. However, with the depletion of petroleum reserves and the increasing BD demand, it is crucial to develop alternative technologies for BD production [3]. It is widely accepted that coal and natural gas will last longer than petroleum, therefore, many new and alternative chemical processes based on coal and natural gas are being developed. Acetylene is one of the most important raw materials for coal and natural gas-based chemistry [4]. In view of this, acetylene has been proposed as a raw material for the production of BD. The process includes the dimerization of acetylene to produce monovinylacetylene (MVA) and the hydrogenation of MVA to produce BD. In the past few years, our group have done much work on the dimerization of acetylene and got remarkable results [5–7]. However, the hydrogenation of MVA has been scarcely reported in the patent and academic literature. The reported works on MVA hydrogenation are mainly focused its use for the purification of the olefin feeding stock which contains trace alkynes [8–11]. In this work, the hydrogenation of MVA with high concentration was investigated for developing an entire process for BD production from acetylene.
Palladium is usually used as the catalyst for the industrial hydrorefining of olefin streams due to its high activity and selectivity for semihydrogenated products [10–12]. In these processes, the low concentration of alkynes in the olefin stream allow the use of a supported Pd catalyst and a fixed bed reactor owing to the small amount of heat released [13]. However, when MVA is present at a high concentration and is hydrogenated in a fixed bed, a large amount of reaction heat will be released, and any poor heat transfer will probably induce a large amount of by products. Quasi-homogeneous catalysis is then the preferred choice for the hydrogenation of high concentration MVA for its efficient mass and heat transfer. An ionic liquid stabilized transition-metal nanoparticles catalytic system is a quasi-homogenous system which has received much interest, especially for catalytic hydrogenation, for the excellent properties of ionic liquids and also for the good catalytic performance of transition-metal nanoparticles synthesized in an ionic liquid [14–17]. At present, almost all of the hydrogenation reactions catalyzed by ionic liquid stabilized transition metal nanoparticles are processed in batch reactors [18–20]. In a batch process, the transition metal nanoparticles can be reused for several times without an appreciable decrease of catalytic activity. However, a batch reaction is not suitable for a large-scale industrial application, and the investigation of the stability of transition metal nanoparticles in a batch process is not enough for predicating the life time of the catalyst in a continuous process. In this work, the continuous hydrogenation of MVA catalyzed by Pd nanoparticles stabilized by an ionic liquid for BD production was investigated and discussed.
2 Experimental
2.1 Materials
Palladium(II) acetylacetonate (Pd content = 34.7 %) was purchased from Alfa Aesar. 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4], purity ≥ 99 %) was purchased from Cheng Jie Chemical Co. Methanol and n-heptane were analytically pure and purchased from Sinopharm Chemical Reagent Co. Ltd. H2 (purity ≥ 99.99 %) was obtained from Beijing Beiwen Gas Factory. Monovinylacetylene was supplied by Shana Chemical.
2.2 Catalysts Preparation and Catalytic Hydrogenation
A methanol solution of palladium(II) acetylacetonate was mixed with [BMIm]BF4 and stirred at a set temperature to obtain a uniform solution. Then, the solution was distilled to remove the methanol. Finally, hydrogen gas was introduced into the ionic liquid solution at 80 °C for the reduction of palladium(II). After 2 h, Pd nanoparticles dispersed uniformly in [BMIm][BF4] were obtained. For the hydrogenation of MVA, the catalyst was placed in a bubble reactor equipped with a gas inlet and outlet, and a mixture of H2 and MVA was continuously bubbled into the catalyst solution. During the reaction, the gas was sampled at the outlet and analyzed by gas chromatography (Shimadzu GC-14B equipped with an Al2O3-PLOT chromatograph column and a hydrogen flame ionization detector).
2.3 Analysis
Powder X-ray diffraction (XRD) patterns were recorded on a Bruker D8 advance X-ray diffractometer with a nickel-filtered CuKα radiation X-ray source at 40 kV and 20 mA. Transmission electron microscope (TEM) images were taken using a JEM-2010 TEM. Fourier transform infrared (FTIR) spectra were recorded using a Nicolet Nexus spectrometer.
3 Result and Discussion
3.1 Structure of Pd Nanoparticles
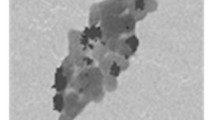
The Pd nanoparticles obtained after the reduction of palladium(II) acetylacetonate by hydrogen at 80 °C for 2 h were washed with ethanol and separated from [BMIm][BF4]. In the XRD pattern of the Pd nanoparticles shown in Fig. 1, the peaks at 40.15°, 46.73°, 68.20° and 82.13° indexed to the 111, 200, 220 and 311 planes of Pd(0) (JCPDS PDF#65-2867) indicated the formation of Pd(0) with the FCC structure. A TEM image of the Pd nanoparticles is presented in Fig. 2. It was observed that the Pd nanoparticles were dispersed uniformly and the average diameter of the nanoparticles was 10 nm.
Figure 3 displays the FTIR spectra of the Pd nanoparticles and [BMIm][BF4]. The absorption peaks of the Pd nanoparticles showed that organic groups were adsorbed on the surface of the Pd nanoparticles. The interaction of the organic group with the metal surface is an effective interaction responsible for the stabilization of the nanoparticles.
The FTIR spectrum of the Pd nanoparticles was further compared with that of [BMIm][BF4]. In the spectrum of [BMIm][BF4], the absorption peaks at 3,161 and 3,121 cm−1 were assigned to the vibration of aromatic C–H bonds, the absorption peaks at 2,941 and 2,876 cm−1 are attributed to the vibration of aliphatic C–H, and the absorption peak at 1,061 cm−1 was the characteristic absorption peak of B–F [21]. In the spectrum of the Pd nanoparticles, the absorption peaks attributed to the vibration of aliphatic C–H bonds and B–F remain unchanged compared with that of [BMIm][BF4], but the absorption peaks assigned to the vibration of the aromatic C–H bonds exhibited a red shift. This indicated that the organic group adsorbed on the surface of Pd nanoparticles was N-heterocyclic carbene as suggested by Finke and co-authors [22] and Dupont and co-authors [23].
3.2 Catalytic Hydrogenation of MVA
The Pd nanoparticles stabilized by [BMIm][BF4] synthesized at 80 °C were used in situfor the catalytic hydrogenation of MVA. MVA and H2 were simultaneously bubbled into the Pd/[BMIm][BF4] through a 3 mm tube, The reaction temperature was gradually raised from 40 to 80 °C with the increase of reaction time. The result shown in Fig. 4 showed that the conversion of MVA increased from 12 to 23 % with the increase of reaction temperature, and the selectivity for BD remained around 72 %. Unfortunately, after reaction for 3 h, it was found that Pd nanoparticles were suspended on the liquid surface and could not be re-dispersed into the liquid phase. Due to this, the Pd nanoparticles exhibited weak catalytic activity. Therefore, the suspended nanoparticles were separated and characterized by FTIR. The FTIR spectrum of the Pd nanoparticles shown in Fig. 3 showed the disappearance of the absorption peaks assigned to the vibration of the aromatic C–H bonds and the stronger absorption peaks attributed to the vibration of the aliphatic C–H bonds indicated that the N-heterocyclic carbene layer adsorbed on the surface of Pd nanoparticles had been possibly replaced by a hydrophobic by product.
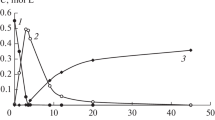
Based on the above result, the process was changed to be improved in two aspects. First, MVA and H2 were bubbled into Pd/[BMIm][BF4] through a sintered plate, which was used as a gas distributor. Second, some n-heptane, which is immiscible with [BMIm][BF4], was added into the Pd/[BMIm][BF4] system to form a two-phase reaction system. Comparing of the new catalytic result (Fig. 5) with the original catalytic result, it was showed that the conversion of MVA remained around 20 % but the selectivity of BD was increased from 73 to 80 %. The gas distributor is proved effective for improving mass transfer and BD selectivity. In addition, in the reaction process, Pd nanoparticles were transferred from the [BMIm][BF4] phase to the n-heptane phase, and were dispersed in n-heptane uniformly. Thus, the Pd nanoparticles remained a high catalytic activity for more than 20 h as shown in Fig. 5.
In fact, for the semi-hydrogenation of alkynes catalyzed by pure palladium, the selectivity for semi-hydrogenated products is very low. Pd-containing bimetallic alloys and ligand-modified palladium are usually used for promoting the selectivity of palladium catalyst [24–26]. In this work, the Pd nanoparticles was not doped with other metals and showed high selectivity toward BD. Are the N-heterocyclic carbene adsorbed on the surface of the Pd nanoparticles responsible for the high selectivity?
Lopez investigated the influence of hydrides and carbides on the selectivity of palladium. The results showed that the carbides of palladium displayed no influence on the adsorption of H atoms on the surface of Pd nanoparticles but impeded the adsorption of H atoms on the deep layer of Pd structure [24]. The H atoms adsorbed on the deep layer of Pd structure are unselective for semi-hydrogenated products [27]. In this work, the N-heterocyclic carbene adsorbed on the surface of the Pd nanoparticles via Pd–C bonds, which prevented the formation of hydrides on the deep layer of Pd structure. Thus, the Pd–C bonds between the Pd nanoparticles and the N-heterocyclic carbene are responsible for the high selectivity of fresh catalyst.
However, in the hydrogenation process, the Pd nanoparticles transferred from the ionic liquid phase to the n-heptane phase due to the hydrophobic by product covered on the Pd nanoparticles. The hydrophobic by product was probably oligomer of BD because BD was identified as the precursor of oligomer formation in the acetylene hydrogenation process [28]. Although theoretic study showed that the barrier for oligomerization increased in the presence of carbides [24], the oligomerization was inevitably in presence of much BD. After reaction, the n-heptane phase was collected, and then n-heptane was distilled, with an agglomeration left over. It was the produced oligomer in the hydrogenation process. The diffusion of the oligomer from the surface of the Pd nanoparticles to the n-heptane phase made it possible to remain origin activity. The separated Pd nanoparticles after reaction were characterized by XRD and XPS. The XRD pattern displayed in Fig. 1 indicated that the Pd nanoparticles remained the FCC structure. In the XP spectra shown in Fig. 6, only three strong peaks assigned to C12, O1s and F1s were observed. It deduced that the Pd nanoparticles were covered by the oligomer. In the Pd3d XP spectra, the peaks at 335.8 and 337.7 eV were attributed to the Pd(0) and the Pd bonding with C. The Pd–C bonds made it possible to remain high selectivity. In a word, the [BMIm][BF4]-n-heptane system provided an catalytic system with possible long life time.
4 Conclusion
Pd nanoparticles stabilized by [BMIm][BF4] were synthesized by the reduction of palladium(II) acetylacetonate dissolved in [BMIm][BF4]. The N-heterocyclic carbene species that covered the surface of the nanoparticles was effective for the stabilization of the nanoparticles. In the hydrogenation of MVA catalyzed by Pd/[BMIm][BF4], a high reaction temperature led to a high MVA conversion but low BD selectivity. When a gas distributor was used to introduce the feed gases, the BD selectivity was increased from 73 to 80 %. In the catalytic reaction process, with the increase of reaction time, the N-heterocyclic carbene layer on the Pd nanoparticles was replaced by a hydrophobic byproduct, which induced the separation of Pd nanoparticles from [BMIm][BF4] and made the Pd nanoparticles ineffective. N-heptane was introduced into the catalytic system, which could dissolve the hydrophobic by product and this induced the dispersion of Pd nanoparticles in n-heptane. In the [BMIm][BF4]-n-heptane two phase system, the Pd nanoparticles maintained a high catalytic activity for more than 20 h.
References
Chae HJ, Kim TW, Moon YK, Kim HK, Jeong KE, Kim CU, Jeong SY (2014) Appl Catal B-Environ 150–151:596–604
Yan W, Kouk QY, Luo J, Liu Y, Borgna A (2014) Catal Commun 46:208–212
Lewandowski M, Babu GS, VezzOli M, Jones MD, Owen RE, Mattia D, Plucinski P, Mikolajska E, Ochenduszko A, Apperley DC (2014) Catal Commun 49:25–28
Trotuş IT, Zimmermann T, Schüth F (2014) Chem Rev 114(3):1761–1782
Liu JG, Zuo YZ, Han MH, Wang ZW, Wang DZ (2012) J Nat Gas Chem 21(5):495–500
Liu JG, Zuo YZ, Han MH, Wang ZW (2013) J Chem Technol Biotechnol 88(3):408–414
Liu JG, Han MH, Wang ZW (2013) J Energy Chem 22(4):599–604
Masashi A, Higashio Y (1984) US Patent, 4469907, 1984
Setiawan I, Cavell KJ (1995) Appl Catal A-Gen 131(2):225–241
Molnár Á, Sárkány A, Mónika V (2001) J Mol Catal A-Chem 173(1–2):185–221
Bridier B, Karhánek D, Pérez-Ramírez J, López N (2012) ChemCatChem 4(9):1420–1427
Crespo-Quesada M, Cardenas-Lizana F, Dessimoz AL, Kiwi-Minsker L (2012) ACS Catal 2(8):1773–1786
Teschner D, Vass E, Havecker M, Zafeiratos S, Schnorch P, Sauer H, Knop-Gericke A, Schloegl R, Chamam M, Wootsch A (2006) J Catal 242(1):26–37
Umpierre AP, Machado G, Fecher GH, Morais J, Dupont J (2005) Adv Synth Catal 347(10):1404–1412
Scheeren CW, Machado G, Teixeira SR, Morais J, Domingos JB, Dupont J (2006) J Phys Chem B 110(26):13011–13020
Beier MJ, Andanson JM, Baiker A (2012) ACS Catal 2(12):2587–2595
Banerjee A, Theron R, Scott RWJ (2012) ChemSusChem 5(1):109–116
Hu Y, Yang HM, Zhang YC, Hou ZS, Wang XR, Qiao YX, Li H, Feng B, Huang QF (2009) Catal Commun 10(14):1903–1907
Zhu WW, Yu YY, Yang HM, Hua L, Qiao YX, Zhao XG, Hou ZS (2013) Chem Eur J 19(6):2059–2066
Zeng Y, Wang YH, Xu YC, Song Y, Jiang JY, Jin ZL (2013) Catal Lett 143(2):200–205
Suarez PAZ, Dullius JEL, Einloft S, DeSouza RF, Dupont J (1996) Polyhedron 15(7):1217–1219
Ott LS, Cline ML, Deetlefs M, Seddon KR, Finke RG (2005) J Am Chem Soc 127(16):5758–5759
Prechtl MHG, Campbell PS, Scholten JD, Fraser GB, Machado G, Santini CC, Dupont J, Chauvin Y (2010) Nanoscale 2(12):2601–2606
Garcia-Mota M, Bridier B, Perez-Ramirez J, Lopez N (2010) J Catal 273(2):92–102
Vile G, Almora-Barrios N, Mitchell S, Lopez N, Perez-Ramirez J (2014) Chem Eur J 20(20):5926–5937
Witte PT, Boland S, Kirby F, van Maanen R, Bleeker BF, de Winter DAM, Post JA, Geus JW, Berben PH (2013) ChemCatChem 5(2):582–587
Borodzinski A (2006) Catal Rev Sci Eng 48(2):91–144
Yang B, Burch R, Hardacre C, Hu P, Hughes P (2014) J Phys Chem C 118(3):1560–1567
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhen, B., Chen, W., Jia, Z. et al. Continuous Hydrogenation of Monovinylacetylene for 1,3-Butadiene Production Catalyzed by Ionic Liquid Stabilized Pd Nanoparticles. Catal Lett 144, 2216–2220 (2014). https://doi.org/10.1007/s10562-014-1385-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1385-3