Abstract
Purpose of Review
Antibodies to human leukocyte antigens (HLA) are associated with adverse patient and allograft outcomes after heart transplantation. Non-HLA antibodies have increasingly been recognized as important mediators of rejection, cardiac allograft vasculopathy (CAV), and allograft injury. In particular, these antibodies have been implicated in a subset of heart transplant recipients who have clinical and/or pathologic evidence of antibody-mediated rejection in the absence of detectable antibodies against HLA.
Recent Findings
Non-HLA antigens have been identified to have important roles in both the innate and adaptive immune response in transplantation. These antigens are predominantly expressed on vascular endothelium of the donor heart and include major histocompatibility class I related chain A (MICA), G protein coupled receptor angiotensin II type 1 receptor (AT1R), cytoskeletal elements such as myosin and vimentin. A growing number of studies have demonstrated antibodies to these antigens in rejection and development of CAV. At present, non-HLA antibodies are not routinely monitored post-transplant, and laboratory evaluation remains non-standardized. Further investigation is required to improve the detection of non-HLA antibodies, define pathophysiological mechanisms involved in allograft injury, and better understand their impact on clinical outcomes.
Summary
Non-HLA antibodies have been identified as important mediators of rejection, allograft dysfunction, and CAV in heart transplantation. Ongoing investigations and improving laboratory detection methods will determine their potential role in post-transplant risk stratification and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survival and allograft outcomes after heart transplantation are limited by rejection and the development of cardiac allograft vasculopathy (CAV) [1]. Antibody-mediated rejection (AMR) is triggered by circulating antibodies in the transplant recipient directed against donor human leukocyte antigens (HLA). In addition, there is increasing recognition of an important pathogenic role for non-HLA antibodies. This is perhaps best supported by the demonstration of AMR in HLA identical sibling kidney transplants [2]. While several non-HLA antibodies have been implicated in AMR, their precise function and relative contribution to rejection, CAV, and allograft dysfunction remain unclear. Furthermore, potential adverse additive and synergistic interactions between non-HLA and HLA antibodies have been described but not well elucidated [2, 3]. In this paper, we will review key non-HLA antibodies in heart transplantation, their postulated mechanisms of injury, laboratory testing, and impact on allograft outcomes.
Non-HLA Antibody Mechanisms of Injury
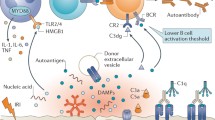
The development of non-HLA antibodies is dependent on cell injury, particularly ischemia reperfusion injury and cell apoptosis. Ischemia reperfusion injury during transplantation is a complex pathophysiological process, involving the production of reactive oxygen species, complement activation, endothelial cell activation, and leukocyte recruitment [2, 8]. This causes vascular injury, endothelial dysfunction, and ultimately cell death. It is postulated that donor-derived apoptotic cells and/or their related antigens released during injury are presented to B cells, with subsequent Th17 cell-mediated propagation of the autoimmune response and autoantibody production. Hence, these autoantibodies are produced as a result of vascular and tissue injury occurring at the time of or after transplantation [2, 6, 7, 9•]. Given the broad spectrum of non-HLA antigens, there are likely multiple pathways involved in autoimmune activation.
Non-HLA antibodies primarily target donor endothelial cells, causing allograft injury through a variety of mechanisms. Complement dependent cytotoxicity has been demonstrated for non-HLA antibodies to the G-protein coupled receptors and vimentin, which elicit C4d and C3d deposition, respectively [6, 10••, 11]. The binding of antibody to specific antigens can also trigger intracellular pathways that affect endothelial cellular function and survival, for example, binding of angiotensin to the AT1R receptor leads to proinflammatory and procoagulant responses [2, 4, 6]. In vitro studies have shown anti-endothelial cell antibodies inducing apoptosis and graft thrombosis [2]. Anti-angiotensin 1 receptor (AT1R) antibodies are capable of directly activating transcription factors leading to increased pro-inflammatory cytokine production [6]. The presence of non-HLA antibodies may be a biomarker for cell stress or underlying immune status; however, their pathophysiological mechanisms of action have not been fully elucidated
Non-HLA Antibody Detection
The identification of non-HLA antibody has proven to be challenging [7, 8]. Their detection has been limited by lack of clarity on whether the observed immune responses are due to a polymorphic alloantigen or a specific autoantigen [2, 8]. Recent availability of high-throughput protein microarrays as well as solid phase assays has improved the sensitivity of detection of non-HLA antibodies. However, their utilization remains primarily in the research arena and has not been routinely applied clinically due to high cost, lack of validation for use in large cohorts, and limited specificity [8, 12]. Certain methods are limited to the detection of novel non-HLA antibodies. This includes protein microarray which is a high-throughput method that mass produces selected proteins from library clones [2, 8]. Table 1 summarizes the current commercially available tests including enzyme-linked immunosorbent (ELISA) and single antigen bead assays [2, 8, 12, 13]. Importantly, standardization and validation of laboratory testing for non-HLA antibodies are lacking. As such, the majority of transplant programs do not perform routine testing for non-HLA antibodies.
Non-HLA Antibodies in Heart Transplantation
Several types of non-HLA antibodies have been identified in solid organ transplant recipients. In most studies, significance of a particular non-HLA antibody has been inferred through their detection and correlation to concurrent clinical events. The data for non-HLA antibodies in heart transplantation is limited and their precise impact on patient and allograft outcomes remain incompletely understood [2, 12,13,14]. Table 2 summarizes potentially relevant non-HLA antibodies in heart transplantation, including antibodies to major histocompatibility complex class 1 related chain A (MICA), vimentin, myosin, angiotensin II type I receptor (AT1R), and endothelin type A receptor (ETAR).
Polyreactive Antibodies
Polyreactive or naturally occurring antibodies have critical functions in maintaining immune protection, but their role in transplantation is less well defined. Polyreactive antibodies are postulated to be produced as part of a B cell response, developing spontaneously in healthy individuals without the need for prior immunization. These antibodies are predominantly IgG isotype and are capable of binding multiple structurally different ligands including self-antigens [15]. Pathogenic polyreactive antibodies are involved in various inflammatory conditions including autoimmune disease and the systemic inflammatory response syndrome [2, 15]. They have also been implicated in ischemia reperfusion injury wherein endothelial cells expose altered self-antigens that are recognized by polyreactive antibodies leading to allograft damage. Murine studies have demonstrated that tissue injury peri-transplant prompts the production of exosome-like vesicles that promote autoantibody production [16•]. All polyreactive antibodies appear to react to determinants on apoptotic but not viable cells [13,14,15, 16•]. Data from kidney transplant studies identified the development of polyreactive antibodies as a risk factor independent to the presence of HLA-DSA for allograft loss (hazards ratio (HR) 2.07, 95% confidence interval (CI) 1.03–4.17, p = 0.04) [17•]. Polyreactive antibodies are mainly IgG3 and IgG1 isotypes with the ability to activate complement [15]. However, the detection of polyreactive antibodies in the absence of evidence of complement activation suggests involvement of complement independent pathways [17•].
Ventricular assist device (VAD) support prior to transplant has been associated with increased levels of polyreactive antibodies and subsequent adverse post transplant outcomes. See et al. examined sera for polyreactive antibodies and reactivity to apoptotic cells in a cohort of 206 heart transplant recipients, including 128 patients bridged with a VAD [18]. Apoptotic cells were produced by exposure to ultraviolet light and heat to mimic ischemia reperfusion injury. There was no difference in IgM polyreactive antibody levels between patients with or without a VAD. Levels of IgG3 and IgG1 polyreactive antibodies increased 10% post VAD implant, unrelated to the duration of VAD support, but no significant changes were seen in patients without a VAD. Increased polyreactive antibody reactivity with apoptotic cells in VAD patients was associated with the development of primary graft dysfunction post transplant (HR 1.88, 95% CI 1.23–2.87, p < 0.05), but not rejection. The authors hypothesized that VAD-related biomaterials may be responsible for the observed systemic immune activation with broad B cell activation leading to the development of polyreactive antibodies.
Allograft infiltrating B cells that secrete polyreactive antibodies have been associated with the development of CAV. In allografts affected by CAV, memory CD27+ B cells and IgG secreting plasma cells predominate with T cell activation and polyreactive antibody production involved in injury [19••]. Chaterjee et al. compared cardiac biopsy samples and sera from 56 patients with CAV to 49 native failed hearts and 25 normal cardiac autopsy specimens [20•]. They demonstrated significantly increased infiltrating secretory B cells around coronary arteries in allografts with CAV, independent of the presence of HLA donor specific antibody (DSA) and AMR. The observed B cell infiltration around the coronary arteries was significantly greater than within the endomyocardium, with the majority secreting polyreactive antibodies reactive to apoptotic cells. As CAV involves endothelial cell injury with accumulation of apoptotic bodies, the authors postulated that endothelial cell apoptosis was responsible for recruitment and local retention of secretory B cells contributing to progressive CAV.
MICA Antibodies
Major histocompatibility complex class 1 related chain A (MICA) is expressed on endothelial cells, monocytes, keratinocytes, dendritic cells, fibroblasts, and epithelial cells. It bears a similar structure to class I HLA but does not associate with β-2 microglobulin and therefore cannot bind peptides. MICA is not constitutively expressed on immunocompetent cells but can be induced by activation of CD4+ and CD8+ T cells [4, 6]. MICA is highly polymorphic with several alleles identified and its expression is upregulated by stress cytokines, including tumor necrosis factor-alpha (TNFα) [4, 21]. MICA may be a stress marker given the increased expression with inflammation, DNA damage, and ischemia reperfusion injury [23]. A soluble isoform of MICA (sMICA) has also been discovered and is derived from the shedding of membrane-bound MICA into serum. The interaction between sMICA and natural killer (NK) cells leads to suppression of the innate immune response [5].
Approximately 30% of heart transplant recipients have MICA antibodies [5]. Risk factors for the development of antibodies to MICA and HLA are similar and include prior pregnancy, blood transfusion, and previous transplantation. Despite their association with pregnancy, MICA antibodies have been predominantly found in men in both heart and kidney transplant cohorts [5]. As an example, a study of 494 renal transplant recipients reported a prevalence of antibodies to MICA of 14% in men and 7% in women [22]. MICA antibodies have donor specificity and have been associated with increased rates of rejection in heart transplantation [5, 6]. In addition, renal data suggests synergism of MICA and HLA antibodies that leads to allograft loss [4, 5].
Suarez-Alvarez et al. assessed MICA and sMICA antibodies in 31 heart transplant recipients including 8 patients who had severe cellular rejection in the first year of transplant [24]. Compared to patients without rejection, a higher proportion of patients with rejection had MICA antibodies: 63% versus 17% (odds ratio (OR) 7.9, p < 0.03). In contrast, there was an inverse relationship between sMICA and recurrent severe rejection (OR 8.5, p < 0.03). The authors speculated that sMICA may inhibit the humoral response to MICA antibodies by suppressing B cell function or MICA antibody recognition. Furthermore, MICA is a ligand for the natural-killer group 2 member D (NKGD2) receptor expressed on NK cells and CD8+ T cells. The NKGD2 receptor is involved in activation of NK cells and functions as a co-stimulatory signal for CD8+ T cells. Cancer studies demonstrate that sMICA induces endocytosis and degradation of NKGD2 receptors [5, 7, 23, 24]. Similarly, MICA antibodies have been associated with AMR [25]. In one study of 168 heart transplant recipients, a significantly higher proportion of patients with AMR were positive for MICA DSA compared to patients without AMR: 26% versus 2%, respectively [26].
Small studies have also suggested an association between antibodies to MICA and CAV [26, 27]. Nath et al. followed 52 heart transplant recipients more than 1 year post-transplant, of whom 12 patients developed moderate or severe angiographic CAV [27]. The development of HLA DSA alone, MICA antibodies alone, as well as both HLA DSA and MICA antibodies were strongly correlated with development of CAV. This suggests both independent and additive roles of MICA antibodies to HLA DSA in CAV.
In contrast, a larger study of 491 heart transplant patients demonstrated no correlation between MICA antibodies and cellular rejection or CAV [28]. Pre-transplant sera was available from all heart transplant recipients, and no correlation was found between cellular rejection or CAV with antibodies to MICA. A subset of 196 patients had post-transplant sera analyzed for MICA antibodies annually for 5 years post transplant. Patients with HLA antibodies were excluded. Distinct to findings from other studies, there was no significant association between post transplant MICA antibodies and development of cellular rejection or CAV. The differences in results may be related to the use of the Luminex single antigen bead assay for detection of MICA antibodies compared to ELISA in most other studies.
G-Protein Coupled Receptor Antibodies
G-protein coupled receptors are the largest family of cell surface receptors [2, 4, 5, 12]. The two most widely studied in solid organ transplantation, predominantly kidney transplant, are angiotensin II type 1 receptor (AT1R) and endothelin type A receptor (ETAR) [7, 29•]. Antibodies to AT1R have also been implicated in autoimmune diseases such as systemic sclerosis, and in preeclampsia [2, 4, 29•]. Antibodies to ETAR and associated potential mechanisms of injury are less well characterized [2, 4, 12].
Angiotensin II type 1 receptor (AT1R) is expressed on endothelial cells, monocytes, B cells, and T cells [2, 4]. It mediates angiotensin II effects, promoting vasoconstriction, inflammation, proliferation, and fibrosis. Kidney transplant data shows strong associations between anti-AT1R antibodies and vascular injury, malignant hypertension, and rejection [12]. Antibodies to AT1R are predominantly IgG3 and IgG1 isotypes; however, C4d has not always been detected suggesting involvement of complement independent pathways [2, 12]. Proposed mechanisms involved in the formation of these antibodies include loss of self-tolerance and exposure of a neoantigen due to shear stress induced clipping of the extracellular loop of AT1R from the cell surface [2, 4].
Antibodies to AT1R are elevated in LVAD patients; however, no clear impact on rejection or survival post transplant has been demonstrated. In a recent study of 88 LVAD patients, antibodies to AT1R increased post-implant, but there was no association between AT1R levels and freedom from rejection or CAV in the 75 patients who subsequently underwent heart transplantation [30•]. Similarly, Urban et al. evaluated sera from 69 LVAD patients for antibodies to AT1R before and after transplantation, observing no difference in 5-year survival between patients with and without AT1R antibodies [31]. Notably, 25% of transplant recipients, who had both AT1R and HLA antibodies at time of transplant, subsequently developed high-grade acute cellular rejection with allograft dysfunction. This suggests synergistic action of anti-AT1R and HLA antibodies. Similar findings were demonstrated by Reinsmoen and colleagues in a study of 200 heart transplant recipients in whom patients with both HLA and AT1R antibodies had lower 2-year freedom from AMR: 50% for patients with antibodies versus 89% for patients without antibodies (HR 8.0, 95% CI 2.3–27.9, p < 0.01) [32]. In lung transplantation, elevated levels of antibodies to AT1R and ETAR at time of transplant significantly increased the risk of subsequently developing HLA DSA and was also associated with lower freedom from acute cellular rejection and AMR [33].
Antibodies to ETAR can occur concomitantly with AT1R antibodies. In animal studies, upregulation of ETAR is associated with vasoconstriction, vasospasm, and vascular remodeling [2, 4]. In heart transplantation, ETAR antibodies have been associated with early onset microvasculopathy, acute cellular, and antibody-mediated rejection [6]. Hieman and colleagues demonstrated an increase in both ETAR and AT1R antibodies in patients on LVAD support and post transplantation [34]. The highest levels of these antibodies were observed within 24 h of transplant, with a nadir at 1 month followed by further increases at 6 and 12 months. These antibodies were associated with microvasculopathy on cardiac biopsy: 67% of patients with ETAR and AT1R antibodies developed microvasculopathy compared to 23% of patients without antibodies. During the first year post-transplant, significantly higher titers of both ETAR and AT1R antibodies were detected in patients with rejection compared to patients without rejection.
Vimentin and Myosin
Myosin belongs to a family of proteins that bind the actin cytoskeleton and move cargo proteins through ATP hydrolysis. Anti-myosin antibodies are associated with AMR and CAV [2, 4]. Vimentin is the sub-unit of an intermediate filament which is part of the cytoskeleton. It is secreted by macrophages, endothelial cells, vascular smooth muscle cells, activated platelets, apoptotic T cells, and neutrophils. Vimentin is not expressed by adult cardiac myocytes but is strongly expressed in the coronary intima and media [4, 6, 10••, 36]. Secretion is increased by TNFα and inhibited by IL-10 [4]. Aberrant expression of vimentin can occur secondary to ischemia reperfusion injury and promote the formation of anti-vimentin antibodies [2, 4, 36]. This is supported by the detection of antibodies to vimentin on the surface of damaged cells within solid organ allografts [2, 35, 36].
Antibodies to vimentin are associated with increased risk of developing CAV, but no association has been found with rejection or survival post heart transplantation [13]. A study of 50 heart transplant recipients showed the presence of antibodies to vimentin in 17 (34%) patients with trends towards higher prevalence in females and younger patients. However, rejection-free graft survival at 1 year was similar for patients with and without vimentin antibodies [36]. Jurcevic and colleagues demonstrated that vimentin antibodies were an independent predictor of CAV. In this study cohort of 109 heart transplant recipients followed up to 5-year post transplant, 38 patients developed CAV and had significantly higher titers of vimentin antibodies [37]. A prothrombotic state induced by the interaction of vimentin antibodies, neutrophils, and platelets has also been associated with CAV [36].
Temporal relationships with the development of HLA antibodies and according to time from transplant have been described for antibodies to vimentin and myosin. Nath et al. performed serial antibody monitoring post-heart transplant, demonstrating antibodies to vimentin and myosin preceding the detection of anti-HLA DSA by 3.0 ± 0.4 and 1.7 ± 0.3 months, respectively. Additionally, patients with AMR were more likely to have anti-HLA DSA and had significantly higher levels of antibodies to both vimentin and myosin. Patients who developed CAV also had significantly elevated levels of antibodies to myosin and vimentin [11]. More recently, in a study of 161 heart transplant patients, the persistence of myosin antibodies at 12 months post transplant was independently associated with the composite endpoints including death and re-transplantation (HR 2.9, 95% CI 1.0–8.5, p < 0.01) [38••]. These findings support potential added prognostic value of antibodies to myosin and vimentin post-heart transplant.
Non-HLA Antibodies in Non-cardiac Transplantation
Other non-HLA antibodies important in solid organ transplant outcomes include anti-endothelial cell antibodies and perlecan in kidney transplantation, and collagen V and K-α 1 tubulin in lung transplantation [4, 4,5,6, 9•, 12, 33]. Antibodies to several anti-endothelial cell antigens have been discovered in kidney transplantation including endoglin, epidermal growth factor-like repeats, discoidin I-like domains 3 (EDIL3), and intercellular adhesion molecule 4 (ICAM-4). These have been linked to rejection and allograft loss but data in heart transplantation is lacking [4, 6, 9•]. Perlecan is a critical component of the endothelial basement membrane with multiple functions. Antibodies to the LG3 fragment of perlecan are associated with rejection in kidney transplant recipients [4, 12]. Antibodies to collagen V and K-α 1 tubulin induce fibrogenesis and contribute to bronchiolitis obliterans in lung transplant [5, 33].
Future Directions
The increasing availability of commercial detection methods for non-HLA antibodies may expand current knowledge on their potential role for immune risk stratification in the clinical setting. Technological advances will allow longitudinal patient evaluation and determine the prevalence of non-HLA antibodies in larger cohorts. Moreover, an improved understanding of the pathophysiological functions of non-HLA antigens may assist future development of targeted therapeutic strategies. For example, losartan and plasmapheresis have been shown to prevent inflammation and rejection in kidney transplant patients [4]. This raises the question of whether treatment with losartan could improve allograft survival in patients with AT1R or ETAR antibodies. Non-HLA antibody-associated effects on inflammatory cytokines, such as IL-10, may serve as a target for modulation. Soluble MICA appears to play a protective role and may therefore serve as a potential target for immunosuppression development.
Conclusion
There is emerging evidence that non-HLA antibodies have an important role in heart transplantation, including potential mediators of AMR, CAV, and allograft dysfunction. The relative clinical significance of specific non-HLA antibodies remains to be determined. Future investigation and standardization of laboratory testing are needed to fully elucidate the mechanisms of allograft injury and the true clinical impact of non-HLA antibodies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report—2013; Focus Theme: Age. J Heart Lung Transplant. 2013;32(10):951–64.
Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. 2016;12(8):484–95.
Béland S, Désy O, Vallin P, Basoni C, De Serres SA. Innate immunity in solid organ transplantation: an update and therapeutic opportunities. Expert Rev Clin Immunol. 2015;11(3):377–89.
Gates KV, Pereira NL, Griffiths LG. Cardiac Non-Human Leukocyte Antigen identification: Techniques and Troubles. Front Immunol. 2017;8:1332.
Delville M, Charreau B, Rabant M, Legendre C, Anglicheau D. Pathogenesis of non-HLA antibodies in solid organ transplantation: Where do we stand? Hum Immunol. 2016;77:1055–62.
Matsuda Y, Sarwal MM. Unraveling the Role of Allo-Antibodies and Transplant injury. Front Immunol. 2016;7:432.
• Padet L, Dieudé M, Karakeussian-Rimbaud A, et al. New insights into immune mechanisms of antiperlecan/LG3 antibody production: Importance of T cells and innate B1 cells. Am J Transplant. 2019;19:699–712 This article reviews human and murine studies on the role of antiperlecan/LG3 in renal transplantation.
•• Divanyan T, Acosta E, Patel D, et al. Anti-vimentin antibodies in transplant and disease. Hum Immunol. 2019. https://doi.org/10.1016/j.humimm.2019.03.017. This article provides information on the pathophysiology of vimentin antibodies and their role in rejection as well as other inflammatory diseases.
Nath DS, Basha HI, Tiriveedhi V, et al. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29:1277–85.
Zhang X, Reinsmoen NL. Impact of Non-Human Leukocyte Antigen-Specific Antibodies in Kidney and Heart Transplantation. Front Immunol. 2017;8:434.
Dragun D, Catar R, Philippe A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int Rep. 2016;90:280–8.
Reinsmoen NL, Zhang X. Non-human leukocyte antigen-specific antibodies in thoracic transplantation. Curr Opin Organ Transplant. 2016;21(4):350–4.
Vallin P, Désy O, Béland S, Wagner E, de Serres SA. Clinical relevance of circulating antibodies and B lymphocyte markers in allograft rejection. Clin Biochem. 2016;49:385–93.
Zorn E, See SB. Polyreactive natural antibodies in transplantation. Curr Opin Organ Transplant. 2017;22(1):8–13.
• Dieudé M, West LJ, Muruve DA, et al. New Answers to Old Conundrums: What Antibodies, Exosomes and Inflammasomes Bring to the Conversation. Canadian National Transplant Research Program International Summit Report. Transplantation. 2018;102(2):209–14. This article focuses on recent advancements in the understanding of antibody mediated rejection. It provides in depth knowledge on immune response in relation to certain non-HLA antibodies.
• See SB, Aubert O, Loupy A, et al. Post-Transplant Natural Antibodies Associate with Kidney Allograft Injury and Reduced Long-Term Survival. J Am Soc Nephrol. 2018;29(6):1761–70. This article looks at polyreactive antibodies and outcomes in renal transplantation.
See SB, Clerkin KJ, Kennel PJ. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant. 2017;36(8):862–70.
•• Zorn E. Effector B cells in cardiac allograft vasculopathy. Curr Opin Transplant. 2019;24(1):31–6. This article summarises the role of effector B cells and polyreactive antibodies in CAV.
• Chatterjee D, Moore C, Gao B, et al. Prevalence of polyreactive innate clones among graft--infiltrating B cells in human cardiac allograft vasculopathy. J Heart Lung Transplant. 2018;37(3):385–93. This study reviews the role of polyreactive antibodies and secretory B cells in cardiac allograft vasculopathy.
Luo L, Li Z, Wu W, Luo G, Xu C, Sun Z, et al. Role of MICA antibodies in solid organ transplantation. Clin Transpl. 2014;28:152–60.
Mehra NK, Baranwal AK. Clinical and immunological relevance of antibodies in solid organ transplantation. Int J Immunogenet. 2016;43(6):351–68.
Lemy A, Andrien M, Wissing KM, Ryhahi K, Vandersarren A, Racapé J, et al. Major histocompatibility complex class 1 chain-related antigen a antibodies: sensitizing events and impact on renal graft outcomes. Transplantation. 2010;90(2):168–74.
Suárez-Alvarez B, López-Vázquez A, Díaz-Peña R, et al. Post-transplant soluble MICA and MICA antibodies predict subsequent heart graft outcome. Transpl Immunol. 2006;17(1):43–6.
Pavlova YA, Malek I, Honsova E, Netuka I, Sochman J, Lodererova A, et al. Hepatocyte growth factor and antibodies to HLA and MICA antigens in heart transplant recipients. Tissue Antigens. 2010;76(5):380–6.
Qiuheng Z, Cecka M, Gjertson DW, et al. HLA and MICA: Targets of Antibody-Mediated Rejection in Heart Transplantation. Transplantation. 2011;91:1153–8.
Nath DS, Angaswamy N, Basha HI, Phelan D, Moazami N, Ewald GA, et al. Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major histocompatibility complex class I–related chain A in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol. 2010;71(12):1191–6.
Smith JD, Brunner VM, Jigjidsuren S, Hamour IM, McCormack A, Banner NR, et al. Lack of Effect of MICA Antibodies on Graft Survival Following Heart Transplantation. Am J Transplant. 2009;9:1912–9.
• Gareau AJ, Wiebe C, Pochinco D, et al. Pre-transplant AT1R antibodies correlate with early allograft rejection. Transpl Immunol. 2018;46:29–35. In this study of renal transplant recipients, the presence of AT1R antibodies was associated with cellular rejection but not graft survival.
• Zhang X, Mirocha J, Aintablian T, et al. Revealing a new mode of sensitization induced by mechanical circulatory support devices: Impact of anti-AT1 R antibodies. Clin Transpl. 2018;32(2). This study explores the association between durable mechanical support and development of antibodies to AT1R.
Baranwal AK, Mehra NK. Major Histocompatibility Complex Class I Chain-Related A (MICA) Molecules: Relevance in Solid Organ Transplantation. Front Immunol. 2017 28;8:182. https://doi.org/10.3389/fimmu.2017.00182
Urban M, Slavcev A, Gazdic T, et al. The impact of angiotensin II type 1 receptor antibodies on post-heart transplantation outcome in Heart Mate II bridged recipients. Interact Cardiovasc Thorac Surg. 2016;22(3):292–7.
Reinsmoen NL, Lai C-H, Mirocha J, et al. Increased Negative Impact of Donor HLA-Specific Together With Non-HLA Specific Antibodies on Graft Outcome. Transplantation. 2014;97:595Y601.
Reinsmoen NL, Mirocha J, Ensor CR, Marrari M, Chaux G, Levine DJ, et al. A 3-Center Study Reveals New Insights Into the Impact of Non-HLA Antibodies on Lung Transplantation Outcome. Transplantation. 2017;101(6):1215–21.
Hiemann NE, Meyer R, Wellnhofer E, Schoenemann C, Heidecke H, Lachmann N, et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation. 2012;94(9):919–24.
Rose ML. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013;74(11):1459–62
Young RK, Dale B, Russell SD, Zachary AA, Tedford RJ. Incidence and early outcomes associated with pre-transplant anti-vimentin antibodies in the cardiac transplantation population. Clin Transpl. 2015;29(8):685–8.
Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71(7):886–92.
•• Stehlik J, Armstrong B, Baran DA, et al. Early immune biomarkers and intermediate-term outcomes after heart transplantation: Results of Clinical Trials in Organ Transplantation-18. Am J Transplant. 2019 May;19(5):1518–1528. https://doi.org/10.1111/ajt.15218. Up to date analysis of the role of myosin antibodies in heart transplantation in a cohort derived from the CTOT-15 study.
Acknowledgments
Sharon Chih is supported by a Heart and Stroke Foundation Canada Ontario Clinician Scientist Phase I award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Faith Njue declares no conflict of interest.
Sharon Chih reports salary support from Heart and Stroke Foundation Canada Ontario Clinician (Scientist Phase I award), during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Thoracic Transplantation
Rights and permissions
About this article
Cite this article
Njue, F., Chih, S. The Importance of Non-HLA Antibodies After Heart Transplant. Curr Transpl Rep 6, 300–306 (2019). https://doi.org/10.1007/s40472-019-00254-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-019-00254-1