Abstract
This study was aimed to establish whether the cryopreservation procedure we currently use in clinics can modify arterial homograft antigenicity. To this purpose, we performed an immunohistochemical study on fresh and cryopreserved human arterial homografts to visualize the expression of HLA class I heavy and light chains “in situ” by using the HC-10 and Namb-1 monoclonal antibodies. Human femoral arteries and thoracic aortas were harvested from 18 heart-beating donors and sampled before and after cryopreservation. Arterial segments were frozen in liquid nitrogen vapors in a controlled rate freezing system. After thawing, samples were processed for routine immunohistochemistry. To standardize immunostaining, flow-cytometry indirect immunofluorescence analysis was performed on HUVEC; immunohistochemistry of human ovarian cortical vessels was performed as an additional positive control. Negative controls were performed by omitting tissue incubation with primary antibodies. HLA-class I antigens were markedly expressed by endothelial cells lining surface intima and adventitial vasa vasorum; a moderate expression was found in medial smooth muscle cells. Except for the surface unreactivity caused by loss of endothelium, results from cryopreserved arterial allografts were strictly comparable to those observed in fresh, unfrozen tissues. These results support the view that cryopreserved arterial allografts are immunogenic as their fresh counterparts; apart from smooth muscle cells which retained a moderate expression of HLA class I antigens following cryopreservation, our study suggests that the highly HC-10 positive endothelial cells we found to line the rich adventitial network of vasa vasorum are expected to be one of the major targets of the serological response in the recipient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serological studies report that cryopreserved arterial allografts, which have proven to be effective in the surgical treatment of arterial infections (Gabriel et al. 2004; Teebken et al. 2004), are immunogenic since they induce a strong and long-lasting donor-specific anti-HLA antibody response in the recipient (Smith et al. 1998; Welters et al. 2002; Mirelli et al. 2005). In most clinical settings, this serological reaction has been implicated in the process of chronic graft rejection; as to the arterial transplant, it is not yet clear whether this anti-HLA class I antibody response could further contribute to the degradation of the arterial homograft and its late clinical failure.
Arteries are truly sensitive conduits which can be structurally damaged in any phase related to cryopreservation; critical steps include preliminary graft handling (Song et al. 1994), cryoprotectant exposure (Song et al. 1995), cooling and warming rates (Hunt et al. 1994; Pegg et al. 1997). Recently we found that the standard cryopreservation protocol we use for reconstructive surgery results in irreversible damage of smooth muscle cells from multi-organ donor human thoracic aortas (Pasquinelli et al. 2006). This finding is not surprising since post-thawing functional studies performed on cryostored human arteries have documented a reduced performance of contractility in the graft (Müller-Schweinitzer et al. 1997; Vàzquez et al. 2004a; Vàzquez et al. 2004b; Stanke et al. 1998; Langerak et al. 2001) and human cryopreserved homografts are histologically found essentially acellular after implantation (Vogt et al. 1999).
However, the specific serological response against HLA class I antigens implies the presence of cells expressing HLA class I antigens.
The main goal of the present study was to establish whether the cryopreservation procedure we currently use for clinical applications can modify or maintain arterial homograft expression of HLA class I antigens. To this purpose, we performed an immunohistochemical study on routinely processed fresh and cryopreserved human arterial homografts to visualize the expression of HLA class I heavy chain and β2-microglobulin “in situ” by using the HC-10 and Namb-1 MoAbs, respectively.
Materials and methods
Fresh and cryopreserved arterial tissue
Human femoral arteries (n. 12) and thoracic aortas (n. 6) were harvested from 18 heart-beating donors (mean age 44 years; 11 females). After collection, the arteries were kept in a sterile box with Celsior media (IMTIX SANGSTAT, Lyon France), and transferred to the Cardiovascular Tissue Bank in isothermal boxes. Under a laminar flow, a 1 cm-long ring was cut from each artery and immediately fixed in 10% neutral buffered formaldehyde and routinely processed. These samples represented the fresh controls. After decontamination in RPMI 1640 with antibiotics for 72 h at 4°C, the remaining arterial segments were transferred into sterile bags containing 100 ml of fresh cryoprotectant solution, i.e., RPMI 1640 with human albumin (Kedrion, Lucca, Italy) and DMSO at a final concentration of 10%. The bags were frozen in liquid nitrogen vapors in a controlled rate freezing system (IceCube 1860, Sy-Lab, Wien Austria). The cryopreserved homografts were stored in the vapour phase of liquid nitrogen. Before any clinical use, cryopreserved arterial homografts were quickly submerged in a water bath at 39°C and let thaw for 10 min. When thawing was achieved, the bags were cut under sterile conditions and the samples were washed in cooled saline solution for 3 times to remove the cryoprotectant. After thawing, a 1 cm-long ring was removed from the graft, fixed in 10% neutral buffered formaldehyde and routinely processed.
HC-10 and Namb-1 antibodies
The HC-10 and Namb-1 MoAbs were generated, purified and characterized as described elsewhere (Perosa et al. 2003; Pellegrino et al. 1982; Stam et al. 1986). The HC-10 antibody recognizes an epitope expressed on virtually all β2-microglobulin-free HLA-B heavy chains and on β2-microglobulin-free HLA-A10, HLA-A28, HLA-A29, HLA-A30, HLA-A31, HLA-A32 and HLA-A33 heavy chains. The Namb-1 antibody recognizes both free and HLA class I heavy chain-associated human β2-microglobulin.
Flow-cytometry analysis
To standardize surface immunofluorescent staining, flow cytometry assay was performed on HUVEC (in vitro-cultured cord-derived endothelial cells expressing HLA class I antigens) obtained from umbilical cord samples as previously described (Jaffe et al. 1973). The HUVEC (gift of Dr. M. Brigotti, Dept. of Experimental Pathology, University of Bologna, Bologna, Italy) were maintained in Tc199 medium containing VEGF, heparin and 10% FBS and harvested after trypsin EDTA treatment. Cells were then washed twice with PBS containing 2% FBS and utilized for MoAb staining. In brief indirect fluorescence was performed as follows: 2–3 × 105 cells were first incubated with 5 μl of appropriate dilutions of monoclonal antibodies against heavy and light chain of HLA class I antigens. After 20 min at room temperature, cells were washed twice with PBS and incubated as above with FITC F(ab′)2 goat anti mouse Ig (FITC-GAM Ig; Beckman Coulter-Miami FL). After two washes cells were analysed with a FC500 flow cytometer (Beckman Coulter). Control samples were run with FITC-GAM Ig alone and positive controls with the W6/32 MoAb (Macdonald et al. 2002), recognizing the HLA class I complex. The W6/32 MoAb reacts on fresh intact cells or frozen tissues only, not on routine-processed tissues.
Immunohistochemical staining
About 5-μm sections from fresh and cryopreserved arterial segments were dewaxed with xylene and rehydrated through decreasing concentrations of ethanol. Endogenous peroxidase activity was blocked by a 20-min incubation at room temperature with absolute methanol containing 1.5% H2O2. To recover tissue antigenicity, tissue sections were immersed in a jar containing a Tris/HCl EDTA buffer (pH 9) and treated at 98°C in a water bath for 20 min. After rinsing in PBS (pH 7.4) tissue sections were incubated with HC-10 (1:600) and Namb-1 (1:50) MoAbs in a humidified chamber overnight at room temperature. Monoclonal antibody tissue labelling was revealed by using a non-biotin polymeric system (BioGenex Super SensitiveTM Polymer-HRP IHC Detection System, Biogenex, San Ramon, CA). Briefly, tissue sections were treated with the Super EnhancerTM Reagent for 20 min, washed in PBS and incubated with SS-HRP polymeric complexes for 30 min. After several washings in PBS, peroxidase activity was detected by incubating tissue sections for 2–3 min with a freshly prepared solution of 3,3′-diaminobenzidine (Sigma Chemicals Co., St. Louis, MO; 40 mg of 3,3′-diaminobenzidine in 100 ml of PBS containing 100 μl of H2O2). Tissue sections were counterstained with Mayer’s haematoxylin (Sigma Chemicals). Negative controls were done by omitting incubation with the primary antibody. Tissue sections were read independently by two investigators (GP and LF). Endothelial cells from venous, arterial and capillary vessels from samples of normal human ovarian cortex were considered as positive controls. The ovarian tissue was selected from the archives of our Institution.
Results
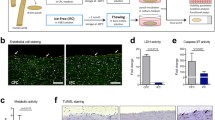
At flow-cytometry analysis, HUVEC were stained with HC-10, W6/32 and Namb-1 MoAbs (Fig. 1a–d). The whole cell population was found positive for HLA class I antigens with each MoAb, without any subset.
Representative examples of flow-cytometry (a–d) and immunohistochemistry (e–f) analyses used to standardize immunostaining with HC-10 and Namb-1 MoAbs. Immunofluorescence staining of unfixed HUVEC: (a) negative control; (b) HC10 MoAb; (c) W6/32 MoAb; (d) Namb-1 MoAb. Immunohistochemistry performed on fixed and routinely processed human ovarian cortical vessels: (e) HC-10 MoAb; (f) Namb-1 MoAb. Original magnification (e, f) 250 ×
By using the polymer technology of antigen–antibody revelation, endothelial cells from human cortical ovarian tissues showed an intensity of HC-10 (Fig. 1e) and Namb-1 (Fig. 1f) immunostaining which was comparable to that previously seen in ovarian (Vitale et al. 2005) and submandibolar gland (Mirandola et al. 2006) tissues.
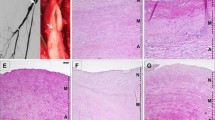
As to the comparison between fresh and cryopreserved samples, we found that in the intima of fresh arterial homografts, HLA class I antigens were markedly expressed by luminal endothelial cells; the positivity was intense and found both on cell membrane and in the cytoplasm; in the media smooth muscle cells showed a diffuse and moderate staining with HC-10; in the adventitia we found a well-developed network of vasa vasorum whose endothelial cell lining was intensely stained with a pattern similar to that of luminal endothelium; when compared to endothelial cells, smooth muscle cells of the same vessels showed a lower expression of the HLA class I antigens. The cell staining pattern of Namb-1 was similar to that of HC-10. Representative results are shown in Figs. 2a, c, e and 3a, c, e.
Representative examples illustrating the HC-10 immunoreactivities seen in the different layers of fixed and routinely processed samples from fresh (a, c, e) and cryopreserved (b, d, f) human arterial allografts. Note that no significant difference of HLA I heavy chain antigen expression was found between fresh and cryopreserved arterial allografts. HC-10 immunostaining is intense in endothelial cells lining the surface intima (a) and adventitial vasa vasorum (c, e, f), moderate and diffuse in medial smooth muscle cells (b, c, d). Original magnification (a–f) 100 ×
Representative examples illustrating the Namb-1 immunoreactivities seen in the different layers of fixed and routinely processed samples from fresh (a, c, e) and cryopreserved (b, d, f) human arterial allografts. Alike Fig. 2 no significant difference of HLA I light chain antigen expression was found between fresh and cryopreserved arterial allografts. Namb-1 pattern of immunostaining is comparable to that of HC-10. Original magnification (a–f) 100 ×
In cryopreserved arterial allografts the expression of HLA class I antigens was comparable to that observed in fresh, unfrozen tissues; however, as a consequence of surface endothelium damage induced by cryopreservation we found a decreased expression of HLA class I antigens in the luminal intima; no difference of HLA class I and β2-microglobulin expression was found in the adventitia with respect to fresh arterial tissue. Representative results are illustrated in Figs. 2b, d, f and 3b, d, f.
Discussion
It is yet unclear whether cryopreserved arterial allografts are less immunogenic than fresh tissue (Nagasaka et al. 2005; Oei et al. 2002; Solanes et al. 2004); in fact cryopreservation damages structurally and functionally endothelial and smooth muscle cells (Song et al. 1994; Song et al. 1995; Hunt et al. 1994; Pegg et al. 1997; Faggioli and Ricotta 1994; Pasquinelli et al. 2006; Müller-Schweinitzer et al. 1997; Vàzquez et al. 2004a; Vàzquez et al. 2004b; Stanke et al. 1998; Langerak et al. 2001). Conseguently, this procedure reduces the number of grafted cells which constitutively express HLA class I antigens (crucial for tissue histocompatibility); moreover, damage of surface endothelium, caused by cryopreservation (Faggioli and Ricotta 1994) might lead to the loss of a potential alternative source of antigen presentating cells.
However, the possible immunological advantage of cryopreservation is apparently in contrast with the serological results described in clinical studies. Immunological follow-ups of patients treated with cryopreserved arterial allografts unequivocably demonstrates that both humoral and cellular specific responses are raised in the recipients against donor cells within 30–60 days from surgery (Smith et al. 1998; Welters et al. 2002; Mirelli et al. 2005; Welters et al. 2001). Thus, taking these results altogether, it is conceivable to hypothesize that, although cryopreservation alters the number and viability of immunogenic cells, the immunogenic properties of the transplanted vessels are still maintained in the allograft. A straightforward conclusion could be that the residual cells in the allograft are fully capable to induce in the recipient an immune response against HLA class I antigens.
Very few studies have been performed on the “in situ” detection of HLA class antigens in cryopreserved arterial allografts (Oei et al. 2002; Verghese and Cherian 2002). In fact HLA class II antigen expression has been documented and this finding represents a major point to support the view that cryopreserved allografts are immunogenic. Instead the issue of HLA class I “in situ” expression has not yet fully addressed. HLA class I antigens are essential to initiate allograft rejection and recent reports suggest that, under proper experimental conditions, the interaction of endothelial cells with anti HLA class I antibodies induces the activation of Akt phosphorilation. This latter finding could be strictly related to proliferation or apoptosis of cell targets, depending on the involved cytotype as well as on the local microenvironment settings (Jin et al. 2002). In addition it might also be involved in the mechanisms of graft remodelling after implantantion.
To the best of our knowledge, we found a single report addressing this issue; according to Verghese and Cherian (2002) cryopreserved human aortic and pulmonary homografts do not express HLA class I antigens by immunohistochemistry analysis; our results are instead apparently different since we found that human aortic and femoral arteries still retain HLA class I expression following cryopreservation. Several reasons could explain these differences; in the Verghese and Cherian study the arterial tissues were obtained from cadaveric donors and consequently the initial cell integrity remains questionable; then direct immersion of arterial tissue in liquid nitrogen was used; however, this is a suboptimal method of cryopreservation which is known to deteriorate the tissue structure significantly; moreover, the Authors used a different MoAb to label HLA class I complexes; in fact the clone W6/32 recognizes a monomorphic conformational epitope on the 45 kDa polypeptide products of the HLA-A, B and C loci which is associated in a non-covalent manner to β2-microglobulin (Macdonald et al. 2002); finally, tissue processing was different since frozen sections and a conventional immunohistochemical method of revelation were used. In contrast, in our study arteries were obtained from heart-beating multiorgan donors and cryopreservation was achieved by using an automated controlled-rate freezing apparatus which allows the maintenance of improved tissue structure and function; moreover MoAbs specifically directed against heavy and light chains of HLA class I antigens and able to react on fixed, routinely processed samples were used. In particular, HC-10 recognizes an epitope expressed on virtually all β2-microglobulin-free HLA-B heavy chains and on selected β2-microglobulin-free HLA-A heavy chains whereas Namb-1 recognizes human free- and heavy chain associated-beta 2-microglobulin (Perosa et al. 2003; Pellegrino et al. 1982; Stam et al. 1986). The fact that both epitopes were found expressed in cryopreserved arteries could lead to the hypothesis that HLA class I molecules might be present in their native conformation. Finally, it should be noted that a new technology based on a non-biotin polymeric system aimed at increasing specificity of the reaction while decreasing the background staining was used to reveal the immunological reaction “in situ”.
In the present study we have demonstrated that cryopreserved arteries express HLA class I antigens. Besides surface endothelium, even smooth muscle cells and vasa vasorum stained positive with HC-10 and Namb-1 MoAbs. Although injured by cryopreservation (Pasquinelli et al. 2006) smooth muscle cells still retain a HLA class I expression comparable to that of fresh tissues; this finding is in agreement with the observation that smooth muscle cells cultured from cryopreserved human aortic homografts express HLA class I antigens on their surface as demonstrated by enzyme-linked immunoassay and immunohistochemistry (Salomon et al. 1993). An impressive finding was the high numbers of vasa vasorum found in our samples. Arterial allografts are prepared by removing adventitia from media; however this surgical procedure is not sufficiently adequate to remove the vasa vasorum which are intimately connected to the medial layer; in our arterial samples vasa vasorum were found between media and adventitia and in the outermost portion of the media; these vessels were lined with continuous endothelium highly expressing HLA class I antigens.
In conclusion by demonstrating the “in situ” expression of HLA class I antigens we provide further support to the view that cryopreserved arterial allografts are immunogenic as their fresh counterparts; medial smooth muscle cells retain moderate expression of HLA class I antigens and therefore are able to initiate the serological response against donor tissue; although the surface of arterial allografts was found to be largely deprived of endothelial cells, the vasa vasorum network lined with highly HC-10 positive endothelial cells is expected to be one of the major targets of the “in vivo” immunological response.
Abbreviations
- MoAbs:
-
Monoclonal antibodies
- HUVEC:
-
Human umbilical venous endothelial cells
- VEGF:
-
Vascular endothelial growth factor
- FBS:
-
Fetal bovine serum
References
Faggioli G, Ricotta JJ (1994) Cryopreserved vein homografts for arterial reconstruction. Eur J Vasc Surg 8:661–669
Gabriel M, Pulaski F, Dzieciuchowicz L, Oszhinis G, Checinski P (2004) Cryopreserved arterial allografts in the treatment of prosthetic graft infections. Eur J Vasc Endovasc Surg 27:590–596
Hunt CJ, Song YC, Bateson EAJ, Pegg DE (1994) Fractures in cryopreserved arteries. Cryobiology 31:506–515
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. J Clin Invest 52 (11):2745–2756
Jin Y-P, Singh RP, Du Z-Y, Rajasekaran AK, Rozengurt E, Reed EF (2002) Ligation of HLA class I molecules on endothelial cells induces phopshorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol 168: 5415–5423
Langerak SE, Groenink M, van der Wall EE, Wassenaar C, Vanbavel E, van Baal MC, Spaan JAE (2001) Impact of current cryopreservation procedures on mechanical and functional properties of human aortic homografts. Transpl Int 14:248–255
Macdonald W, Williams DS, Clements CS, Gorman JJ, Kjer-Nielsen L, Brooks AG, McCluskey J, Rossjohn J, Purcell AW (2002) Identification of a dominant self-ligand bound to three HLA B44 alleles and the preliminary crystallographic analysis of recombinant forms of each complex. FEBS Lett 527(1–3): 27–32
Mirandola P, Sponzilli I, Solenghi E, Micheloni C, Rinaldi L, Gobbi G, Vitale M (2006) Down-regolation of human leukocyte antigen class I and II and β2-microglobulin expression in human herpesvirus-7-infected cells. J Infec Dis 193:917–926
Mirelli M, Buzzi M, Pasquinelli G, Tazzari V, Testi G, Ricchi E, Conte R, Stella A (2005) Fresh and cryopreserved arterial homografts: immunological and clinical results. Transplant Proc 37: 2688–2691
Müller-Schweinitzer E, Mihatsch MJ, Schilling M, Haefeli W (1997) Functional recovery of human mesenteric and coronary arteries after cryopreservation at − 196°C medium. J Vasc Surg 25:743–750
Nagasaka S, Taniguchi S, Nakayama Y, Skaguchi H, Nishizaki K, Naito H, Morioka H (2005) In vivo study of the effects of cryopreservation on heart valve xenotransplantation. Cardiovasc Pathol 14:70–79
Oei FB, Stegmann AP, van der Ham F, Zondervan PE, Vaessen LM, Baan CC, Weimar W, Bogers AJ (2002) The presence of immune stimulatory cells in fresh and cryopreserved donor aortic and pulmonary valve allografts. J Heart Valve Dis 11:315–324
Pasquinelli G, Foroni L, Buzzi M, Tazzari PL, Vaselli C, Mirelli M, Gargiulo M, Conte R, Stella A (2006) Smooth muscle cell injury after cryopreservation of human thoracic aortas. Cryobiology 52:309–316
Pegg DE, Wusteman MC, Boylan S (1997) Fractures in cryopreserved elastic arteries. Cryobiology 34:183–192
Pellegrino MA, Ng AK, Russo C, Ferrone S (1982) Heteregeneous distribution of the determinants defined by monoclonal antibodies on HLA-A and B antigens bearing molecules. Transplantation 34:18–23
Perosa F, Luccarelli G, Prete M, Favonio E, Ferrone S, Dammacco F (2003) β2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 171: 1918–1926
Salomon RN, Friedman GB, Callow AD, Payne DD, Libby P (1993) Cryopreserved aortic homografts contain viable smooth muscle cells capable of expressing transplantation antigens. J Thorac Cardiovasc Surg 106:1173–1180
Smith JD, Hornick PI, Rasmi N, Rose ML, Yacoub MH (1998) Effect of HLA mismatching and antibody status on “homovital” aortic valve homograft performance. Ann Thorac Surg 66:S212–S215
Solanes N, Rigol M, Castellà M, Khabiri E, Raminez J, Segalés J, Roqué M, Agustì E, Pérez-Villa F, Roig E, Pomar JL, Sanz G, Heras M (2004) Cryopreservation alters antigenicity of allografts in a porcine model of transplant vasculopathy. Trasnplant Proc 36: 3288–3294
Song YC, Hunt CJ, Pegg DE. (1994) Cryopreservation of the common carotid artery of the rabbit. Cryobiology 31:317–329
Song YC, Pegg DE, Hunt CJ (1995) Cryopreservation of the common carotid artery of the rabbit: optimization of dymethyl sulfoxide concentration and cooling rate. Cryobiology 32:405–421
Stam NJ, Spits H, Ploegh HL (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 137:2299–2306
Stanke F, Riebel D, Carmine S, Cracowski JL, Caron F, Magne JL, Egelhoffer H, Bessard G, Devillier P (998) Functional assessment of human femoral arteries after cryopreservation. J Vasc Surg 28:273–283
Teebken OE, Pichlmaier MA, Brand S, Haverich A (2004) Cryopreserved arterial allografts for in situ reconstruction of infected arterial vessels. Eur J Vasc Endovasc Surg 27:597–602
Vázquez MER, Rodriguez Carbarcos M, Martínez Santos MV, Fernández Mallo RO et al (2004a) Functional assessment of cryopreserved human aortas for pharmaceutical research. Cell Tissue Bank 5:119–123
Vázquez MER, Rodriguez Carbarcos M, Fernández Mallo RO et al (2004b) Functional assessment of human femoral arteries after cryopreservation. Cryobiology 49:83–89
Verghese S, Cherian KM (2002) HLA expression in aortic and pulmonary homografts: effects of cryopreservation. Indian Heart J 54:394–398
Vitale M, Pelusi G, Taroni B, Gobbi G, Micheloni C, Mezzani R, Donato F, Wang X, Ferrone S (2005) HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clinical Cancer Res 11:67–72
Vogt PR, Stallmach T, Niederhäuser U, Schneider J, Zünd G, Lachat M, Künzli A, Turina MI (1999) Explanted cryopreserved allografts: a morphological and immunohistochemical comparison between arterial allografts and allografts heart valves from infants and adults. Eur J Cardio-Thoracic Sur 15:639–645
Welters MJ, Oei FB, Vaessen LM, Stegmann AP, Bogers AJ, Weimar W (2001) Increased numbers of circulating donor-specific T helper lymphocytes after human heart valve transplantation. Clin Exp Immunol 124:353–358
Welters MJ, Oei FB, Witvliet MD, Vaessen LM, Cromme-Dijkhuis AH, Bogers AJ, Weimar W, Claas FH (2002) A broad and strong humoral immune response to donor HLA after implantation of cryopreserved human heart valve allografts. Hum Immunol 63: 1019–1025
Acknowledgements
This work was partially funded by RFO (ex quota 60%)—Università degli Studi di Bologna, Bologna, Italy and by PRIN 2005—MIUR, Italy
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pasquinelli, G., Pistillo, M., Ricci, F. et al. The “in situ” expression of Human Leukocyte Antigen Class I antigens is not altered by cryopreservation in human arterial allografts. Cell Tissue Banking 8, 195–203 (2007). https://doi.org/10.1007/s10561-006-9025-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-006-9025-9