Abstract
Introduction
The goals of our study were to evaluate the biomechanical differences between five tendons and the changes in biomechanical properties caused by irradiation.
Methods
Achilles, quadriceps, semitendinosus + gracilis (STG), tibialis anterior (TA) and the peroneus longus (PL) were harvested from 30 donors. Group A contained 50 tendons without gamma irradiation. The groups were irradiated with a dose of 21 kGy (group B 50 tendons) and with a dose of 42 kGy (group C 50 tendons). The grafts were soaked in a radio-protectant solution and frozen at −80 °C. Cyclic loading tests were performed followed by load to failure tests. Young modulus of elasticity, maximum force, strain at tensile strength and strain at rupture were calculated.
Results
The Achilles tendons had significantly lower Young modulus than the TA (p = 0.0036) in group A. The Achilles showed significantly lower than PL (p = 0.000042) and TA (p = 0.00142) in group B and C. The quadriceps and the ST (p = 0.0037) provided poorer values than the TA (p = 0.0432) in group C. We found no difference in maximum loads among the tendons in group A. The maximum load of the Achilles and quadriceps showed better results than the PL (p = 0.0016), (p = 0.0018) and the STG (p = 0.0066), (p = 0.0019) in group C. The TA had similar results like the Achilles and quadriceps.
Discussion and conclusions
The vulnerability of gamma irradiation of TA was less than Achilles and quadriceps tendons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ACL reconstruction has become a common procedure for ACL-deficient knees [1]. The incidence of ACL injuries is estimated at about 250,000 cases per year [2], and from this approximately 130,000 ACL reconstructions are undertaken in the US [3]. The demand of ACL allografts has increased in recent years, and the post-operative results are promising. It is visible in the allografts use from 2% (between 1986 and 1996) to 14% (between 1996 and 2001) [4]. Cvetnovich et al. carried out a meta-analysis comparing the clinical outcomes of patients undergoing ACL reconstruction with hamstring autografts and those undergoing ACL reconstruction with soft-tissue allografts and found no significant differences [5]. The comparison of functional outcome, re-operations, septic complications and arthrofibrosis showed no significant differences. Usage of allografts could decrease the operation time, post-operative bleeding and donor site pain [6]. However, there are risks associated with the use of allografts, most notably disease transmission—both bacterial and viral, such as human immunodeficiency virus (HIV) and hepatitis [7], and in some cases fatal septic complications could be observed [8]. Several efforts were implemented to eliminate this adverse effect of allografts, among other antibiotic soaks or washing of tendons in ethanol solutions [9]. The penetration of these liquids into the graft tissue is questionable, and it has no antiviral effect. Chemical sterilization with ethylene oxide also had several side effects. It caused chronic synovitis, delayed incorporation or even dissolution of the grafts [9]. Electron beam irradiation was also a promising technique, but its effect is questionable. Once it was reported e-beam severely damaged the biomechanical properties of the tendons [10], on the other hand its damage effect on tendons is less than gamma irradiation [4].
One of the most accepted procedures to minimize the risk of disease transmission by allograft tissue, is gamma irradiation. The pathogen inactivation is dose dependent. Greaves et al. found that lower doses of gamma irradiation (10–15 kGy) had only a bactericide effect [11]. For complete virucidal sterilization, a radiation dosage of 30–50 kGy is required [12]. This method can damage the structure of the tendons and can decrease their biomechanical properties [9] and it could result and increseasd laxity or early tear of the graft and these could decrease the outcome of ACL reconstructions. Irradiation affects the biomechanical properties of allografts through two mechanisms. Firstly, gamma rays split the polypeptide chains of collagen fibres in a direct manner [13]. Secondly, gamma rays indirectly lead to radiolysis of water molecules and then release free radicals which injure the collagen [14]. This side effect could be decreased by using a radio-protectant solution, scavenging the free radicals [15].
The first purpose of our study was to biomechanically evaluate the initial biomechanical properties of five different types of freshly frozen tendon allografts used in knee ligamentous reconstruction: Achilles, doubled semitendinosus and gracilis (STG), doubled tibialis anterior (TA), doubled peroneus longus (PL) and quadriceps. Our hypothesis was there is no difference between the grafts in the initial biomechanical properties. Secondly, we evaluate the changes in graft biomechanical properties caused by gamma irradiation. Our hypothesis was there is no difference in biomechanical properties of five different types of tendon allografts caused by gamma irradiation. We hope our results can provide help in choosing the best tendon allograft for ACL reconstruction.
Materials and methods
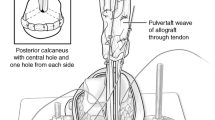
Our study included 30 human cadavers, from which 150 grafts were collected. From every donor, five types of grafts were harvested: Achilles, quadriceps, semitendinosus + gracilis (STG), tibialis anterior (TA) and peroneus longus (PL). The tendons were harvested from donors within 24 hours from death. The TA, STG, PL tendons were harvested from the musculotendinous junction. All soft tissue—including the paratenon—was removed from around the tendons. The mid-thirds of the quadriceps and the Achilles tendons were used. We used only the free ends of the grafts, because of measuring difficulties, as we described previously [16]. All tendons were visually and mechanically screened for degenerative changes. There was no previous history or evidence of injury or disease of the tendons in the patient’s documentation. The grafts were soaked in a radio-protectant solution that contained 16.7% 1,2-propanediol, 24.2% dimethyl-sulfoxide, 3.8% D-trehalose, 2.7% D-mannitol all w/w (Sigma-Aldrich, Saint Louis, USA) for 4 h at 40 °C with agitation and 24 hours at 4 °C according to Grieb et al. [15]. Each specimen was labelled in a separate container and frozen slowly at −80 °C [15]. The grafts were divided into three groups. Group A contained 50 frozen specimens (average age of donors 79.9 ± 16.82 years). Another 50 tendons of group B (average age of donors 83.61 ± 8.64 years) were irradiated with a target dose of 21 kGy (dose range 18–24 kGy, this is bactericidal dose) and the last 50 specimens (group C, average age of donors 81.55 ± 10.14 years) were irradiated with a target dose of 42 kGy (dose range 38–46 kGy, this is virucidal dose). We found no significant difference between the ages of groups. The irradiations were done on frozen grafts. Great care was taken not to elevate the temperature of the tendons. The grafts were thawed at room temperature on the day of biomechanical testing. Before testing, the cross-sectional area and the inter-clamp length of the tendons was measured. We used a micrometric caliper. We averaged the cross-sectional area of three levels along the tendons, to decrease the measurement mistakes (Table 1). Instron 8872 servo-hydraulic load frame (Instron Ltd., High Wycombe, UK) equipped with a 25 kN load capacity Instron Dynacell load cell and an Instron Fasttrack 8800 control unit was used for the endurance tests. The grafts were fixed into the frozen clamps (Fig. 1) [16]. During our pre-tests three minutes of freezing was ideal to reach the appropriate fixation and prevent the freezing of the grafts [16]. Before tensile testing, pre-tensioning was applied with 50 N for 30 seconds. During cyclic loading tests, the specimens were cycled between 50 and 250 N for 1000 cycles at 2 Hz frequency, and then a load to failure test was performed. The test speed was 20 mm/minutes.
Biomechanical parameters were calculated based on measured geometry data, crosshead displacement and force–elongation curves registered by the tensile tester. Young’s modulus values were evaluated as the slope of the linear region of the stress–strain curves. The first progressive linear region of the curves has been manually selected for each curve between typically 25 and 45% of the maximum force. Between these points the moduli have been calculated by fitting a line with linear regression to the measured curve sections. The coefficient of correlation was used to validate the selection of the linear region.
Statistical analysis
Statistical analyses were performed with Statistica 8.0 (Statsoft Inc, Tulsa, OK, USA). Data were presented as median with the corresponding interquartile range (25–75% percentile). For group comparisons of variables the Kruskal-Wallis test were used. Multiple comparisons of mean ranks for all groups were applied for post hoc analyses. In all analyses, a p value of less than 0.05 was considered statistically significant.
Results
To compare the biomechanical properties of the specimens, the following four parameters were used: Young modulus of elasticity, maximum load, strain at tensile strength and strain at rupture.
In group A, we compared the Young modulus of elasticity of the tendons, which resulted in significantly lower values of the Achilles tendon as compared to the TA (p = 0.0036). Additionally, we found no difference in maximum loads among the tendons, but the results of the STG were inferior to the other four tendons. The strain at tensile strengths of the Achilles tendons was significantly higher than that of the ST (p = 0.0016), TA (p = 0.042) and the quadriceps (p = 0.002). The strain at rupture of the Achilles was inferior to the ST (p = 0.0103) and TA (p = 0.0199) (Tables 2 and 3, Figs. 2, 3, 4 and 5).
Values of tendons of Young’s moduli of elasticity. Median, 25%, 75% percentile minimum and maximum values are used. The letters show the significant difference between the tendons. The exact p values are written in the text. The A-B-C letters on the X axis shows the 3 different groups: A non-irradiated, B irradiated with 21 kGy, C irradiated with 42 kGy
Values of tendons of load to failure forces. Median, 25 and 75% percentile are used. Median, 25%, 75% percentile minimum and maximum values are used. The letters show the significant difference between the tendons. The exact p values are written in the text. The A-B-C letters on the X axis shows the 3 different groups: A non-irradiated, B irradiated with 21 kGy, C irradiated with 42 kGy
Values of tendons of strain at tensile strength. Median, 25 and 75% percentile are used. Median, 25%, 75% percentile minimum and maximum values are used. The letters show the significant difference between the tendons. The exact p values are written in the text. The A-B-C letters on the X axis shows the 3 different groups: A non-irradiated, B irradiated with 21 kGy, C irradiated with 42 kGy
Values of tendons of strain at rupture. Median, 25 and 75% percentile are used. Median, 25%, 75% percentile minimum and maximum values are used. The letters show the significant difference between the tendons. The exact p values are written in the text. The A-B-C letters on the X axis shows the 3 different groups: A non-irradiated, B irradiated with 21 kGy, C irradiated with 42 kGy
In group B, we first compared the Young modulus of elasticity of the tendons; we found that the Achilles reached significantly lower values than the quadriceps (p = 0.0042), the PL (p = 0.028) and the TA (p = 0.0001). After evaluating the maximum loads, the results of the STG tendons were significantly lower than those of the Achilles (p = 0.002) and quadriceps (p = 0.01). There was no difference between the Achilles, quadriceps and the TA. The strain at tensile strength of the Achilles were inferior compared to the quadriceps (p = 0.017) and the STG tendons (p = 0.000056). The strain at break of the Achilles demonstrated significantly inferior results compared to the STG (p = 0.000029), PL (p = 0.000032) and TA (p = 0.00044). Likewise, the quadriceps provided poorer results when compared to the STG (p = 0.0215) and TA alone (p = 0.0232) (Tables 2 and 3, Figs. 2, 3, 4 and 5).
Similarly, in group C we compared the Young modulus of elasticity, and found that the Achilles reached significantly lower values than the PL (p = 0.000042) and TA (p = 0.00142). The quadriceps performed worse than the TA (p = 0.0037), and the STG also provided poorer values than the TA (p = 0.0432). The maximum load of the Achilles showed better results than the PL (p = 0.0016) and the STG (p = 0.0066). The quadriceps also showed better results than PL (p = 0.0018) and STG (p = 0.0019). The TA had similar results as the Achilles and quadriceps. The strain at tensile strengths of Achilles were significantly less resistant to the tensile forces than the STG (p = 0.0166), PL (p = 0.0039) and TA (p = 0.004). Also, the quadriceps were inferior compared to TA (p = 0.00226) or to the PL (p = 0.0006). The strains at rupture of the Achilles had significantly lower values compared to the PL (p = 0.00045) and TA (p = 0.00066) and similarly, the quadriceps were significantly inferior to the PL (p = 0.00045) and TA (p = 0.00066) (Tables 2 and 3, Figs. 2, 3, 4 and 5).
Comparing the effect of gamma irradiation on each tendon the worst results were found in the case of the quadriceps tendons. Young modulus of elasticity significantly decreased, however only when group B vs. C were compared (p = 0.048). Both the 21 kGy and the 42 kGy gamma irradiation decreased the strain at tensile strength (group A vs. group C p = 0.0021, group B vs. group C p = 0.015) and strains at rupture (group A vs. group C p = 0.0108, group B vs. group C p = 0.0048). The maximum load was not affected by the irradiation. In the case of the other four tendons we found no difference in the examined parameters.
Discussion
The rises in the number of ACL reconstructions and the increasing use of allografts in such repairs have driven the need for an effective sterilization method that preserves the biomechanical integrity of the allografts [17]. The current study biomechanically evaluated five allografts for potential ligamentous reconstruction, which underwent different doses of gamma irradiation. Our results indicate that different tendons have different initial biomechanical properties. In our opinion the most important factor is Young modulus of elasticity, which describes the elasticity of the tendons at normal loading. Gamma irradiation of 42 kGy significantly decreased the Young modulus of elasticity in the case of the quadriceps. However, when the comparison was carried out between the groups, TA and PL were superior to Achilles at lower doses. At higher doses, the Achilles, STG and quadriceps were all inferior to TA in this parameter. Conrad et al. also reported a significant decrease in Young modulus of elasticity in the case of Achilles allografts after 15–25 kGy irradiation (292, 154 and 129 MPa) [18]. The doubled TA and PL tendons demonstrated the Young modulus of elasticity, maximum load, strain at tensile strength and strain at rupture that were equal to or better than all the other currently described ACL grafts. The Achilles had poorer endurance properties than the others. Similar results were published by Almqvist [19] and Pearsall [20]. They found that the biomechanical properties of TA tendons were superior compared to the bone-patellar tendon-bone allografts [19]. In addition, our grafts were obtained in older patients, indicating that grafts harvested from younger donors may show even better biomechanical properties. Previously, it was reported that higher [21]—or in some studies also lower [17]—doses of irradiation could decrease these parameters.
The values of the load failure forces of the grafts obtained in this study were consistent with those in the literature [19–21]. Due to the fact that the load to failure force highly depends on the diameter of the tendon, statistical analysis of this parameter is questionable, but we used this parameter as a benchmark to other studies. The median values were above 2200 N in every type of tendon except STG in group A. The forces that affect the ACL during ground level walking (303–355 N) [22] are far from those load-to-failure forces that we measured in the three groups. The minimal criterion for expected loading during an aggressive early rehabilitation program is 450 N [23]. These forces are far from our maximum load to failure forces. Noyes [23] found the ultimate load of the native ACL in young adults to approach 2160 N. Another aspect that an orthopedic surgeon has to take into consideration is the endurance properties of the stitch-tendon borderline. Previously it was reported that the strength of stitches ranged from 318 to 381 N [16].
Mabe et al. measured similar strain at tensile strength, as did our study group, after 9–11 kGy irradiation of Achilles and quadriceps tendons (0.15 ± 0.07, 0.16 ± 0.02) [24]. In our study, both low and high doses of irradiation caused significant increases in the strain at tensile strengths, and strain at rupture parameters in the case of the Achilles and the quadriceps. This could mean that the higher the dose of gamma irradiation is, the lower the Achilles and quadriceps tendons’ capability to resist against elongation forces, and their capability of restabilization to their original length is also decreased. Conrad et al. also reported significant (p = 0.0061) increase in strain at failure when he compared the control and irradiated (15–25 kGy) tendons [18]. This could lead to an increased laxity of the knee after ACL reconstruction with allografts [5]. During these aforementioned loading forces, the Young’s modulus of elasticity and strain at maximal forces play a critical role in joint laxity. We found no difference in the case of the TA. In these force ranges, the irradiated allografts have the same elasticity properties as the controls. We measured similar results in the case of PL, however the 42 kGy irradiation significantly increased the strain at rupture parameter.
The measured main endurance parameters in the case of the TA did not change. Samsell reported that low-dose gamma irradiation did not affect the biomechanical properties of tibialis anterior allografts [25]. In our study we used 21 kGy target dose. Nevertheless, in the case of 42 kGy dose, we did not measure any changes of TA tendons with respect to Young modulus of elasticity, maximum load, strain at tensile strength and strain at rupture parameters.
There are certain limitations to this study that have to be mentioned. First, the efficiency of pathogen inactivation was not evaluated in this study; it was based on the previously reported references. We plan to perform further studies to explore the effect of virus and bacteria inactivation of this process. Also, we used a micrometric caliper to measure the length and cross-sectional area of the graft, whereas a laser micrometer system is more accurate. We tried to eliminate any measurement errors by averaging the cross-sectional area of three different levels along the tendons.
After low and high doses of gamma irradiation, the quadriceps and Achilles suffered the greatest decrease in biomechanical parameters. The effect of gamma irradiation on the STG and PL was slight, but the initial properties were not as good as the TA. Additionally, semitendinosus tendons—if used alone—have to be folded into four strings; or when used with the gracilis, the two tendons have to be sutured together during the operation. In the case of the TA and PL, only a single loop is used.
The results of this study indicate that different types of tendons react differently to gamma irradiation. Achilles and quadriceps grafts were the most sensitive to gamma irradiation. It seemed that the vulnerability of irradiation of TA and slightly of the PL were less than that of the Achilles and quadriceps tendons.
These results indicate that our hypothesis cannot be validated.
Some conclusions can be drawn from our results. If a surgeon wants to use a fresh-frozen graft, then with the exception of the Achilles, all four kinds of grafts can be recommended. If a bacteria-free graft is the focus then TA, PL and STG can be used. If the highest level of microbiological safety is preferred, usage of TA graft can be the best choice. Certainly, additional donor screening can improve the safety of the grafts.
References
Takigami J, Hashimoto Y, Yamasaki S, Terai S, Nakamura H (2015) Direct bone-to-bone integration between recombinant human bone morphogenetic protein-2-injected tendon graft and tunnel wall in an anterior cruciate ligament reconstruction model. Int Orthop 39:1441–1447
Badran MA, Moemen DM (2016) Hamstring graft bacterial contamination during anterior cruciate ligament reconstruction: clinical and microbiological study. Int Orthop. doi:10.1007/s00264-016-3168-5
Schwarting T, Benölken M, Ruchholtz S, Frink M, Lechler P (2015) Bone morphogenetic protein-7 enhances bone-tendon integration in a murine in vitro co-culture model. Int Orthop 39:799–805
Hoburg A, Keshalf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss C, Scheffler S (2015) High-dose electron beam sterilization of soft tissue grafts maintains significantly improved biomechanical properties compared to standard gamma treatment. Cell Tissue Bank 16:219–226
Cvetanovich GL, Mascarenhas R, Saccomanno MF et al (2014) Hamstring autograft versus soft-tissue allograft in anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy 30:1616–1624
Schandl K, Horváthy DB, Doros A, Majzik E, Schwarz CM, Csönge L, Abkarovits G, Bucsi L, Lacza Z (2016) Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int Orthop. doi:10.1007/s00264-016-3246-8
Yanke AB, Bell R, Lee A et al (2013) The biomechanical effects of 1.0 to 1.2 Mrad of gamma irradiation on human bone-patellar tendon- bone allografts. Am J Sports Med 41:835–840
Kainer MA, Linden JV (2004) Closridium infections associated with musculoskeletal-tissue allografts. N Engl J Med 350:2564–2571
Scheffler SU, Scherler J, Pruss A, von Versen R, Weiler A (2005) Biomechanical comparison of human bone-patellar tendon- bone grafts after sterilization with peracetic acid ethanol. Cell Tissue Bank 6:109–115
Schmidt T, Hoburg A, Broziat C et al (2012) Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL). Cell Tissue Bank 13:387–400
Greaves L, Hecker A, Brown C (2008) The effect of donor age and low-dose gamma irradiationon the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med 36:1358–1366
Ng K, Wanivenhaus F, Chen T et al (2013) Differential cross-linking and radio-protective effects of geninpin on mature bovine and patella tendons. Cell Tissue Bank 14:21–32
Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W (2005) Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 6:201–219
Wei W, Liu Y, Yang X et al (2013) Fraction of 50 kGy electron beam irradiation: effects on biomechanich of human flexor digitorum superficialis tendons treated with absorbate. J Biomech 46:658–661
Grieb TA, Forng RY, Bogdansky S, Ronholdt C, Parks B, Drohan WN, Burgess WH, Lin J (2006) High-dose gamma irradiation for soft tissue allografts: high margin of safety with biomechanical integrity. J Orthop Res 24:1011–1018
Gy H, Pánics G, Szebényi G, Kiss R, Hangody L, Pap K (2016) Pitfalls during biomechanical testing—evaluation of different fixation methods for measuring tendons endurance properties. Acta Physiol Hung 103:86–93
Jung HJ, Vangipuram G, Fisher MB et al (2011) The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J Orthop Res 8:1193–1198
Conrad BP, Rappé M, Horodyski M, Farmer KW, Indelicato PA (2013) The effect of sterilization on mechanical properties of soft tissue allografts. Cell Tissue Bank 14:359–366
Almqvist KF, Jan H, Vercruysse C, Verbeeck R, Verdonk R (2007) The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: biomechanical properties. Knee Surg Sports Traumatol Arthrosc 15:1326–1330
Pearsall AW, Hollis MJ, Russel GV, Scheer Z (2003) A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthroscopy 19:1091–1096
Hoburg AT, Keshlaf S, Schmidt T et al (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allograft in anterior cruciate reconstruction. Am J Sports Med 38:1134–1140
Nagura T, Matsumoto H, Kiriyama Y, Chaudhari A, Andriacchi TP (2006) Tibiofemoral joint contact force in deep knee flexion and its consideration in knee osteoarhrosis and joint replacement. J Appl Biomech 22:305–313
Noyes F, Butler D, Grood E et al (1984) Biomechnical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66:344–352
Mabe I, Hunter S (2014) Quadriceps tendon allografts as an alternative to Achilles tendon allografts: a biomechanical comparison. Cell Tissue Bank 15:523–529
Samsell BJ, Moore AM (2012) Use of controlled low dose gamma irradiation to sterilize allograft tendons for ACL reconstruction: biomechanical and clinical perspectives. Cell Tissue Bank 13:217–223
Acknowledgements
This work was supported by the Hungarian Scientific Research Found (OTKA101070 and 116189 grants).
Gábor Szebényi acknowledges the financial support received through János Bolyai Scholarship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Funding
There is no other funding source than that mentioned in the acknowledgment.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hangody, G., Szebényi, G., Abonyi, B. et al. Does a different dose of gamma irradiation have the same effect on five different types of tendon allografts? — a biomechanical study. International Orthopaedics (SICOT) 41, 357–365 (2017). https://doi.org/10.1007/s00264-016-3336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3336-7