Abstract
Acid orange 7, chemically known as sodium 4-[(2E)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]benzenesulfonate, is extensively used for dyeing textiles, paper and leather. The discharge of wastewater containing this dye, causes environmental and health related problems. Therefore, in the present research, we have developed optimum conditions for the facile oxidative decolorization of this dye with sodium N-chlorobenzenesulfonamide or chloramine-B (CAB). The kinetics and mechanism of oxidative decolorization of acid orange 7 dye with CAB in acidic medium have also been studied spectrophotometrically at 303 K in the presence and absence of RuCl3 catalyst. Under similar experimental conditions, the reaction exhibits a first-order dependence of rate each on [CAB]o and [dye]o, and an inverse-fractional-order dependence on [H+] for both the RuCl3 catalyzed and uncatalyzed reactions. The order with respect to RuCl3 is fractional. Activation parameters have been computed. Dielectric effect is negative in both the cases. Oxidation products of the acid orange 7 dye are identified as 1,2-naphthoquinone and benzenesulfonic acid by GC–MS data. The RuCl3 catalyzed reaction is about four fold faster than the uncatalyzed reaction. The chemical oxygen demand value of the dye was determined. The mechanistic pathways and kinetic modelings have been computed based on experimental results. The developed oxidative decolorization method is expected to be helpful to treat acid orange 7 dye present in wastewater after suitable modifications.
Graphical Abstract
The stoichiometry of the reaction is in the mole ratio of 1:1(AO7:CAB) in both the cases as shown given below

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acid orange 7 is chemically known as sodium 4-[(2E)-2-(2-oxonaphthalen-1-ylidene)hydrazinyl]benzenesulfonate and it is widely used for dyeing textiles, paper and leather [1, 2]. The industrial wastewater bearing this dye causes environment and health problems. Oxidation decolorization is considered a simple and economic technology for the removal of dyestuffs from wastewater. Though there are many reports in literature on the oxidative decolorization of this dye [3–5], similar studies have not yet been carried out by keeping its kinetic and mechanistic aspects. The chemistry of organic N-haloamines is of great interest due to their diverse behavior [6]. The important chloramine compounds of this class are sodium N-chloro-p-toluenesulfonamide or chloramine-T (CAT) and sodium N-chlorobenzenesulfonamide or chloramine-B (CAB). The mechanistic aspects of many of these reactions have been documented [7–16]. The decolorization of acid orange 7 dye was tested with both CAT and CAB in acid medium. It was observed that the decolorization of this dye is most effective with CAB.
To the best of our knowledge, the impact of platinum group metal ions on the oxidation of acid orange 7 dye has not yet been investigated. Among platinum group metal ions, ruthenium (III) chloride (RuCl3) has been extensively employed as a homogeneous catalyst in redox reactions [17–19] and some of these systems have proved suitable for kinetic analysis [20–23]. Our preliminary experimental results indicated that a micro-quantity of RuCl3 effectively catalyzes the oxidation of acid orange 7 dye with CAB in acid medium. Therefore, in the present work, we have explored the kinetics and mechanism of the acid orange 7—CAB redox system in acid medium in the absence and presence of RuCl3 catalyst. The other objective of the present study is to optimize the reaction conditions for the efficient decolorization of acid orange 7 dye with CAB in acid medium.
2 Experimental and Methods
2.1 Materials and Reagents
Chloramine-B was obtained from Sigma and purified by the method of Morris et al. [24]. An aqueous solution of CAB was prepared afresh whenever required, standardized by iodometric method and stored in brown bottles until further use to prevent its any photochemical deterioration. Acid Orange 7 (SD Fine Chem. Ltd., India) was used as received and an aqueous solution of the desired strength of the dye was prepared afresh each time. A solution of RuCl3 (Merck) was prepared in 20 mM HCl and employed as a catalyst. Allowance was made for the amount of acid present in the catalyst solutions while preparing reaction mixtures for kinetic runs. All the chemicals used were of analytical grade and used as such. All the solutions were prepared using double distilled water.
2.2 Kinetic Measurements
Kinetic measurements were carried out using a UV–Vis spectrophotometer. (SL 159. Elico Ltd). Kinetic runs were performed under pseudo-first-order conditions by keeping an excess of CAB over dye in HClO4 medium with and without RuCl3 at 303 K and the detailed procedure which was followed is similar to that reported earlier [25]. Absorbance measurements were made at 484 nm (λmax for the dye) for more than two half-lives. The absorbance readings at t = 0 and t = t are D0 and Dt and plots of log Do/Dt versus time were made to evaluate the pseudo-first-order rate constants (k′ s−1). All the kinetic runs were carried out twice to check the reproducibility, and was found to be reproducible within ±5 % error. Calculations of the regression coefficients (R2) were carried out using fx—100 W scientific calculator.
3 Results
3.1 Reaction Stoichiometry and Product Analysis
The stoichiometry of the reaction was determined by equilibrating varying ratios of [CAB]o and [dye]o in presence of 3.0 × 10−3 mol dm−3 HClO4 (2.0 × 10−5 mol dm−3 RuCl3 for the catalyzed reaction) at 303 K for 24 h Determination of the residual oxidant showed that the stoichiometry of the reaction is in the mole ratio of 1:1 in both the cases as shown in Eq. 1.

The reaction mixture in the stoichiometric ratio was allowed to progress for 24 h in the presence of HClO4 (RuCl3 for the catalyzed reaction) at 303 K under stirred conditions. After completion of the reaction (monitored by TLC), the reaction products were neutralized with dilute NaOH and extracted with ethyl acetate. Separation of these products was achieved using silica gel (60–100 mesh) column chromatography with hexane/ethyl acetate (8:6, v/v) as mobile phase. The oxidation products were identified as 1,2-naphthoquinone and benzenesulfonic acid for the RuCl3 catalyzed and uncatalyzed reactions. These are confirmed by GC–MS analysis. GC–MS data were obtained on a 17A Shimadzu gas chromatograph with a QP-5050A Shimadzu mass spectrometer. The mass spectra showed a molecular ion peak at 158 and 158 amu, clearly conforming 1,2-naphthoquinone and benzenesulfonic acid, respectively. It was also noticed that there was no further reaction of these oxidation products under the present set of experimental conditions. Benzenesulfonamide (BSA or PhSO2NH2), the reduction product of CAB, was detected [26] by TLC using light petroleum-chloroform-butan-1-ol (2:2:1 v/v/v) as the solvent and iodine for detection. The Rf value was found to be 0.88, which is in good agreement with the literature value [26]. Nitrogen was detected by the conventional test.
3.2 Kinetic Orders
The kinetics of oxidative decolorization of acid orange-7 (hereafter abbreviated as dye) with CAB has been investigated spectrophotometrically at several initial concentrations of the reactants in HClO4 medium with and without RuCl3 catalyst. The standard experimental conditions established for the facile oxidative decolorization of the dye are: [CAB]o = 3.0 × 10−3 mol dm−3, [dye]o = 2.0 × 10−4 mol dm−3, [HClO4] = 3.0 × 10−3 mol dm−3, and [RuCl3] = 2.0 × 10−5 mol dm−3 (in case of catalyzed reaction) at T = 303 K. Under these conditions, CAB decolorizes the dye completely within 25 and 90 min in the presence and absence of RuCl3 catalyst. The pseudo-first order rate constants obtained for standard run in the presence and absence of RuCl3 catalyst are 16.2 × 10−4 and 3.80 × 10−4 s−1, respectively. Hence, it can be said that RuCl3 acts as an effective catalyst for the facile oxidative decolorization of acid orange-7 by CAB in acid medium. Unless otherwise noted, the other attributes of the reactions were unchanged by the presence of RuCl3.
The kinetic runs were performed under pseudo-first-order conditions of [CAB]O ≫ [dye]O in both the cases. At constant [CAB]O, [HClO4], RuCl3 (in case of catalyzed reaction) and temperature, plots of log(absorbance) versus time were linear (R2 > 0.9884), indicating a first-order dependence of rate on [dye]o for both RuCl3 catalyzed and uncatalyzed reactions. The values of pseudo-first order rate constants (k′ s−1) were found to be independent of [dye]o, confirming the first-order dependence of rate on [dye]o. The values are given in Table 1. The rate increased with the increase in [CAB]o (Table 1) in both the cases and log–log plots of rate versus [CAB] were linear (Fig. 1; R2 > 0.9926) with a slope of unity. This ensures that the kinetics exhibits a first-order dependence on [CAB]o. Further, plots of k′ versus [CAB] were linear (R2 > 0.9939) passed through the origin, confirming the first-order dependence of rate on [CAB]o and also showing the transient nature of the intermediate formed with the dye.
The reaction rate decreased with increase in [HClO4] (Table 1) and plots of log k′ versus log [HClO4] were linear (Fig. 2; R2 > 0.9904) with fractional slopes of −0.50 and −0.86 for RuCl3 catalyzed and uncatalyzed reactions, respectively. This clearly indicates a negative fractional-order dependence of rate on [HClO4] in both the cases. The rate increased with an increase in [RuCl3] (Table 1) and the slope of log k′ versus log [RuCl3] plot (Fig. 3; R2 = 0.9990) was found to be 0.48, indicating a fractional-order dependence of rate on [RuCl3]. The dielectric constant of the medium was varied using different amounts of methanol and H2O (0–30 % v/v). The reaction rate decreases with an increase in methanol content in both the cases (Table 2). Plots of log k′ versus 1/D were found to be linear (Fig. 4; R2 > 0.9960) with negative slopes. Values of dielectric constant of methanol–water mixture reported in literature were employed [27]. Blank experiments performed showed that MeOH was not oxidized significantly by CAB under the prevailing experimental conditions (without substrate). No attempt was made to keep the ionic strength of the system fixed for both the reactions, since the pseudo-first-order rate constants remains unaltered in presence of 0.2 mol dm−3 NaClO4 solution.
Addition of 5.0 × 10−3 mol dm−3 benzenesulfonamide (BSA or PhSO2NH2), the reduction product of CAB, to the reaction mixture showed negligible influence on the rate of the reaction in both the cases. It signifies that the BSA is not involved in any step prior to the rate determining steps (rds) in the reaction schemes. Similarly, addition of Cl− ion in the form of NaCl (2.0 × 10−3 mol dm−3) does not have any pronounced effect on the rate of the reaction, indicating that the Cl− ion plays no role in the reaction sequence. Activation parameters (Ea, ΔH≠, ΔG≠, ΔS≠ and log A) for the reaction have been evaluated from Arrhenius plots of log k′ versus 1/T (Fig. 5; R2 > 0.9939) by studying the reactions at different temperatures (293–313 K). These results are summarized in Table 3. On adding a small amount of the reaction mixture to acrylonitrile, there was no polymerization indicating the absence of free radical species in the reaction sequence.
4 Discussion
As CAT and CAB exhibit similar chemical properties, it is expected that identical equilibria exist in aqueous solutions of these compounds [28, 29]. In general, CAB undergoes a two electron change in its reactions [30]. The redox potential of CAB/BSA couple is pH dependent and decreases with increase in pH of the medium [30].
4.1 Reactive Species of CAB
Chloramine-B (PhSO2NClNa) behaves as a strong electrolyte [28] and depending on the pH of the medium, it furnishes different equilibria in aqueous solutions [24, 28, 29]. The possible oxidizing species in acidified CAB solutions are PhSO2NHCl, PhSO2NCl2, HOCl and possibly H2O+Cl, and in alkaline solutions they are PhSO2NHCl, PhSO2NCl−, HOCl and OCl−. The present redox system was carried out in acid medium and hence from the four possibilities, the reactive species of CAB can be decided based on the observed kinetic data.
4.2 Tautomerism of Acid Orange 7 Dye
Azo dyes, such as Acid Orange 7, containing hydroxyl groups conjugated to azo group [31] exhibit azo-hydrazone tautomerism as shown below:

In the present studies, the azo form of acid orange 7 is involved in the reaction.
4.3 Reactive Species of RuCl3
RuCl3 catalysis in redox reactions involves different degrees of complexicity, due to formation of different intermediate complexes, free radicals and its variable oxidation states. Cady and Connick [32], and Connick and Fine [33] have investigated aqueous RuCl3 complex species using the ion exchange resins and UV-spectral studies. They found that the octahedral complex species [RuCl5(H2O)]2−, [RuCl3(H2O)3], [RuCl2(H2O)4]+ and [RuCl(H2O)5]2+ may not exist in aqueous solution of RuCl3. Other studies [17, 34, 35] have shown the existence of following equations for RuCl3 in acidic solutions:
In the present study, the absence of chloride ion on the rate indicates that the equilibrium (4) does not play any role in the reaction. Hence, the complex ion, [RuCl5 (H2O)]2−, is assumed to be the reactive catalyst species.
4.4 Reaction Scheme and Rate Law for Uncatalyzed Reaction
The probable reactive species in acidic solutions of CAB are PhSO2NHCl, PhSO2NCl2, HOCl and possibly H2O+Cl. The first-order dependence of rate on [CAB]o and the addition of PhSO2NH2 (benzenesulfonamide) having no effect on the reaction rate both indicate that PhSO2NCl2 and HOCl may not be the reactive species. Hardy and Johnston [29], who have studied the pH-dependent relative concentrations of the species present in acidified CAB solutions of comparable molarities and have shown that PhSO2NHCl is the likely oxidizing species in acid medium. Narayanan and Rao [36] and, Subhashini et al. [37] have reported that CAB can further be protonated at pH < 2 to give PhSO2N+H2Cl. In the present case, protonated oxidant (PhSO2N+H2Cl) generates the free conjugate acid (PhSO2NHCl) in the acid retarding step and hence PhSO2NHCl is the active oxidizing species. In view of these points, Scheme 1 can be formulated for the oxidation of acid orange 7 dye with CAB in acidic medium.
Here X is the complex intermediate species whose structure is shown in Scheme 2. In Scheme 2, in an initial equilibrium [step(i)], deprotonation of PhSO2N+H2Cl generates the conjugate free acid PhSO2NHCl. In the next slow and rds [step(ii)], the azo form of the dye reacts with the conjugate acid of the oxidant to form the dye-CAB complex (X) with the elimination of PhSO2NH2. The complex X in the presence of a molecule of water through the several fast steps [step(iii)] yields the ultimate products viz., 1,2-naphthoquinone and benzenesulfonic acid with the elimination of a molecule each of HCl and N2.
If [CAB]t is the total effective concentration of CAB, then based on Scheme 1, we can write
From step (i) of Scheme 1,
On substituting the value of PhSO2N+H2Cl from Eq. 6 into Eq. 5, and solving for PhSO2NHCl, we get
From slow/rds of Scheme 1,
On substituting for [PhSO2NHCl] from Eq. 7 into Eq. 8, the rate of reaction is given as
Rate law 9 holds good agreement with the experimental results, wherein a first-order dependence of rate each on [CAB]o and [dye]o, and an inverse-fractional order on [H+] was noted.
4.5 Reaction Scheme and Rate Law for RuCl3 Catalyzed Reaction
The reactive oxidizing species in this case also PhSO2NHCl, which accounts for the observed inverse-fractional-order dependence of rate on [H+]. The proposed mechanism for RuCl3 catalyzed oxidation of acid orange 7 by CAB in acidic medium is presented in Scheme 3.
Here X′ and X″ represent the complex intermediate species whose structures are shown in Scheme 4. In Scheme 4, in the initial equilibrium [step(i)] deprotonation of PhSO2NH2Cl+ generates the conjugate free acid PhSO2NHCl. In the next fast pre-equilibrium step [step(ii], the donor nitrogen atom of the oxidizing species coordinates to the metal centre of the active catalyst species and gives an intermediate complex X′. In the next slow and rds step [step (iii)], another intermediate complex X″ is formed from the reaction between X′ and dye. Finally, the complex X″ undergoes decomposition through several fast steps in the presence of H2O, yielding the final products 1,2-naphthoquinone and benzenesulfonic acid with the elimination of PhSO2NH2, RuCl3, HCl and N2.
Evidence for the formation of complex between oxidant and catalyst is obtained from the UV–Visible spectra of CAB, RuCl3 and the mixture of both. Absorption maxima appear at 224 nm for CAT, 215 nm for RuCl3 in aqueous acidic medium, and 230 nm for their mixture. A bathochromic shift of 15 nm from 215 to 230 nm of RuCl3 suggests that complexation occurs between CAB and RuCl3 in the present case.
Based on the above Scheme 3, the total CAB concentration is
By substituting for [PhSO2N+H2Cl] and [PhSO2NHCl] from steps (i) and (ii) of Scheme 3 and solving for X′, we get
From slow/rds of Scheme 3,
By substituting for [X′] from Eq. 11 into Eq. 12 we get
Rate law 13 is in accordance with the experimental findings. The proposed schemes and the derived rate laws in both the cases are also substantiated by the experimental observations discussed below:
4.6 Effect of Dielectric Constant
In order to find out the nature of reactive species, the dielectric constant (D) of the medium was varied by adding different amounts of methanol (0–30 % v/v) to the reaction mixture. The rate decreased with increasing methanol content (Table 2). Several approaches [38–41] have been made to explain quantitatively the effect of dielectric constant of the medium on the rates of reactions in solutions. Amis and Jaffe [38] predicted a linear relation between log k′ versus 1/D. The slope of such a plot should be negative for a reaction between a negative ion and a dipole or between two dipoles, while a positive slope obtained for positive ion–dipole reactions. In the present investigations, plots of log k′ versus 1/D were linear with negative slopes in both cases, thus supporting the participation of the two dipoles in the rate-determining steps (Schemes 2, 4).
4.7 Effect of Ionic Strength
The proposed reaction mechanisms are also evinced by the observed zero effect of ionic strength on the rate of the reaction. The primary salt effect on the reaction rates has been described by Bronsted and Bjerrum theory [42]. In the present investigations, in both the cases, neutral molecules are involved in the rate determining steps of Schemes 2 and 4. Hence, variation of the ionic strength of the medium does not alter the rate in both the cases clearly conform to the above theory [42].
4.8 Catalytic Activity of RuCl3
The general equation relating for uncatalyzed and catalyzed reactions have been derived from Moelwyn – Hughes [43] and can be correlated as:
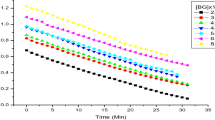
Here k1 is the observed pseudo-first-order rate constant obtained in the presence of RuCl3 catalyst, ko is that for the uncatalyzed reaction, KC is the catalytic constant and x is the order of the reaction with respect to RuCl3. In the present case, x value for the standard run was found to be 0.48 for RuCl3 catalyst. Then the Kc has been evaluated using the equation:
The values of K C have been evaluated at different temperatures (293, 298, 303, 308 and 313 K), and Kc was found to vary with temperature. Further, a plot of log K C versus 1/T was linear (Fig. 5; R 2 = 0.9972) and values of energy of activation and other activation parameters with respect to RuCl3 catalyst were evaluated. All these results are tabulated in Table 3.
4.9 Comparison of RuCl3 Catalyzed and Uncatalyzed Reactions
RuCl3 catalyzed oxidative decolorization of the dye by CAB with that of uncatalyzed reaction (without RuCl3 catalyst) under an identical set of experimental conditions was compared. The observed rates of oxidative decolorization of the dye in the presence of RuCl3 catalyst revealed that the reactions are about four-fold faster than the uncatalyzed reactions (Table 3). This was also confirmed by the calculated activation energies. The difference in activation energies for the catalyzed and uncatalyzed reactions explained the catalytic effect on the rate of the reaction. This may be attributed to the formation of the intermediate complex X′ between RuCl3 and the oxidant, which increases the oxidizing property of the oxidant than without RuCl3 catalyst. Further, RuCl3 favorably modifies the reaction path by stabilizing the transition state, which in turn provides an alternative pathway having lower activation energy for the reaction. Consequently, it can be said that RuCl3 is an efficient catalyst for the present redox system.
4.10 Activation Parameters
The variation of rate constants with temperatures and values of activation parameters are shown in Table 3. Values of ΔH≠ indicates that the reactions are enthalpy controlled. The more positive values of ΔG≠ points out a highly solvated transition state. The large negative values of ΔS≠ signifies that the transition state is more rigid than the initial state with less degrees of freedom. The values of frequency factor (log A) specify the frequency of collisions and orientation of the reaction molecules.
4.11 Determination of Chemical Oxygen Demand Value
In the present research, an effort has been made to determine the chemical oxygen demand (COD) for acid orange 7 dye. COD is a measure of oxidizable matter in dye stuff. The COD of acid orange 7 dye was determined using the standard dichromate method. The procedure followed to determine COD value was according to a literature procedure [44]. Under the prevailing experimental conditions, COD of acid orange 7 dye sample was found to be 1168.10 mg/lit.
5 Conclusion
Different kinetic conditions were experimented and better optimum conditions were established, for the facile oxidative decolorization of acid orange 7 dye with CAB. The rate laws obtained are: −d[CAB]/dt = k[CAB]o [dye]o [H+]−0.50 [RuCl3]0.48 for RuCl3 catalyzed reaction and −d[CAB]/dt = k[CAB]o [dye]o [H+]−0.86 for uncatalyzed reaction. Activation parameters have been evaluated. 1,2-naphthoquinone and benzenesulfonic acid were identified as the oxidation products of acid orange 7 dye. RuCl3 catalyzed reaction is about four-fold faster than the uncatalyzed reaction. Based on the kinetic results, reaction mechanisms and rate laws have been worked out. The COD value of acid orange 7 dye was also determined. The present redox-system can be adopted for treating acid orange 7 dye present in industrial wastewater with suitable modifications to minimize the toxicity caused by it.
References
Zollinger H (1981) Color chemistry: synthesis, properties and applications of organic dyes and pigments. VCH, New York
Silva JP, Sousa S, Rodrigues J, Antunes H, Porter JJ, Goncalves I, Ferreira-Dias S (2004) Sep Purify Technol 40:309–315 and references therein
Li G, Wang N, Liu B, Zhang X (2009) Desalination 249:936–941
Yang S, Yang X, Shao X, Niu R, Wang L (2011) J Hazard Mater 186(1):659–666
Chen X, Qiao X, Wang D, Lin J, Chen J (2007) Chemosphere 67(4):802–808
Campbell MM, Johnson G (1978) Chem Rev 78:65–79
Banerji KK, Jayaram B, Mahadevappa DS (1987) J Sci Ind Res 46:65–76
Armesto XL, Canle L, Garia MV, Santaballa JA (1998) Chem Soc Rev 27:453–460
Agnihotri G (2005) Synlett 18:2857–2858
Kolaveri E, Ghorbeni-Choghamarani A, Salehi P, Shirini F, Zolfigol MA (2007) J Iran Chem Soc 4:126–174
Vinod KN, Puttaswamy, Gowda KNN (2009) Inorg Chim Acta 362:2044–2051
Puttaswamy, Sukhdev A, Shubha JP (2012) Prog React Kinet Mech 37:42–58
Puttaswamy, Jagadeesh RV (2006) Int J Chem Kinet 38:48–56
Puttaswamy, Jagadeesh RV (2005) Eur J Chem 3:482–501
Puttaswamy, Shubha JP (2008) Prog React Kinet Mech 33:313–330
Puttaswamy, Sukhdev A (2009) Indian J Chem 48:339–345
Griffith WP (1967) The chemistry of rare platinum metals. Interscience, New York
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York
Mallesh RT, Bellakki MB, Nandibewoor ST (2004) Catal Lett 97:91–98
Bhat KR, Jyothi K, Gowda BT (2002) Oxid Commun 25:117–141 and references therein
Rashmi R, Sushma G, Upadyay SK (1990) Indian J Chem 29A:847–851
Mulla RM, Hiremath GC, Nandibewoor ST (2004) Monatshefte fur Chemie 135:1489–1502
Jagadeesh RV, Puttaswamy (2008) J Phys Org Chem 21:844–858
Morris JC, Salazar JA, Wineman MA (1948) J Am Chem Soc 70:2036–2041
Puttaswamy, Shubha JP, Jagadeesh RV (2007) Trans Met Chem 32:991–999
Venkatesha BM, Ananda S, Mahadevappa DS (1992) J Phys Org Chem 5:373–381
Akerloff G (1932) J Chem Soc 54:4125–4139
Bishop E, Jennings VJ (1958) Talanta 1:197–199
Hardy FF, Johnston JP (1973) J Chem Soc Perkin Trans 2:742–745
Murthy ARV, Rao BS (1952) Proc Ind Acad Sci 35:69–72
Oakes J, Gratton P (1998) J Chem Soc Perkin Trans 2:2201 and reference therein
Cady HH, Connick RE (1958) J Am Chem Soc 80:2646–2652
Connick RE, Fine DA (1960) J Am Chem Soc 82:4187–4192
Backhouse JR, Dwyer FD, Shales N (1950) Proc R Soc 83:146–155
Davfokratova T (1963) Analytical chemistry of ruthenium. Academy of Sciences, USSR
Narayanan SS, Rao VRS (1983) Radiochem Acta 32:211–212
Subhashini M, Subramanian M, Rao VRS (1985) Talanta 32:1082–1085
Amis ES, Jaffe G (1942) J Chem Phys 10:598–604
Laidler KJ, Landskroener PA (1956) Trans Faraday Soc 52:200–210
Tanford C, Kirkwood JG (1957) J Am Chem Soc 79:5333–5339
Reihardt C (2003) Solvent and solvent effects in organic chemistry, 3rd edn. Wiley, New York
Laidler KJ (1995) Chemical kinetics, 2nd edn. Tata Mc-Graw Hill, New Delhi
Moelwyn-Hughes EA (1947) Kinetics of reactions in solutions. Oxford University Press, London
Gomati Devi L, Mohan Reddy K (2010) Appl Surf Sci 256:3116–3122
Acknowledgments
The authors greatly acknowledge the University Grant Commission, New Delhi for the award of UGC-Major Research Project [F. No. 39-721/2010 (SR)]. We thank Prof. M. A. Pasha of this department for his valuable suggestions regarding the reaction schemes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjunatha, A.S., Puttaswamy RuCl3 Catalyzed and Uncatalyzed Oxidative Decolorization of Acid Orange 7 Dye with Chloramine-B in Acid Medium: Spectrophotometric, Kinetic and Mechanistic Study. Catal Lett 145, 1312–1321 (2015). https://doi.org/10.1007/s10562-015-1526-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1526-3