Abstract
Patellar tendon auto- and allo-grafts are commonly used in orthopedic surgery for reconstruction of the anterior cruciate ligaments (ACL). Autografts are mainly used for primary reconstruction, while allografts are useful for revision surgery. To avoid the risk of infectious disease transmission allografts should be radiation-sterilised. As radiation-sterilisation supposedly decreases the mechanical strength of tendon it is important to establish methods of allograft preservation and sterilisation assuring the best quality of grafts and their safety at the same time. Therefore, the purpose of this study was to compare the tensile strength of human patellar tendon (cut out as for ACL reconstruction), preserved by various methods (deep fresh freezing, glycerolisation, lyophilisation) and subsequently radiation-sterilised with doses of 0, 25, 50 or 100 kGy. Bone-Tendon-Bone grafts (BTB) were prepared from cadaveric human patella tendons with both patellar and tibial attachments. BTB grafts were preserved by deep freezing, glycerolisation or lyophilisation and were subsequently radiation-sterilised with doses of 0 (control), 25, 50 or 100 kGy. All samples were subjected to mechanical failure tensile tests with the use of Instron system in order to estimate their mechanical properties. All lyophilised grafts were rehydrated before performing of those tests. Obtained mechanical tests results of examined grafts suggest that deep-frozen irradiated grafts retain their initial mechanical properties to an extent which does not exclude their clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patellar tendon auto- and allografts are commonly used in orthopedic surgery for reconstruction of the anterior cruciate ligaments (ACL). Autografts are mainly used for primary reconstruction, while allografts are useful for revision surgeries (Schrock and Jackson 1995; Beasley et al. 2005). During the last 30 years, there were over 400 surgical techniques developed and five conservative methods established for knee joint treatment.
Up to the 1980s surgical complications were observed in more than 50% of operated patients. Introduction of arthroscopic techniques has decreased surgical complications to 10–50% (Ferrari et al. 1999; Kartus et al. 2001). However, the longer the life-time of transplanted graft the higher the risk of its potential degeneration (Lee et al. 2004). Nowadays indications for revision surgery seem to appear in 5–25% patients (Beasley et al. 2005).
The risk of infectious disease transmission from donor to recipient limits the use of fresh frozen human tissue grafts. To avoid this risk, allografts should be radiation-sterilised (Fideler et al. 1995; Dziedzic-Gocławska and Kamiński 2004a). As radiation-sterilisation supposedly decreases the mechanical strength of tendon tissue, it is important to establish such methods of allografts preservation and sterilisation that would assure the best quality of grafts and their safety at the same time (Dziedzic-Gocławska et al. 2004b; Kamiński et al. 2004; Sherman and Banffy 2004; Schrock and Jackson 1995).
The aim of the study was to compare the tensile strength of human patellar tendon (cut out as for ACL reconstruction—i.e., the central part of it), preserved by various methods (deep freezing, glycerolisation or lyophilisation) and subsequently radiation-sterilised with doses of 0 (control), 25, 35, 50 or 100 kGy.
The doses in the range of 25–35 kGy are routinely used in many countries for sterilisation of tissue grafts (Schrock and Jackson 1995; Dziedzic-Gocławska and Kamiński 2004a; McDermott and Thomas 2005). Higher doses guarantee better safety but the possibility of collagen damage limits their use for radiation-sterilisation of grafts (Fideler et al. 1995; Dziedzic-Gocławska et al. 2004b; Kamiński et al. 2004).
Material and methods
Patellar tendons harvested from 25 male cadaveric donors aged 17–84 years were used for this experiment. Potential donors were qualified as tissue donors according to tissue bank standard procedure. Tissue procurement procedure, as well as the whole experiment, were approved by the Local Ethical Committee.
Left and right patellar tendons were procured up to 48 h after death. Bone-tendon-bone (BTB) allografts with both patellar and tibial attachments were prepared by removing lateral parts of the tendon and of the bone attachments so that only the central longitudinal part of the tendon (width 12 mm) with attachments remained—medial one-third of the initial tendon width (Fig. 1). Next, the fragments of tendons were processed at the Central Tissue Bank in Warsaw.
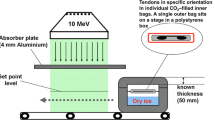
The grafts were divided into pairs to establish seven groups as described in Table 1. They were preserved by either deep freezing or glycerolisation or lyophilisation and subsequently radiation-sterilised with doses of 0, 25, 35, 50 or 100 kGy. All tendons except for the lyophilised ones were frozen at temperature −70°C. For lyophilised tendons residual water was <5% (ranged 2.9–3.4, mean 3.4%). Glycerolised tendons were incubated in 10% glycerol for 1 h and then freezed in −70°C with glycerol solution. One graft from each pair was radiation-sterilised, while the second one, which served as a control, was not irradiated (dose: 0 kGy). The sterilisation procedure was performed by electron beam at the Institute of Nuclear Chemistry and Technology in Warsaw.
All samples were subjected to failure tensile tests with the use of Instron testing machine (Matava and Hutton 1995) to estimate their mechanical properties. Special clamps were constructed to hold bone attachments in a way enabling imitation of mechanical challenges to which the graft is exposed in the recipient knee. Before mechanical tests were performed all lyophilised grafts were rehydrated by incubation in buffered saline over night till they regained wet weight comparable to the initial wet weight.
Anova test was used for statistical analysis of the obtained results.
Results
During mechanical testing of grafts, damages in attachment areas affecting both: bone (fractures) and tendon (fibres destruction) were relatively often observed. Failures located in tendon only, i.e., not affecting the bone attachments, were rare. On the whole, among 50 failed tendons 24 failures were localized in tibia attachment area, 20 in patellar attachment area, and only 6 in tendon area. What concerns the occurrence of failure localized only in tendon area in relation to the irradiation dose used the data are as follows: the failures were observed in two grafts irradiated with 25 kGy, one graft irradiated with 50 kGy, one graft irradiated with 100 kGy, one lyophilised non-irradiated graft and one lyophilised graft irradiated with 35 kGy from the same pair.
Tensile strength was calculated for each graft from values of tensile force measured and tendon cross section area.
In the first stage of the experiment the relation between donor’s age and graft tensile strength was estimated. The experimental group included 17 deep-frozen non-irradiated grafts. The donors of those grafts were divided into six age groups. Decrease in tensile strength was observed in grafts harvested from donors aged over 60 years (Fig. 2).
In the second stage of the experiment the influence of various irradiation doses on tensile strength was assessed. The experimental group included deep-frozen grafts exposed subsequently to irradiation. Values of tensile strength of an irradiated graft and its non-irradiated control, both harvested from the same donor, were compared. Tensile strength of an irradiated graft was presented as a percentage of tensile strength of its control, i.e., of a non-irradiated graft. Only statistically non-significant decrease in tensile strength was observed. This decrease ranged from 20 to 40% of the values for non-irradiated controls and it was not dose-dependant (Fig. 3).
In the third stage of the experiment the influence of different preservation methods on the tensile strength of grafts was assessed. Grafts preserved by different methods and subsequently irradiated with the same dose of 35 kGy were subjected to the failure tensile test. The controls were non-irradiated grafts preserved by the same method. Observed decrease in tensile strength was lower in case of grafts preserved by deep freezing and sterilised on dry-ice (−70°C). Tensile strength decrease was higher when grafts were preserved by lyophilisation or glycerolisation (Fig. 4). In all the compared groups differences in values of tensile strength were not statistically significant.
Tensile strength of grafts preserved by different methods and subsequently irradiated with the dose of 35 kGy in different temperatures, expressed as % of non-irradiated controls. Presented values indicate mean decrease of tensile strength. Bars indicate maximal and minimal values of calculated tensile strength decrease. A fresh frozen and sterilised on dry-ice; B fresh frozen and sterilised in room temperature; C glycerolised and sterilised on dry-ice; D lyophilised and sterilised in room temperature
Conclusions
The tensile strength of frozen BTB grafts retrieved from donors aged over 60 years was decreased. The tensile strength of fresh frozen radiation-sterilised BTB grafts was approximately 20% lower as compared to non-irradiated controls. There were no significant dose-related differences in a range of investigated doses (25–100 kGy).
The preservation method (deep freezing, glycerolisation or lyophilisation) and the temperature during irradiation (dry-ice temperature or room temperature) may affect the tensile strength. Deep freezing seems to be the most efficient method for preservation of BTB allografts if subsequently radiation-sterilised.
Obtained mechanical tests results of examined grafts suggest that deep-frozen irradiated grafts retain their initial mechanical properties to an extent which does not exclude their clinical application.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- BTB:
-
Bone-tendon-bone graft
- Gy:
-
Grey
References
Beasley LS, Weiland DE, Vidal AF et al (2005) Anterior cruciate ligament reconstruction: a literature review of anatomy, biomechanics, surgical considerations, and clinical outcomes. Oper Tech Orthop 15:5–19. doi:10.1053/j.oto.2004.11.003
Dziedzic-Gocławska A, Kamiński A (2004) Procedury bankowania tkanek. In: Rowiński W, Wałaszewski J, Pączek L (eds) Transplantologia kliniczna. PZWL, Warszawa, pp 617–623
Dziedzic-Gocławska A, Kamiński A, Uhrynowska-Tyszkiewicz I (2004) Wpływ metod konserwacji i warunków sterylizacji radiacyjnej na degradację kolagenu. In: Dziedzic-Gocławska A, Ostrowski K (eds) 40 lat bankowania i sterylizacji radiacyjnej tkanek w Polsce. Zakład Transplantologii i Centralny Bank Tkanek, Warszawa, pp 313–318
Ferrari JD, Bush-Joseph CA, Bach BR (1999) Anterior cruciate ligament reconstruction using bone-patellar tendon-bone grafts. Oper Tech Sports Med 4(7):156–171. doi:10.1016/S1060-1872(99)80022-7
Fideler BM, Vangsness CT, Lu B et al (1995) Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med 5(23):643–646. doi:10.1177/036354659502300521
Kamiński A, Komender A, Dziedzic-Gocławska A (2004) Wpływ metod konserwacji i warunków sterylizacji radiacyjnej na wytrzymałość mechaniczną kości. In: Dziedzic-Gocławska A, Ostrowski K (eds) 40 lat bankowania i sterylizacji radiacyjnej tkanek w Polsce. Zakład Transplantologii i Centralny Bank Tkanek, Warszawa, pp 313–318
Kartus J, Movin T, Karlsson J (2001) Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 9(17):971–980. doi:10.1053/jars.2001.28979
Lee CA, Meyer JV, Shilt JS et al (2004) Allograft maturation in anterior cruciate ligament reconstruction. Arthroscopy 20(Suppl 2):46–49. doi:10.1016/j.arthro.2004.04.009
Matava MJ, Hutton WC (1995) A biomechanical comparison between the central one-third patellar tendon and the residual tendon. Br J Sports Med 3(29):178–184
McDermott I, Thomas NP (2005) Tendon allografts in the knee. Knee 12:401–404
Schrock KB, Jackson DW (1995) Allograft reconstruction of the anterior cruciate ligament: basic science. Oper Tech Sports Med 3(3):139–147. doi:10.1016/S1060-1872(95)80002-6
Sherman OH, Banffy MB (2004) Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy 9(20):974–980
Author information
Authors and Affiliations
Corresponding author
Additional information
All conducted experiments were approved by the Local Ethical Committee.
Rights and permissions
About this article
Cite this article
Kamiński, A., Gut, G., Marowska, J. et al. Mechanical properties of radiation-sterilised human Bone-Tendon-Bone grafts preserved by different methods. Cell Tissue Bank 10, 215–219 (2009). https://doi.org/10.1007/s10561-008-9112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-008-9112-1