Abstract
Degradation of Acid Red 88 (AR 88) azo dye in water was investigated in laboratory-scale experiments in presence of three metal oxide supported catalyst. Oxidation process was monitorized in terms of colour, chemical oxygen demand (COD) and total organic carbon (TOC) removals. Influence of catalysts type and aqueous solution pH were studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Azo dyes are used extensively in the textile industry, photographic industry, coating industry and photochemical applications [1]. Disperse azo dyes like Acid Red 88 (AR 88) induce a significant threat to human health and ecological systems due to their widespread use, carcinogenic characteristic of degradation byproducts (such as toxic aromatic amines) and low biodegradability. Different treatment methods are currently used for dyes removal from waste effluents such us coagulation, adsorption on activated carbon, reverse osmosis and ultra filtration, etc. but these processes assure only the transfer from liquid media to a solid one and lead to formation of solid waste. Chemical oxidation is more efficient but has to be optimized in order to accomplish a high removal rate along with low energy consumption. In this respect, in recent years, ozonation is emerging as a potential process for removal of dyes from water, since the chromophore groups with conjugated double bonds, can be broken down by ozone directly or indirectly forming smaller molecules and decreasing the color of the effluents [2–4].
According to some authors [5–8] ozonation process can be significantly improved by use of different catalysts. It has been emphasized that catalytic ozonation comparing with single ozonation allow the oxidation of recalcitrant compounds and lead to a considerable chemical oxygen demand (COD) and total organic carbon (TOC) reduction.
This study deals with the comparative investigation of AR 88 ozonation from aqueous solutions in presence and absence of MeO/Al2O3 (Me = Ni, Cu, Co). Experiments were conducted to investigate the effects of catalyst type and solution pH which is assessed in term of decolourization, TOC removal, and COD removal.
2 Experimental
2.1 Catalytic Systems
The studied catalysts (Table 1) were prepared by impregnating γ-Al2O3 with nickel, copper or cobalt nitrate aqueous solutions so that the metal content on the catalyst attain 10%.
Metal content in prepared solids was controlled using an atomic absorption spectrophotometer (Solar M5, Thermo Electron) after a prior digestion with a microwave digester (Ethos Sel, Milestone).
Following impregnation, catalysts were dried for 4 h at 110 °C and calcined for 2 h at 550 °C. Phase composition was emphasized by X-ray diffraction with a Shimadzu XRD 6000 and the specific surface area of the supported catalysts was determined by nitrogen adsorption using a Sorbty 1750 (Carlo Erba). In order to assess the interactions of AR 88 molecules with catalyst surface DRIFT studies were carried using a Varian 3100 Excalibur spectrometer equipped with a Harrick Praying Mantis diffuse reflectance accessory. DRIFT spectra of samples were collected in the region 4,000–500 cm−1 at a resolution of 2 cm−1 and the spectra of fresh catalyst were subtracted from the spectra of catalyst with adsorbed dye. The point of zero charge (PZC) for all samples was estimated using salt addition method [9].
2.2 Materials
Acid Red 88 azoic dye [4-(2-hydroxy-1-naphthylazo)-1-naphthalenesulfonic acid sodium salt, C·I. 1,658-56] was obtained from Aldrich (Fig. 1). Nickel, cooper and cobalt nitrates were purchased from Merk.
2.3 Ozonation Procedure
Ozone was generated from dried air by an ozone generator (COM-AD-01, Anseros). Ozone concentration in gas phase was determined by iodometric method. Concentration of dissolved ozone was determined by the indigo method [10]. Ozonation tests were performed in a column glass reactor by bubbling the ozone/air mixture in the solution at a volume stream of 20 L/h through a sintered glass filter fixed at the bottom (Fig. 2). In a typical catalytic ozonation test, 2 g/L of catalyst and 800 mL of synthetic solution of AR 88 (C = 500 mg/L) were mixed under stirring and thermostatic control. At given intervals, 6.0 mL of samples were taken from the reactor and were immediately centrifuged at 6,000 rpm for 10 min to completely remove catalyst particles.
2.4 Analytical
Concentrations of AR 88 samples were determined by measuring the absorbance of the solutions at 505 nm using a Helios α (Unicam) spectrophotometer. Total organic carbon (TOC) was measured using a (HiPerTOC, Thermo Electron) equipment and COD was measured by standard method [11].
The mean oxidation state of carbon (MOC) (MOC values oscillate between −4 and +4) was calculated from the values of TOC and COD using equation:
3 Results and Discussions
3.1 Catalyst Characterization and Surface Properties
From the Table 1 it can be seen that after impregnation and calcination the surface of obtained catalysts decrease comparing with bare alumina. Surface decrease is observed mainly for nickel based catalyst (18%) and to a lesser extent for copper (9%) and cobalt (12%) catalysts and can be most probably attributed to oxides crystallites formation which partially plug the support pores. In all diffractograms of prepared catalysts (Fig. 3) peaks of γ-Al2O3 and of supported metallic oxides can be seen.
Low resolution of characteristic peaks of nickel oxide suggests a high dispersion of active component on the alumina surface. On the contrary for cooper and cobalt catalysts higher peaks suggest that the size of crystallites increase. The PZC values of catalysts and support are also presented in Table 1 and were obtained by plotting ΔpH (changes in pH after addition of solid catalyst into NaNO3 solution) against initial pH (Fig. 4). Only minor shift in PZC values of catalyst compared with PZC of alumina (~7.5) was observed and it can be explained by similar values of PZC for all oxides involved [12–14].
From the adsorption data (Fig. 5) it can be seen that irrespective of working pH alumina and nickel and cobalt based catalysts (C1, C3) present a moderate adsorption capacity in contrast with cooper based catalyst (C2) probably due to the formation of surface complexes. Moreover C1 catalyst shows the lowest adoption capacity from all tested solids.
Also it was found that sorption equilibrium data are well fitted with the Freundlich model in logarithmic form as follows:
K F (mg1−1/n/L1/ng) and n (dimensionless), related to adsorption capacity and adsorption intensity, respectively, are presented in Table 2. From these data it is obvious that adsorption capacity of solids increase as the solution pH decrease and C2 catalyst has a superior adsorption capacity towards other catalysts.
DRIFT spectra (Fig. 6) reveal several bands which are assigned as follow: the band 1,615 and 1,502 cm−1 corresponds to the aromatic ring stretching vibration and 1,047 cm−1 band to ring C–H plane bending vibration and a relatively broad band at 1,209 cm−1 which can be assigned both to C–O or C–N stretching vibration.
Of particular interest for our work is the presence of prominent bands at 1,353 cm−1 (assigned to phenolic group) and 1,298 cm−1 only for C2 catalyst. The band at 1,298 cm−1 is characteristic for the phenol group involved in the adsorption process as it is also emphasized in the study of Bandara et al. [15]. Also it can be observed that intensity of bands at 1,615 and 1,502 cm−1 decrease significantly with pH increase. These experimental findings together with the high pk a value for AR88 (10.7) [16] allow us to assume that below pH = 7.5 adsorption occur by means of negative ions of dye onto positive sites of alumina surface:

Adsorption is weaker in case of C1 and C3 catalysts comparing with alumina probably due to covering of support surface by nickel and respective cobalt oxide and less affinity of these oxides for AR 88 molecule. For C2 catalyst adsorption is stronger comparing with alumina and seems to occur through –SO3H and –OH groups resulting in complex formation on solid surface.
3.2 Catalytic Activity
3.2.1 Influence of Catalyst Type
Dye removal from aqueous solutions in absence and in presence of prepared catalysts can follow simultaneously several possible routes such as: (1) adsorption on the catalyst surface; (2) direct oxidation by molecular ozone in the bulk solution; (3) oxidation by radicals formed from ozone decomposition at high pH; (4) oxidation in the bulk solution by radicals formed from catalytic ozone decomposition; (5) oxidation on the catalyst surface.
From the experimental tests it can be seen that all three catalytic systems are able to achieve complete decolourisation of AR 88 from the bulk solution and on catalyst surface. Moreover from our previous studies [17] in similar condition it can be emphasized that for the selected experimental parameters the oxidation process is chemically controlled.
Assessing the performance of prepared catalysts it can be seen from Fig. 7 that reaction time for complete dye removal is considerable longer (~40 min) for single ozonation comparing with catalytic ozonation (~20 min). From the experimental data previously presented it seems that catalytic ozonation process occur differently for prepared catalysts. C2 catalysts appear to be the most active among the others. However adsorption data reveal that a large amount of dye is adsorbed on catalyst surface thus, in this case, dye removal is completed trough a combined process: adsorption followed by oxidation on solid surface and in bulk solution. Moreover, after complete oxidation of dye molecules, a change in reaction mechanism in terms of ozone decomposition and radical formation on catalyst surface may occur. These assumptions are in accordance with a study which emphasizes that oxidation byproducts desorbs from cooper based catalyst surface [18]. Conversely, C1 catalyst exhibit a low adsorption capacity and, more important, ozone decomposition capability as shown in our previous work [17] therefore it can be assumed that dye molecules are oxidized mainly by means of hydroxyl radicals formed on catalyst surface or direct ozonation. C3 catalyst is less active than C1 catalyst however the reaction route probably is similar considering the low adsorption capacity of this solid as resulted from adsorption data.
These assumptions are further supported by the evolution of TOC and COD with reaction time presented in Fig. 8. Hence for C2 catalyst two phases in the oxidation process can be observed: a rapid decrease of TOC and COD values followed by removal of organic substrate which account for large amount of dye adsorbed on catalyst surface and oxidation in bulk solution and/or on solid surface. C1 and C3 catalysts show a steady decrease of the two parameters meaning that adsorption process of dye molecules on catalyst surface play a minor part. Also it can be observed that in absence of prepared solids TOC and COD parameters present a very slow decrease therefore single ozonation process act mainly on chromophores groups of dye molecules and have a low effect on oxidation byproducts.
Regarding the extent of TOC and COD removal it can be noticed that mineralization process is relatively slower even in presence of catalytic systems comparing with oxidation degree of organic substrate.
From different studies [19–21] it is clear that that an accumulation of oxidation-refractory organics occur during the ozonation processes. This behavior it is emphasized by the changes in the MOC (mean oxidation state of carbon) from the initial to the final step of the oxidation processes (Fig. 9).
This parameter is adimensional and ranges between −4 for the minor oxidation state of carbon (CH4) and +4 for the major oxidation state (CO2) and give information about the nature of the organics from measurements of TOC and COD. As it can be expected MOC values increase during the oxidation process due to a change in oxidation state of carbon of dye molecule and the formation of different oxygenates compounds (mainly carboxylic acids) with high mean oxidation states of carbon. Carboxylic acids start to decompose thus MOC values reach a plateau after about 50 min. At this point, the incorporation of oxygen into the molecules (increasing the MOC value) and the removal of carboxylic acid (decreasing the MOC value) seem to balance each other. Lower MOC values obtained in the case of cooper based catalytic system comparing with nickel and cobalt based catalysts can be explained by the rapid adsorption process which induce a “delay” in the change of organic compounds oxidation state. In the case of single ozonation the low level of MOC values can be attributed to slower rate of dye removal (when dye molecule is oxidized MOC increase) and to low concentration of carboxylic acid accumulated in the system (Fig. 10).
3.2.2 Influence of pH
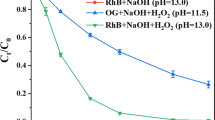
The role of pH on the color removal was assessed in a pH range from 2 to 11. As seen in Fig. 3 enhancement of oxidation process in absence of catalysts in alkaline aqueous solutions may occur as a result of ozone transformation in hydroxyl radicals (Fig. 3a).
In presence of solid catalysts dye removal rate is similar regardless of pH value (Fig. 3b, c). In order to explain this similarity for C2 catalyst it must take in account that for whole pH range adsorption of dye molecule on catalyst surface play an important role and oxidation process occur by direct ozonation on catalyst surface and in bulk solution. Even that at basic pH a fraction of ozone is transformed in hydroxyl radicals this is compensated in acid media by higher oxidation potential of ozone (2.07 V comparing with 1.24 V in alkaline media). As for C1 catalyst this behavior can be attributed to a high ozone decomposition capacity for all considered pH values. A slightest decrease in activity can be observed at very basic pH probably as a result of hydroxyl radicals depletion in non selective reactions.
3.2.3 Ozone Consumption
Ozone consumption related to dye removal it is difficult to evaluate due to simultaneously oxidation of dye molecule and resulted byproducts particularly in catalytic processes. Thus, in this work, oxidation process efficiency in terms of mgO3 consumed per mg of COD or TOC removal was estimated. The mass balance of ozone can be assessed as follow:
-
ozone consumption
$$ ({\text{O}} . {\text{C}} .) = \frac{{n\int_{0}^{t} {(C_{{O_{3} , {\text{in}}}} } - C_{{O_{3} , {\text{out}}}} )dt - C_{{O_{3} {\text{aq}}}} V}}{V} $$ -
specific ozone consumption
$$ ({\text{S}} . {\text{O}} . {\text{C}}.)_{\text{COD}} = \frac{{n\int_{0}^{t} {(C_{{O_{3} ,{\text{in}}}} } - C_{{O_{3} ,{\text{out}}}} ){\text{d}}t - C_{{O_{3} {\text{aq}}}} V}}{{(C_{{{\text{COD}}_{0} }} - C_{\text{COD}} )V}}; $$$$ ({\text{S}} . {\text{O}} . {\text{C}} .)_{\text{TOC}} = \frac{{n\int_{0}^{t} {(C_{{O_{3,} {\text{in}}}} } - C_{{O_{3,} {\text{out}}}} ){\text{d}}t - C_{{O_{3} {\text{aq}}}} V}}{{(C_{{{\text{TOC}}_{0} }} - C_{\text{TOC}} )V}} $$
where (O·C.) stand for ozone consumption (mgO3), (S.O.C.)COD, (S.O.C.)TOC represent the specific ozone consumption (mgO3 consumed per mg of COD or TOC); n is the gas flow rate; \( {\text{C}}_{{{\text{O}}_{ 3 ,} {\text{in}}}} \) and \( {\text{C}}_{{{\text{O}}_{ 3 ,} {\text{out}}}} \) are the concentrations of ozone in the gas inlet and outlet, respectively; \( {\text{C}}_{{{\text{O}}_{ 3} {\text{aq}}}} \) is the concentration of ozone in solution; \( {\text{C}}_{{{\text{COD}}_{\text{o}} }} , \) \( {\text{C}}_{{{\text{TOC}}_{ 0} }} \) and \( {\text{C}}_{\text{COD}} , \) \( {\text{C}}_{\text{TOC}} \) represent COD and TOC values at time 0 and t respectively; V is the reaction volume.
Concentration of ozone in solution attains rapidly a steady state for all experimental tests and varies significantly with pH value (Fig. 11). Also lower values of \( {\text{C}}_{{{\text{O}}_{ 3} {\text{aq}}}} \) can be observed for catalytic test (mainly for C1 catalyst). Ozone consumption (O·C.) is similar for all tests as can be seen in Table 3. Nevertheless, in absence of catalysts, a large amount of ozone is consumed in first 40 min of reaction time and in presence of catalysts a more equilibrated consumption can be observed. Conversely, S.O.C. shows a different pattern. For single ozonation S.O.C. is considerably higher both in terms of COD and TOC removal especially for S.O.C. due to low mineralization degree of organic substrate. C2 catalyst present the lowest specific ozone consumption in terms of COD removal while C1 catalyst show the lowest specific ozone consumption in terms of TOC removal which is in good agreement with MOC evolution.
4 Conclusions
In this study, degradation of AR 88 azo dye in water was investigated using ozone as oxidant agent and three catalytic systems. The obtained results show that single ozonation and catalytic ozonation are efficient processes for removal of dye molecules. However single ozonation represent a poor alternative for removal of TOC and COD comparing with oxidation in presence of catalysts. This aspect is very important since TOC and COD removal represent a real challenge in water treatment processes. Moreover it was emphasized that ozone consumption is very high (3.01 mgO3/mg COD and 132.47 mgO3/mg TOC) for not catalytic ozonation and considerably low in presence of catalysts (C2:0.63 mgO3/mg COD and 3.38 mgO3/mg TOC). Adsorption processes seems to play an important role in catalytic processes mainly in the case of C2 system and to a lesser extent for C1 and C3 catalyst. The order of efficiency, considering the degree of decolourization is as follows: NC < C3 < C1 < C2. In the case of catalytic processes TOC removal range from 24 to 64% and COD removal from 53 to 68%.
References
Eren Z, Acar FN (2006) Desalination 194:1
Madhavan J, Maruthamuthu P, Murugesan S, Anandan S (2008) Appl Catal B Environ 83:8–14
Kepa U, Stanczyk-Mazanek E, Stepniak L (2008) Desalination 223:187
Lackey LW, Mines RO, McCreanor PT (2006) J Hazard Mater B138:357
Dong Y, He K, Yin L, Zhang A (2007) Catal Lett 119:222
Akyol A, Bayramoglu M (2005) J Hazard Mater B124:241
Dong Y, He K, Zhao B, Yin Y, Yin L, Zhang A (2007) Catal Commun 8:1599
Chung HH, Jung J, Yoon JH, Lee MJ (2002) Catal Lett 78:77
Mustafa S, Dilara B, Nargis K, Naeem A, Shahida P (2002) Colloids Surf A Physicochem Eng Asp 205:273
Bader H, Hoigne J (1981) Water Res 15:449
Standard Methods for the Examination of Water and Wastewater (1998) 20th ed., American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC
Ben-Moshe T, Dror I, Berkowitz B, Appl Catal B Environ (in press)
Kaluža L, Zdražil M (2007) React Kinet Catal Lett 91:249
Alvarez PM, Beltran FJ, Pocostales JP, Masa FJ (2007) Appl Catal B Environ 72:322
Bandara J, Mielczarski JA, Kiwi J (2001) Appl Catal B Environ 34:307
Perez-Urquiza M, Beltran JL (2001) J Chromatogr A 917:331
Avramescu SM, Bradu C, Udrea I, Mihalache N, Ruta F (2008) Catal Commun 9:2386
Qu J, Li H, Liu H, He H (2004) Catal Today 90:291
Faouzi M, Canizares P, Gadri A, Lobato J, Nasrc B, Paza R, Rodrigo MA, Saez C (2006) Electrochim Acta 52:325–331
Vogel F, Harf J, Hug A, von Rohr PR (2000) Water Res 34:2689
Martins AO, Canalli VM, Azevedo CMN, al Pires M (2006) Dyes Pigments 68:227
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avramescu, S.M., Mihalache, N., Bradu, C. et al. Catalytic Ozonation of Acid Red 88 from Aqueous Solutions. Catal Lett 129, 273–280 (2009). https://doi.org/10.1007/s10562-008-9812-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9812-y