Abstract
Our laboratory had developed a methodology to expand epithelial cells in culture by growing keratinocyte monolayers, under large volumes of medium that produces large numbers of keratinocytes that leave the monolayer and move into suspension. The cells have been defined as epithelial Pop Up Keratinocytes or ePUKs cells and appear to be highly suitable for clinical applications. In this publication we extend the characterization of the cells with a detailed analysis of the capabilities of the monolayer of a single culture flask to produce, over time, ePUK cells. The cells were characterized using standard epithelial markers for proliferation and differentiation. Analysis of morphology of the monolayer formed and total number of cells produced is presented for a variety of human epithelial cell strains. These keratinocytes provide an additional controlled human cell system for investigation of the mechanisms regulating epithelia cell growth and differentiation and since they are produced in large numbers, they are highly suitable for use in epithelial cell banking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Keratinocytes derived from epidermis, oral mucosa, and urothelium are used in construction of cell based tissue engineering and regenerative medicine applications. Several methods (Oliveira and Hodges 2005; Bavister et al. 2005; Mignone et al. 2010; Lei and Andreadis 2008; Hodgkinson et al. 2010) are being developed to obtain cells with functional plasticity to construct artificial tissue for transplantation, to ‘correct’ specific systemic diseases and as a source for cell-mediated wound healing therapies. It would be advantageous to develop a methodology to grow adult somatic cells with maximum plasticity, from human tissue, to circumvent many of the well-known and currently debated ethical and scientific problems associated with the use of embryonic derived stem cells or induced pluripotent stem cells (Lister et al. 2011).
Previously, we have shown that human epithelia keratinocytes in primary culture can be induced by tissue culture manipulation to produce, without the use of enzymes for passaging, large numbers of small cells in a combined suspension/monolayer culture. The cells, referred to as epithelial Pop Up Keratinocytes or ePUKs cells (Marcelo et al. 2012) leave the culture monolayer and move into suspension and are grown in large amounts. Since this is a completely novel technique to produce large numbers of keratinocytes without the use of enzymes, additional information about the cells and their characteristics is required to evaluate their potential as a source of epithelial cells for clinical and tissue engineering applications. We wanted to study how many of them could be produced over time and whether, and how far, they mantained their basic proliferative and epithelial phenotypes. For this reason, we present an extented study of this novel keratinocyte culturing technique, describing additional characteristics of the cells, with particular focus on the presence of epithelial and differentiation markers. We focus on the detailed characterization of the evolution of a monolayer of cells in a single flask, its ability to produce cells over time and the characterization of the ePUK cells produced. The study is extended to several epithelial cell strains and a brief comparison between them is presented.
While in our initial keratinocyte dissociation from tissues we used standard techniques (Marcelo et al. 1992) requiring an initial enzymatic treatment, combining our ePUK technique with the methodologies described recently by Dragúnová et al. (2012) to obtain the primary keratinocytes directly from skin explants will provide a methodology in which enzymatic treatments are completely avoided, and not just for serial subculturing.
Materials and methods
Primary keratinocytes and ePUK culture preparation
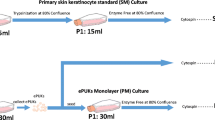
Preparation of the ePUK cultures was done as previously reported (Marcelo et al. 2012). First, primary adult human keratinocytes were obtained with standard techniques using discarded skin tissues obtained from surgeries (Marcelo et al. 1992). Cells were grown in Epilife® medium supplemented with defined growth factors (EDGS) and 0.06 mM Ca2+ (Invitrogen, Carlsbad, CA). Cells were grown at 37 °C, 5 % CO2 gassing and atmospheric oxygen. ePUK culture flasks were fed complete Epilife® Growth medium by using 70 ml/T150; 35 ml/T-75; 15 ml/T-25 flasks, every 24 h. At confluence, the monolayers continued to proliferate, pushing keratinocytes upward into the overlying medium and into suspension. The cells in suspension, referred to as ePUKs (epithelial pop-off keratinocytes) are poured into a new flask, and form new cultures, as depicted in Fig. 1a. In this manuscript, we focus on analyzing the characteristics of the monolayer of the ePUK0 culture and the ePUK cells produced by this culture for a number of days to study their characteristics over time, as conceptualized in Fig. 1b.
a In a previous publication, the overall methodology to produce the ePUKs cells was described (Marcelo et al. 2012) and is represented in this figure. Briefly, when keratinocytes are seeded they form a confluent monolayer that when cultured with high volumes of medium produce cells that go into the overlying medium. The spent medium with the cells in suspension is then poured into another flask and these cells will attach and form another monolayer. The process can be repeated every day. b In this manuscript we study the capabilities of the monolayer of the single flask (labeled ePUK0) (for a variety of cell strains) to produce, over time, a number of ePUK cells and characterize the popped cells that are plated in the new flasks (labeled ePUK1, ePUK2 and ePUK3) using standard epithelial markers. The number of cells produced by each flask every single day was also measured. The cell strains used and the days at which the epithelial markers were analyzed and the ePUK cultures studied are described in detail in Table S1. It is important to emphasize that the cells studied (counting and immunohistochemistry) are always the cells in suspension in the medium and never the cells in the monolayers. Trypsin or enzymatic treatments are never used in this technique
Cultures and creation of ePUK monolayers
Table S1 (Supplementary Material) shows the list of cultured flasks, with information about their confluency, the ePUK flasks produced with ePUK cells and the length of time the culture was maintained as actively producing ePUKs. The days to confluency were determined from the starting day to the day when the flasks were 90 % confluent or more. Counting was always performed after confluency, considering confluency at least 90 % flask surface coverage. Every day during the counting period ePUKs cells popped from the monolayer were collected and counted. The total number of days for cell counting was determined by a cut-off number of produced ePUK cells. This number was experimentally determined to be 10,000 cells per ml or less. If the number of ePUK cells produced were below this threshold or the monolayer was lost the culture was considered finalized. Some flasks were kept for much longer periods of time to analyze what would happen with the monolayers for extended periods of time, but information about epithelial markers and ePUKs cells produced for these long-maintained flasks was not collected. During the culture period light microscopy images of the monolayers were taken to observe their changes and cell morphology. The epithelial cell strains studied were:
-
A.
Keratinocytes from human breast from a previously frozen adult human breast keratinocyte culture at passage 2. The original seeded flask is labeled breast ePUK0. Three ePUK flasks (breast ePUK1, breast ePUK2, breast ePUK3) were created from the cells in suspension at 2, 5, and 8 days after confluency. The number of ePUK cells produced per day and epithelial marker analysis was done for breast ePUK0 and breast EPUK1.
-
B.
Keratinocytes from human neonatal from a previously frozen neonatal keratinocyte culture at passage 2. The original seeded flask is labeled neo frozen ePUK0. Three ePUK flasks were created at 2, 5, and 10 days after confluency, (neo frozen ePUK1, neo frozen ePUK2, neo frozen ePUK3). The analysis of number of ePUK cells produced per day and antibody analysis was done for neo frozen ePUK0 and neo frozen ePUK1.
-
C.
Primary neonatal human keratinocytes obtained from two different foreskins. The original seeded flasks are labeled neo1 fresh ePUK0 and neo2 fresh ePUK0. Two ePUK flasks were created for each of them at 2, 6 and 0 and 4 days after confluency, respectively. Analysis of number of cells popped per day and antibody staining was done for the neo 1 fresh ePUK0, neo2 fresh ePUK0, neo1 freshePUK1 and neo2 fresh ePUK2 cells.
-
D.
Fresh primary oral mucosa human keratinocytes were obtained from a gingiva biopsy from the maxilla. The seeded flask is labeled oral ePUK0. This is a keratinized area of the oral mucosa. Three ePUK flasks, (oral ePUK1, oral ePUK2, oral ePUK3) were created from the cells in suspension at 2, 5, and 8 days after confluency. Analysis of number of cells popped per day and antibody staining was done for the oral ePUK0 and oral ePUK1 cells.
-
E.
Keratinocytes from human skin from a previously frozen keratinocyte culture obtained from a biopsy from the abdomen.
Immunohistochemistry
ePUKs cells were analyzed for presence of markers of basal cells and keratinization (p63, K14/K5, K10); proliferation (Ki67); stem/progenitor cells (α6β4 integrin, CD71) and supra-basal, differentiated cells (loricrin and involucrin). This analysis was performed with ePUK cells collected from the specified culture flask over time and is indicated in Table S1 under the column antibody analysis. For this, ePUK cell suspensions were cytospun at 750 rpm for 5 min onto slides using a ThermoShandon Cytospin 4 (ThermoShandon, Pittsburg, PA; Shandon Cytoslide and ThermoShandon single cytology Funnel, from Fisher Scientific, Pittsburg, PA). Afterwards the cells were fixed and maintained in 95 % ethanol until immunohistochemical analysis was performed. Images were taken using a Nikon E-880 microscope. CD71 was from Novus Biologicals cat #NB100-92243 (dilution factor 1:200); Cytokeratin 14/5 from Novus Biological cat #NC110-59922 (dilution factor 1:200); Cytokeratin 10 from Novus Biologicals cat #NBPI-22537 (dilution factor 1:200); Involucrin from Sigma-Aldrich Cat #19018 (dilution factor 1:1,000); ITGA6 (alpha 6 beta 4 integrin) from Sigma-Aldrich cat # WH0003655M1 (dilution factor 1:200); Ki67 from DAKO Cat# M 7240 (dilution factor 1:50); Loricin from Sigma-Aldrich cat # AV 41738 (dilution factor 1:200); p63 from NeoMarker/LabVision cat# MS 1081 (dilution factor 1:100).
Results
Overall observations and morphology over time of the monolayers producing ePUK cells
In general, the cultured frozen or fresh cell strains reached confluency in a flask between the 3rd and the 7th day after seeding. For all cell strains studied, both the ePUK0 flasks (fresh or frozen) and the ePUK flasks cells formed a monolayer of polygonal cells with an approximate diagonal size of 16–18 microns (first column of Fig. 2, days 3 and 5), that is maintained for 10–15 days and gradually starts disappearing while the keratinocytes change their phenotype to an elongated and curved cell type (beyond day 11 pictures in Fig. 2). For ePUK0 cultures, if the starting keratinocyte cell density in the cell suspension after trypsinization is too low the culture will take longer to reach confluency. In that case, while the phenotype of the monolayer is still polygonal, the number of ePUK cells produced is lower and what we term “giant and big rounded cells” (which are assumed to be differentiated or older cells or at least not “progenitor” cells, shown at day 16 or later in Fig. 2) appear in increased numbers. These giant cells are less common when the monolayer is formed quickly.
ePUK cells are cells that go into the overlying medium after “popping-off” the confluent monolayer in the culture. The morphology of cells in the monolayers of the initial culture flasks, from which the ePUK cells are then obtained, is shown. First row of pictures is for the frozen breast culture monolayer; the second row for the frozen neonatal and the third row for the fresh neonatal. Irrespective of their origins, all cell strains (frozen or fresh) produced a confluent monolayer of polygonal or “cobblestone” cells (black arrows) occupying the whole surface of the flasks. Elongated, curved cells (white arrows) appear when the monolayer starts disappearing. The monolayers may show bigger rounded cells (indicated with *), which will limit the number of ePUKs produced because they occupy a larger area and perhaps because they are no longer capable of replicating. These large cells do not appear to produce ePUK cells. After a period of time, the cells released from the monolayer did not go into the overlying medium, but rather formed a stratified structure with cells aggregating and forming clumps that remained anchored to the monolayer (last column of pictures). Shaking the flasks did not dislodge the cells clumped and anchored. These late cells were not considered ePUKs and were never analyzed in this study. The ePUK cells can be seen in high concentrations in the medium, for instance in the first two columns of the images
The evolution of a typical monolayer is shown in the rows of images in Fig. 2. In the first row the ePUK0 frozen breast strain, at day 5 and 7 with several ePUKs and later days with a changed phenotype and decreased number of cells in suspension. Cells have changed from polygonal to elongate. At day 16, most cells were rounded and big and the rest elongated. This type of morphological changes is observed in all cultured flasks and may happen at different times and with different intensities. If the culture is continuously fed and maintained for long periods of time, two effects take place: first, there is the formation of multiple layers of cells—stratification-, where produced cells appear to concentrate, forming 3D structures and clumps. This starts as early as 2 weeks of culture. Later, the monolayer or multiple layers of cells disappear. These aggregations of cells are mostly anchored cells to the substrate and are not dislodged when shaken (last column of Fig. 2). All cultures will form these aggregations, including oral keratinocytes.
Similar behavior over time was observed for the monolayers formed by the ePUK cells when poured into new flasks (ePUK1 and ePUK2 flasks, Fig. 3). A common feature for all of them was that they formed monolayers with more elongate and larger rounded cells than their ePUK0 cultured monolayers; these ePUK1 monolayers in turn produced a lower number of ePUK cells themselves. This phenomenon was intensified for ePUK2 cultures, as seen in the last row of Fig. 3 for the ePUK2 of the frozen breast strain and ePUK2 of the fresh neonatal strains.
The evolution of some of the ePUK monolayers formed from the original flasks can be seen in these pictures. Some frozen strains may see their capacity to form monolayers decreased and retarded in ePUK cultures. For instance the frozen neonatal ePUK1 took longer to reach confluency and produce ePUKs than the frozen breast EPUK1 (see first and second rows). ePUK monolayers formed from fresh cells (third row) appear to be able to produce a denser monolayer than the frozen strains. An additional observation is that subsequent ePUK monolayers appear to develop more big cells quickly when they come from frozen strains versus fresh strains, as can be seen in fourth row. These observations indicate that to obtain the quicker and more heavily producing ePUK cells the most useful cultures are primary cells during the first week of culture
Number of cells produced
In Table S1 the total number of cells produced during the counting period (15 days for ePUK0 flasks and 11 days for ePUK1 flasks) is reported. Before reaching complete confluency the monolayers would already show cells popping into the medium. Broadly (Fig. 4), the number of ePUK cells increases, reaches a maximum and then steadily declines. The production of ePUKs shows cellular expansion, starting low, reaching a maximum and slowly decreasing afterwards. The maximum of ePUK production appears to occur between the 2nd to the 5th day of monolayer culture, as shown in Table S1. The maximum typically happens two days after starting counting for the ePUK0 flasks, and around the fourth day for the ePUK1 flasks. This is a general observation that occurs for both frozen and fresh, breast, neonatal or oral cells.
The average number of ePUK cells produced by several cultures in this study are compared in these graphs. The first day of counting represents confluency for each flask and the y axis indicates number of cells in the overlying medium per ml of spent medium. ePUK0 cultures produced more cells than the subsequent ePUK1 cultures (b). It is also shown that adult ePUK0 cultures produce more cells at the beginning of the culture than neonatal cells (c), but this effect appears reversed for production of cells of their corresponding ePUK1 cultures (d). While the average production of ePUK0 production appears similar for both fresh and frozen cells at the beginning of the culture, this reverses over time (a) and fresh ePUK1 cultures clearly showed more cell production than the frozen ePUK1 (e). Error bars are SEM
This relatively strong production of ePUK cells will continue for several days, in the range of 10–15 days. After this period the number of cells produced becomes “residual”, below 10,000 cells per ml of medium collected, so this was considered a ‘cut-off’ value for this study. After observing several flasks, however, the strains analyzed continued popping cells even after 2 months of continuous feeding and culturing (every day feeding the same volume) but the number of cells becomes so low that counting is unreliable. In general, the time window where the maximum number of ePUKs occurs is between the second and the eight day after confluency where most of the strains maintained a production of 50,000 cells per ml of medium or more.
The average production of ePUK cells is compared in the graphs of Fig. 4. It indicates that on average (taking together frozen and fresh strains and adult and neonatal) ePUK0 cultures produced more cells than the subsequent ePUK1 cultures (Fig. 4b). It is also shown that adult ePUK0 cultures produce more cells at the beginning of the culture than neonatal cells (Fig. 4c), but this effect appears reversed for production of cells of their corresponding ePUK1 cultures (Fig. 4d). While the average production of ePUK0 production appears similar for both fresh and frozen cells at the beginning of the culture, this reverses over time (Fig. 4b) and fresh ePUK1 cultures clearly showed more cell production than the frozen ePUK1 (Fig. 4e). In general, then, it can be concluded that ePUK1 or ePUK2 cultures always produce less ePUK cells compared to their ePUK0 cultures (individualized details of this can be seen in Figure S1 in the online supplementary material). The differences range from 50 to 300 % less and the decrease is less pronounced when using fresh cultures, instead of frozen cultures), indicating that using previously frozen cells decreases the number of ePUKs produced. In addition, ePUK flasks of previously frozen breast cultures reached confluency on only two cases, and subsequent flasks (POP2 and POP3) did not reach confluency and then no counting was performed (Table S1). The same happened to neonatal frozen cells. In contrast, most of the subsequent ePUK cultures (ePUK1, ePUK2, ePUK3) from fresh cells, including oral keratinocytes reached confluency.
Immunohistochemistry analysis
Figures 5 and 6 show graphs of the percentage of proteins present over time for all strains and some of the most relevant results are also highlighted in pictures in Fig. 7. It summarizes the presence of the proteins for the cell strains used. The cells show high levels of keratinization (K5/K14 remains always highly expressed, over 50 %) for all strains, fresh or cryopreserved. All ePUK cells showed a decline in p63 expression over the time period of the culture, reaching no expression and a loss of proliferative potential with declining Ki67. Keratin 10 was generally expressed at low levels or completely disappeared while the combination integrin α6bright/cd71dimm was generally absent from these cells, except in two strains (cryopreserved breast and neo), while the rest of the strains showed little presence of either protein.
Expression of proliferation (a, b, c, d, e) and differentiation (f, g, h, i, j) markers by cells produced in suspension (ePUKS) by P0 primary monolayers and ePUK1 monolayers. a, f Primary epidermal cell cultures; b, g Primary oral mucosal keratinizing cells. c, h Primary oral mucosal keratinizing ePUK1 cells. d, i Primary neonatal keratinocytes, and e, j Primary neonatal ePUK1cells. Zeros are zero percent expression
Expression of proliferation (a, b, c, d) and differentiation (e, f, g, h) markers by cells produced in suspension (ePUKS) by P3 monolayers and ePUK1 monolayers of previously frozen cells. a, e Frozen epidermal cell cultures; b, f Frozen epidermal ePUK1 cells. c, g Frozen neonatal cells. d, h Frozen neonatal ePUK1 cells. Zeros are zero percent expression
ePUK cells are keratinized cells and maintain high expression levels of K5/K14 for all strains and over the culture period (first column). In general, p63 and Ki67 were the proteins with the highest variability, with a decrease in their expression over time as seen in the examples (1st, 2nd and 3rd in the images refer to the sequential immunohistochemical analysis of ePUKs over time). Involucrin was generally highly expressed in all strains with the peculiarity that cryopreserved strains, in contrast to fresh strains, completely lost involucrin expression over time. The combination integrin α6bright/cd71dimm was generally absent from these cells, except in two strains (cryopreserved breast and neo) while the rest of the strains showed little presence of either protein
The evolution of the protein expression between different cell strains and their corresponding ePUK1 cultures can also be studied by observing Figs. 5 and 6. It can be noted, for instance, that the early ePUK cells produced by the ePUK0 cultures and the early ePUK cells produced by their corresponding ePUK1 flasks have different keratinization profiles, but these profiles, in general, tend to level-off, that is, become similar when the cells become “older”. For instance, the first ePUK cells evaluated from breast and its ePUK1 flask show that ePUK cells from the ePUK1 flasks have less p63 and Ki67 expression than the breast ePUK cells at the same comparative time of culture, or that they completely loss involucrin. These changes appear to depend on cell strain because oral cells did not show the same behavior. It can also be observed that cryopreservation affects more the ePUK cells of the subsequent ePUK1 flasks than the ePUK cells of the ePUK0 flasks. This can be seen in Figure S1, where the proteins expressed initially by the cryopreserved neonatal and the fresh neonatal are similar than the ones expressed by the neonatal and its ePUK1 culture.
Discussion
In a previous report (Marcelo et al. 2012), we presented a technique that produced epithelial cell strains with a high percentage of small diameter cells. These small diameter cells have proliferative potential and grow in a coordinated monolayer/suspension. The cells are fed once a day, with 2–3 times the amount of medium (Epilife®, serum and fatty acid free, low calcium). The cells maintain active cell proliferation at confluence so that the progeny cells are pushed or popped into the overlying medium. The spent medium cell suspension is then poured into a new flask (Fig. 1), where the cells re-attached, forming a new monolayer. When the new flask becomes confluent, the monolayer continues to produce ePUK cells, forming a new monolayer upon transfer to another flask, expanding the cell strain over time. In addition, when the original flask stops producing ePUKs the monolayer is seen to consist of large, apparently “aged” cells after 10–15 days. Of significance is that the original ePUK producing cell monolayer is never trypsinized and is used exclusively to produce ePUKs in the way described. Several other interesting characteristics of this novel culturing technique are (1) high nutrient requirement, as indexed by high glucose utilization and (2) relatively smaller diameter cell fraction (suggesting that an early progenitor or stem-like cell is supported by this culture method since early progenitor/stem-like epithelial cells are theorized to be small diameter cells) or the lack of differentiation.
Given the novelty of the technique, and the fact that it constitutes a completely new way of culturing large numbers of “active” epithelial cells, much remains to be studied regarding this unique culturing technique. All cell strains followed the same pattern of morphological changes over time (Figs. 2, 3) during a period of time that ranges between 10 and 15 days and which varied for different cell strains: breast, neonatal, abdominal or oral mucosa. However, this variability does not prevent the popping of the ePUK cells from the monolayer, just the number of cells and the period of time that may be consider useful. The conclusion then is that the technique can be used to multiply a diverse group of epithelial cells.
The observations that the cells form multiple layers, grow larger in size and start to aggregate by anchoring to the monolayer suggests that the monolayer is following a process of differentiation and stratification and that this process limits the number of days during which the culture maintains an active cellular production. This behavior –stratification- is similar to regularly cultured cells. If the cells are not fed every day they will revert in behavior to a normal passaged cell culture (Marcelo et al. 2012). The cells in the monolayer become similar to terminally differentiated cells although the keratinization profile of the monolayer of aggregated cells was not analyzed. This concept is supported by the fact that the ePUK cells show a general decrease over time in the p63 expression (Figs. 5, 6, 7), meaning that they are becoming less and less “basal-like” cells (Pellegrini et al. 2001). Additional indications for this are seen with the slight increase over time of keratin K5/K14 (Fig. 5) which is considered a fundamental indicator of keratinization in epithelia. The decrease in Ki67 (Fig. 5) indicates that for ePUK cells originating from the same flask, the popped cells progressively have less proliferative capacity. The observations (Figs. 5 and 6), that the first ePUK cells produced by the ePUK0 cultures and the first ePUK cells produced by their corresponding ePUK1 flasks have different initial keratinization profiles suggest that the process of popping from the monolayer and then attaching to a new substrate modifies the keratinocyte replicative process and keratinization profile in a cell-strain dependent manner.
An important question to ask is “What should be the best combination of markers that ePUK cells should have to be used for tissue engineering and clinical applications to maximize their wound healing and regenerative potential?” The observations presented in this work suggest a possible dual use of the cells depending on the date of production. ePUK cells produced during the first 2–4 days after confluency (when they reach the maxima) are more basal-like and proliferative. Cells collected at the end of the period (8–15 days) are more differentiated. We do not know of reports with a possible differential use of the cells depending on collection times when they are cultured using regular culturing techniques but this possibility may be explored in more detail.
A general assumption is that, in tissue engineering and regenerative medicine, it is best to use stem-like or early progenitor cells that have the highest potential for continued growth and repair. In spite of the lack of expression of the combination α6β4 integrinbri/CD71dim phenotype (which is assumed to indicate stem cell-like cells (Webb et al. 2004) the cells during all periods of culture maintained sufficient proliferative capacity and were able to grow on dermal equivalents (Alloderm®) when cultured at the air–liquid interface, as do keratinocytes from traditional cultures that require trypsinization to be harvested from monolayer cultures, as shown in Marcelo et al. (2012). Cryopreserved cells showed staining for involucrin during the first days of culture and almost 100 % of cells expressed involucrin in ePUKs of fresh cultures. This indicated that involucrin expression is also affected by cryopreservation and is a highly preserved marker in these ePUK keratinocytes.
An estimation of the possible use of the ePUK cells can be done by analysis of the number of cells produced over time (Fig. 4). In general, it is thought that for tissue engineering and clinical applications a rapid production of cells is necessary, for example, to cover wounds in burn patients. Based on our results, to maximize production of ePUK cells the strategy would be to use primary strains (instead of cryopreserved) seeded at the highest possible density. This will quickly induce the formation of a packed keratinocyte monolayer reducing the number of cells with a differentiated phenotype. A possible reason for this phenomenon may be that low seeded, isolated cells have signaling mechanisms to “differentiate” faster if they are not surrounded by other cells or a monolayer, to create a more optimal niche, but the reason for this is unknown.
The fact that all strains produced ePUK cells indicates that the technique can be used to expand cells from all epithelial tissues and that this expansion (total number of cells obtained) is similar for all ePUK0 flasks (around 20 million per T75 flask). Experiments using T150 flasks (not shown) indicated that an ePUK0 culture of two or three T150 flasks and their corresponding ePUK flasks can produce 1 billion cells in approximately 2 weeks. To improve this production further and make it in a continuous basis, we have suggested the design and use of bioreactors that could possibly maintain the monolayers in active form for much longer periods of time by continuous feeding of the culture, instead of a single medium change every 24 h (Marcelo et al. 2012). For comparison, the number of cells used in aerosols of keratinocytes to cover wounds in patients is reported to be just 500 K to cover a 25 cm2 area (Duncan et al. 2005). This number of cells can be produced by a single T75 ePUK flask in a single day cultured with 35 ml of medium.
Conclusions
While it is still early to determine all possible uses for the ePUK cultures, investigators considering using this novel culture system for epithelial cell banking and expansion should take into account the following statements that summarize our observations.
-
ePUK cells are produced in large numbers for a few days, normally up to 8–10 days after confluency. They will continue to be produced but at a reduced rate after 10 days. The quality of the cells for specific applications will need to be assessed based on their differential characteristics.
-
ePUK cells from several epithelial tissues can be produced.
-
Cryopreservation is possible but reduces the number of ePUK cells produced by the monolayers.
-
ePUK cells are keratinized cells. The cells produced by a single flask over time maintain the keratinization profile of regular epithelial cells (for instance K5/K14 and some K10 expression) but lower their proliferation potential (less Ki67) and become less basal-like cells (decreased p63) and more differentiated cells (phenotypic observations).
-
ePUK cell characteristics are not affected by enzymatic treatment, which is never used in this culturing technique.
References
Bavister BD, Wolf DP, Brenner CA (2005) Challenges of primate embryonic stem cell research. Clon Stem Cells 7:82–94
Dragúňová J, Kabát P, Koller J (2012) Skin explant cultures as a source of keratinocytes for cultivation. Cell Tissue Bank. doi:10.1007/s10561-012-9330-4
Duncan CO, Shelton RM, Navsaria H, Balderson DS, Papini RP, Barralet JE (2005) In vitro transfer of keratinocytes: comparison of transfer from fibrin membrane and delivery by aerosol spray. J Biomed Mater Res B Appl Biomater 73:221–228
Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ (2010) Genetic engineering of mesenchymal stem cells and its application in human diseases therapy. Hum Gene Ther 21:1513–1526
Lei P, Andreadis ST (2008) Efficient retroviral gene transfer to epidermal stem cells. Methods Mol Biol 433:367–379
Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, Downes M, Yu R, Stewart R, Ren B, Thomson JA, Evans RM, Ecker JR (2011) Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 471:68–73
Marcelo CL, Duell EA, Rhodes LM, Dunham WR (1992) In vitro model of essential fatty acid deficiency. J Invest Dermatol 99:703–708
Marcelo CL, Peramo A, Ambati A, Feinberg SE (2012) Characterization of an unique technique for culturing primary epithelial progenitor/stem-cells. BMC Dermatol 12:8
Mignone JL, Kreutziger KL, Paige SL, Murry CE (2010) Cardiogenesis from human embryoinic stem cells. Circ J 74:2517–2526
Oliveira AA Jr, Hodges HM (2005) Alzheimer’s disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res 2:79–95
Pellegrini G, Dellambra R, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M (2001) P63 identifies keratinocytes stem cells. Proc Natl Acad Sci USA 98:3156–3161
Webb A, Li A, Kaur P (2004) Location and phenotype of human adult keratinocyte strain cells of the skin. Differentiation 72:387–395
Acknowledgments
This work was supported by grant R01AM-26009 to CLM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10561_2012_9343_MOESM2_ESM.tif
Figure S1 Comparison of cell production for the pairs ePUK0-ePUK1 for different cell strains. ePUK production of frozen strains show a substantial decrease in the ePUK1 flasks. In fresh cells, neonatal strains maintain a similar production from the beginning, but oral ePUK1 cells grow more slowly (TIFF 180 kb)
Rights and permissions
About this article
Cite this article
Peramo, A., Feinberg, S.E. & Marcelo, C.L. Characterization of cultured epithelial cells using a novel technique not requiring enzymatic digestion for subculturing. Cell Tissue Bank 14, 423–435 (2013). https://doi.org/10.1007/s10561-012-9343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9343-z