Abstract
LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor (ARNI), is comprised of the angiotensin receptor blocker valsartan and the neprilysin inhibitor pro-drug sacubitril (AHU377). After oral administration, AHU377 is rapidly metabolized to the active neprilysin inhibitor LBQ657. LCZ696 exerts its effects of diuresis, natriuresis, vasodilation and aldosterone secretion inhibition through simultaneous renin-angiotensin-aldosterone system (RAAS) blockade and natriuretic peptides system (NPS) enhancement. Powerful evidence including PARAMETER and PRARDIGM-HF trials have shown that LCZ696 outperforms RAAS inhibition in treating patients with hypertension and heart failure with reduced ejection fraction (HFrEF), and is well tolerated. In addition, accumulating evidence also suggests its potential use in heart failure with preserved ejection fraction (HFpEF), chronic kidney disease (CKD), post-myocardium infarction (post-MI) and stroke. Both the FDA and CHMP have approved LCZ696 for treatment of HFrEF. Despite all this, some special issues (e.g. use in specific subgroups, adverse events, contraindications and cost-effectiveness analysis) should be considered before its implementation in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
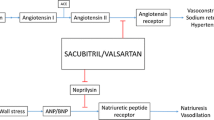
LCZ696(sacubitril/valsartan, a first-in-class angiotensin receptor neprilysin inhibitor (ARNI), consists of the angiotensin receptor blocker (ARB) valsartan and the neprilysin inhibitor prodrug sacubitril (AHU377) in a 1:1 M ratio [1, 2]. The valsartan moiety antagonizes the harmful effects of the renin-angiotensin-aldosterone system (RAAS) activation such as sodium retention, vasoconstriction and maladaptive remodeling via specific blockade of angiotensin II type-1 receptors, while the AHU377 moiety, rapidly enzymatically cleaved to the active metabolite LBQ657, augments the beneficial effects of the natriuretic peptides system (NPS) such as diuresis, natriuresis, vasodilation and RAAS blockade by inhibiting the degradation of natriuretic peptides (NPs) (Fig. 1) [3–5]. The dual action of RAAS blockade combined with NPS augmentation makes LCZ696 a promising therapeutic drug for hypertension, heart failure (HF), renal disease and myocardial infarction. Here, we review the development, pharmacology, and preclinical and clinical data of LCZ696 to date, and evaluate its potential role in future clinical consideration.
Action mechanism of LCZ696 (sacubitril/valsartan). LZC696 is an angiotensin receptor-neprilysin inhibitor composed of the ARB valsartan and the neprilysin inhibitor prodrug sacubitril (AHU377). Neprilysin is a major enzyme in the breakdown of biologically active natriuretic peptides and other vasoactive peptides; hence inhibition of neprilysin increases natriuretic peptides in circulation. Valsartan inhibits the renin-angiotensin-aldosterone system by blocking the angiotensin type I receptor. AngII = angiotensinII; AT1R = angiotensin type I receptor; NEP = neprilysin; NPs = natriuretic peptides; NPR = natriuretic peptide receptor; RAAS = renin-angiotensin-aldosterone system
Development of LCZ696
The most significant difference between LCZ696 and traditional ARB lies in the adjunctive neprilysin inhibitor. Neprilyisin, also known as neutral endopeptidase (NEP), is a membrane-bound enzyme widely distributed in a variety of tissues and particularly abundant in the renal proximal tubule [6]. Previous studies showed that circulating soluble neprilysin is positively associated with cardiovascular death and HF hospitalization in HF patients, supporting NEP inhibition as a potential therapeutic target [7]. In vivo, NEP participates in the breakdown of several endogenous vasopeptides including NPs [6, 8], bradykinin [9], adrenomedullin [10], angiotensin II [11], and endothelin-1 [12]. Therefore, the net effects of NEP inhibition on vascular tone depend on the balance of its effects on the breakdown of vasodilators (i.e., natriuretic peptides, bradykinin, adrenomedullin) versus that of vasoconstrictors (i.e., angiotensin II and endothelin-1). Candoxatrilat, the first orally available NEP inhibitor, although showing a positive effect on diuresis and natriuresis by increasing NPs levels (including atrial NP, B-type NP and C-type NP), failed to lower blood pressure in hypertensive patients [13, 14], partially due to concomitant increases of angiotensin II and endothelin-1levels [15, 16]. The next developed omapatrilat, a dual inhibitor of NEP and angiotensin converting enzyme (ACE), addressed the concern of elevation of angiotensin II and outperformed ACE inhibition (ACEI) alone in lowering blood pressure in an experimental study [17], as well as reducing cardiovascular events in patients with heart failure in the IMPRESS study [18]. Unfortunately, omapatrilat was not approved by the U.S. Food and Drug Administration (FDA) due to unacceptable risk of angioedema observed in the OCTAVE and OVERTURE studies [19, 20]. Accumulation of bradykinin through simultaneous inhibition of NEP and ACE may be responsible for the high incidence of angioedema induced by omapratilat [21, 22]. Thus, a new approach shifting the RAAS target from ACE inhibition to angiotensin receptor blockade effectively addressed the high rate of angioedema and formed the novel ARNI LCZ696. In an animal model, ARNI (using valsartan–candoxatril) exhibited equal BP lowering efficacy to omapatrilat without the risk of angioedema [23].
Pharmacology of LCZ 696
LCZ696 is a supramolecular complex of 6 molecules of valsartan with 6 molecules of sacubitril (AHU377), creating a novel crystalline complex having a molecular weight of 5748 [24]. Following ingestion, the prodrug AUH377 is rapidly metabolized to the active NEP inhibitor LBQ657. Following administration of a single oral dose of LCZ696200–1200 mg, the time to maximum plasma concentration (Tmax) of LBQ657 is 1.9–3.5 h and that of valsartan 1.7–2.2 h. Mean half-life of LBQ657 is 9.9–11.1 h and that of valsartan is 8.9–16.6 h, which makes prescription of LCZ696 once or twice daily reasonable (Table 1) [2]. LCZ696 treatment is associated with an increase in plasma cyclic guanosine monophosphate reflecting augmentation of NPS [25], as well as increases in plasma renin and angiotensin II concentration reflecting the action of ARB [2]. The 103 mg of valsartan component in 200 mg of LCZ696 is bioequivalent to 160 mg of valsartan in Diovan®. Moreover, the pharmacokinetic profile of LCZ696 analytes were shown to be similar between young and elderly, male and female, as well as subjects of Caucasian, Chinese and Japanese origin [26–28].
LCZ696 in Heart Failure with Reduced Ejection Fraction
Heart failure (HF) is the end stage of various kinds of cardiovascular disease, which affects over 23 million patients worldwide [29]. Based on the left ventricular ejection fraction (LVEF), HF can be categorized into HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), with either type making up about half of the overall incidence. Although great advances have been achieved in the treatment for HFrEF in the past three decades, there remains a high mortality rate of 50 % within 5 years [30], more severe than that of the breast, large bowel, prostate, or ovary cancer [31]. The newly released 2016 ESC and ACC/AHA/HFSA guidelines for HF concurrently recommend replacement of ACEI or ARB with LCZ696, in conjunction with beta-blocker and mineralocorticoid receptor antagonist (MRA), to further reduce morbidity and mortality of HFrEF patients [32, 33]. The evidence supporting the guideline recommendation of replacement of ACEI or ARB with LCZ696 for HFrEF patients is derived from the PARADIGM-HF study.
The PARADIGM-HF trial randomized 8399 patients aged 18–96 years, with New York Heart Association (NYHA) class II-IV and LVEF ≤40 % (which was changed to ≤35 % by an amendment to the protocol on December 15, 2010), to receive the ARNI LCZ696200 mg twice daily or enalapril 10 mg twice daily, in addition to recommended therapy (i.e. along with a beta-blocker and an MRA). The primary endpoint was a composite of cardiovascular death or hospitalization for HF. The trial was prematurely stopped after a median follow-up of 27 months for overwhelming advantage of LCZ696 over enalapril. During the course of the study, 914 patients (21.8 %) in the LCZ696 group met the primary endpoint compared with 1117 patients (26.5 %) in the enalapril group (hazard ratio [HR] 0.80; 95 % confidence interval [CI], 0.73–0.87; P < 0.001). Among them, 558 patients (13.3 %) receiving LCZ696 died from cardiovascular causes compared with 693 patients (16.5 %) receiving enalapril (HR, 0.80; 95 % CI, 0.71–0.89; P < 0.001); 537 patients (12.8 %) receiving LCZ696 were hospitalized for HF compared with 658 patients (15.6 %) receiving enalapril (HR 0.79; 95 % CI, 0.71–0.89; P < 0.001). Moreover, death due to any cause was also reduced in patients receiving LCZ696 compared with enalapril (17.0 % versus 19.8 %; HR 0.84; 95 % CI, 0.76–0.93; P < 0.001). Safety analysis showed that LCZ696 was well tolerated except for a higher incidence of symptomatic hypotension (rarely leading to LCZ696 discontinuation) than enalapril [34]. After that, several subsequent analyses of the PARADIGM-HF trial were conducted to address some special issues. Packer et al. focused on the surviving patients in PARADIGM-HF and demonstrated that LCZ696 prevented heart failure deterioration more effectively than enalapril, including reduced need for intensified HF therapy, emergency department visit, intensive care, inotropic agents, or heart transplantation. In addition, patients receiving LCZ696 had an improved quality of life (as assessed by Kansas City Quality of Life Questionnaire) and fewer HF symptoms (as assessed by NYHA functional class) and fewer biomarkers of myocardial wall stress and injury (as assessed by NT-proBNP and troponin T) compared with enalapril [35]. Desai et al. showed that the reduced cardiovascular deaths accounted for 80.9 % of the overall deaths in PARADIGM-HF and were primarily comprised of sudden cardiac deaths and deaths from worsening heart failure [36]. Using actuarial estimates from the PARADIGM-HF trial, Claggett et al. extrapolated that LCZ696 would produce additional 1 to 2 years of life expectancy and survival free from heart failure in the long term as compared with enalapril [37]. Recently, Desai et al. reported that, compared with enalapril, LCZ696 reduced 30-day readmissions for any cause following discharge from HF hospitalization [38]. Furthermore, several post-hoc analyses of PARADIGM-HF showed that the benefits of LCZ696 over enalapril were consistent across the spectrum of LVEF [39], dosage [40], age [41], glycemic status [42], and baseline risk [43] (Table 2). The superiority of LCZ696 over ACEI was indirectly justified in a putative placebo analysis, showing an important reduction in the composite outcome of cardiovascular death or heart failure hospitalization by 39–43 % [44].
LCZ696 in Heart Failure with Preserved Ejection Fraction
Patients with HFpEF have an equally poor prognosis as compared to patients with HFrEF [45], however, there exists no established therapy for HFpEF to date. LCZ696 has been tested in a phase II trial in HFpEF, the Prospective comparison of ARNi with ARB on Management of heart failure with preserved ejection fraction (PARAMOUNT) trial, and showed promise for treatment of HFpEF. In PARAMOUNT, 301 patients with NYHA classII-III, LVEF 45 % or higher, and NT-proBNP greater than 400 pg/mL were enrolled and randomly assigned (1:1) to receive either LCZ696 (titrated up to 200 mg twice daily) or valsartan alone (titrated to 160 mg twice daily) for 36 weeks. The primary endpoint was change in NT-proBNP from baseline to 12 weeks. The results showed that LCZ696 reduced levels of NT-proBNP at 12 weeks (LCZ696: baseline, 783 pg/mL [95 % CI, 670–914], 12 weeks, 605 pg/mL [95 % CI, 512–714]; valsartan: baseline, 862 pg/mL [95 % CI, 733–1012], 12 weeks, 835 pg/mL [95 % CI, 710–981]; ratio LCZ696/valsartan, 0.77, 95 % CI 0.64–0.92, P = 0.005), and reduced left atrial size and improved NYHA class at 36 weeks. LCZ696 was well tolerated with adverse effects similar to those of valsartan [46]. Although the BP reduction was more significant in LCZ696 versus valsartan (−9.3/−4.9 versus −2.9/−2.1 mmHg at 12 weeks; 7.5/5.1 mmHg versus 1.5/0.3 mmHg at 36 weeks, all P < 0.05), the effect of LCZ696 on NT-proBNP, left atrial volume, NYHA class, and estimated glomerular filtration rate (eGFR) showed no association with the reduction in BP [47]. Continual analysis of the PARAMOUNT trial also showed that LCZ696 treatment decreased high-sensitivity troponin T (hs-TnT) to a greater extent at 12 weeks (12 % reduction; P = 0.05) and at 36 weeks (14 % reduction; P = 0.03) compared with valsartan [48]. Whether these effects on surrogate endpoints could translate into improved outcomes needs to be further tested. The ongoing multicenter, randomized, double-blind, parallel group study PARAGON-HF (NCT01920711) is currently enrolling 4600 patients with HFpEF to compare LCZ696 with valsartan regarding their effects on cardiovascular death and HF hospitalization. The trial is expected to be completed in 2019 [49] (Table 3).
LCZ696 in Hypertension
Hypertension was the first indication studied for LCZ 696. In 2010, Ruilope et al. evaluated 1328 patients with mild-to-moderate hypertension for 8 weeks to measure the mean difference of three pairwise comparisons of sitting diastolic BP between LCZ 696 and bioequivalent doses of valsartan (i.e., LCZ 696100 mg versus valsartan 80 mg, LCZ 696200 mg versus valsartan 160 mg, and LCZ 696400 mg versus valsartan 320 mg), as well as a pairwise comparison between AHU 377200 mg and placebo. The trial showed a significantly greater reduction of sitting diastolic BP with 200 mg LCZ696 versus 160 mg valsartan by −2.97 mmHg, and with 400 mg LCZ696 versus 320 mg valsartan by −2.7 mmHg [50]. Of note, the subjects enrolled in the trial of Ruilope et al. were primarily Caucasians and were distinctly different from Asian hypertensive patients who are characterized by higher salt sensitivity. In 2014, Kario et al. evaluated 389 adult Asian patients with mild to moderate hypertension for 8 weeks, and further confirmed the efficacy of LCZ 696 in lowering clinic and ambulatory systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse pressure (PP) compared with placebo. Moreover, the antihypertensive efficacy tended to be greater in Asian hypertensive patients compared with that in Caucasian patients [51]. Both trials mentioned above showed a good tolerance to LCZ696 and no cases of angioedema were reported. However, generalization of the safety data to black people should be done cautiously as these particular patients, proven to be more liable to angioedema incidence, were underrepresented in the trials.
With aging, one of the most common complications of hypertension is the increased arterial stiffness resulting from progressive vascular remodeling [52], which in turn amplifies the pathogenesis of hypertension and links with poor cardiovascular outcomes, such as myocardial infarction, cognitive decline, stroke, and kidney diseases [53–56]. Thus, agents targeted at lowering BP per se and improving vascular elasticity may be a preferred choice for patients with elderly systolic hypertension. An early experimental study in stroke- prone spontaneously hypertensive rats (SHRSP) showed that combined valsartan/NEP inhibition produced comparable efficacy as ACEI/NEP inhibition in lowering BP and improving vascular remodeling, as indicated by increased relaxation responses to acetylcholine and decreased vascular media/lm ratio of resistance arteries [57]. Recently, Kusaka et al. further confirmed that LCZ696 performed better than valsartan in lowering BP and preventing cardiovascular injury in spontaneously hypertensive rats [58]. A recent clinical trial presented at the European Society of Cardiology (ESC) 2015 Congress assessed the efficacy of LCZ696 versus olmesartan on aortic stiffness and central aortic haemodynamics in the elderly [59]. 454 patients aged ≥60 years with mean sitting (ms) SBP >150 mmHg and PP > 60 mmHg were randomly assigned to receive once-daily LCZ696200 mg (n = 229) or olmesartan 20 mg (n = 225) for 4 weeks, followed by a forced-titration to double the initial doses in the next 8 weeks. Add-on hypertension medication was allowed at 12–24 weeks if BP was not under control (msSBP <140 and msDBP <90 mmHg). After 12 weeks, the LCZ696 group showed a greater reduction than the olmesartan group in central aortic systolic pressure (−12.6 versus −8.9 mmHg, P = 0.01), central aortic pulse pressure (−6.4 versus −4 mmHg, P = 0.01), brachial systolic BP (−13.7 versus −9.9 mmHg, P = 0.02), and PP (−7.7 versus −4.9 mmHg, P = 0.01). Throughout the 52 weeks of follow-up, 68 % of the LCZ696 group was able to stay on monotherapy versus 53 % of the olmesartan group. There were no significant between-group differences in safety endpoints. Recently, an 8-week, multicenter, open-label study further demonstrated that LCZ696-based regimen was generally safe and effective, particularly for SBP and PP reductions, in the treatment of severe hypertension in Asian patients [60] (Table 4).
Based on the above evidence, we may suggest that prescribing LCZ696 might be a better choice for elderly systolic hypertension than conventional ARB. Moreover, BP reduction by LCZ696 tended to be greater in salt-sensitive Asian patients compared with Western patients [51]. Furthermore, since numerous in vivo studies [61–64] and limited number of human studies [65] have suggested a potential role of NPs for treatment of resistant hypertension [66], LCZ696 may also be a potential therapeutic strategy for resistant hypertension.
LCZ696 in Chronic Kidney Disease
Chronic kidney disease (CKD) is a common concomitant disorder in patients with cardiovascular disease and is independently predictive of poor outcome. Different from the definite benefits demonstrated in hypertension and heart failure, there exists no direct large-scale trial data supporting the use of LCZ696 in CKD. The evidence for potential renoprotective effects of ARNI is primarily inferred from animal studies using other vasopeptidase inhibitors (i.e. NEP/ACE inhibitor) which demonstrated superiority of the NEP/ACE inhibitor over ACEI in preservation of renal function [67–70]. A second analysis of renal outcomes in the PARAMOUNT trial showed that therapy with LCZ696 for 36 weeks was associated with preservation of eGFR compared with valsartan therapy (−1.5 versus −5.2 mL/min/1.73m2; P = 0.002) [71]. Wang et al. reported that the potentially beneficial effects of ARNI on renal function were associated with inhibited angiotensin II mediated collagen synthesis in renal mesangial cells [72]. A meta-analysis conducted by Bodey et al. included studies of IMPRESS, OVERTURE, PARAMOUNT, and PARADIGM-HF, and demonstrated that NEP–RAAS inhibition (Omapatrilat or LCZ696) in heart failure produced a 32 % relative risk reduction in renal function deterioration compared to ACEI or ARB alone [73]. In an attempt to investigate whether LCZ696 has the potential to protect kidneys better than current standard treatment, the ongoing UK HARP-III Study (ISRCTN 11958993) has randomly assigned 414 participants with eGFR 20–60 mL/min/1.73m2 or urinary albumin to creatinine ratio ≥ 20 mg/mol to receive LCZ696 or irbesartan treatment, to assess the difference in change of renal function over 12 months of follow-up, and the overall trial is expected to be completed in 2017 [74] (Table 5).
LCZ696 in Post-Myocardial Infarction and Stroke
Myocardial infarction (MI) and stroke are severe types of cardio-cerebrovascular diseases characterized by high mortality and disability rate. To the best of our knowledge, there exists no clinical data to date directly exploring the effects of LCZ696 on MI and stroke except two experimental animal studies. Von Lueder et al. demonstrated that LCZ696 attenuated cardiac remodeling after experimental MI through greater inhibition of cardiac hypertrophy and fibrosis compared with NEPI or ARB alone [75]. Bai et al. administered eight-week-old male C57BL/6 J mice with valsartan (3 mg/kg per day) or LCZ696 (6 mg/kg per day) for 2 weeks before middle cerebral artery occlusion, and showed that the preventive effect of LCZ696 on ischemic brain damage after stroke was more marked than that of valsartan.
LCZ696 in Clinical Practice
Based on the quite robust results of PARADIGM-HF, the FDA has approved the combination tablet sacubitril/valsartan (LCZ696) for use in chronic HFrEF patients with NYHA class II-IV symptoms [76]. Not long thereafter, the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) also recommended marketing authorization for LCZ696 use in HF patients [77]. The 2014 CCS, 2016 ESC and 2016 ACC/AHA/HFSA guidelines for HF have recommended conditionally replacing an ACEI or ARB with LCZ696 when the drug is available, with close surveillance of serum potassium and creatinine [32, 33, 78]. It is to be expected that guidelines for HF or even hypertension management in other countries will also be updated, considering the use of LCZ696 in the near future. Last but not least, the following notes should be taken into account before implementation of LCZ696 in clinical practice:
-
1.
Who will benefit?
Recently, a concern was raised about possible attenuated response of LCZ696 in HFrEF patients with lower levels of circulating NEP activity [79], which may occur secondary to elevated BNP levels [80]. Thus, the issue that should NEP catalytic activity be routinely measured before prescribing LCZ696 needs to be further elucidated [81]. Moreover, patients with obesity also merit specific consideration as their lower levels of endogenous NPs suggest a particular benefit from neprilysin inhibition [82].
-
2.
Initial dose and dose titration
The recommended starting dose is 100 mg (sacubitril 49 mg and valsartan 51 mg) twice a day, which is titrated based on patient tolerability after 2 to 4 weeks to a dose of 200 mg (sacubitril 97 mg and valsartan 103 mg) twice a day. The initial dose of sacubitril/valsartan should be decreased to 50 mg (sacubitril 24 mg and valsartan 26 mg) twice a day for patients who are not currently treated with an ACE inhibitor or ARB or treated with low doses of these agents, have severe renal impairment (eGFR <30 mL/min/1.73 m2), or have moderate hepatic impairment (Child-Pugh B). In the Safety and Tolerability of Initiating LCZ696 in Heart Failure Patients (TITRATION) study, Senni et al. showed that initiation/uptitration of sacubitril/valsartan from 50 to 200 mg twice daily over 3 or 6 weeks had a tolerability profile in line with other concomitant HF treatments, including β-blockers, aldosterone antagonists and diuretics, and a more gradual initiation/uptitration (i.e., over 6 weeks) maximized attainment of target dose in patients receiving lower doses of ACEI/ARBs (ACEI/ARBs were stopped for a 36-h washout period before initiating sacubitril/valsartan) [83].
-
3.
Implementation in specific subgroups
Implementation of LCZ96 in clinical practice may face challenges in specific subgroups such as patients with marginally low blood pressure, hospitalized for acute decompensated HF, with asymptomatic left ventricular systolic dysfunction (i.e., NYHA Iclass) or advanced heart failure (i.e., NYHA IV class), and black people [84], for these patients were excluded or underrepresented in the published trials associated with LCZ696 and might be more susceptible to some adverse events.
-
4.
Safety concerns
Nearly 20 % of patients were unable to tolerate LCZ696 or enalapril treatment during the run-in period of the PARADIGM-HF trial, especially those with lower blood pressure, lower glomerular filtration rate, and more severe heart failure [85]. In view of the common adverse events of LCZ696 including angioedema, hypotension, hyperkalemia and impaired renal function, the CHMP recommends that treatment should not be started in patients with low blood pressure (SBP < 100 mmHg) or high potassium levels (K+ > 5.5 mmol/L) [77].
-
5.
Contraindications
Contraindications of LCZ696 include patients with a history of angioedema related to previous ACEI or ARB therapy, concomitant use with ACEI, and pregnancy. For patients currently using ACEI, provide a 36-h washout period when switching from ACEI to LCZ696 to avoid serious angioedema. Additionally, LCZ696 is also contraindicated in patients with hypersensitivity to any of its components and in diabetic patients when concomitantly prescribed with aliskiren (Table 6).
-
6.
Drug-drug interaction assessment
Dedicated drug interaction studies demonstrated that coadministration of LCZ696 with warfarin, digoxin, atorvastatin, hydrochlorothiazide, amlodipine, carvedilol, omeprazole, metformin, or levonorgestrel-ethinyl estradiol was not associated with any clinically relevant pharmacokinetic drug interactions [86–90].
-
7.
Cost-effectiveness analysis
The price of LCZ696 is quite high especially compared with that of enalapril. The wholesale cost for a year of LCZ696 is $4560 per person as of 2015 [91]. However, given the substantial health benefits of LCZ696 relative to enalapril [92], as well as having an incremental cost-effectiveness ratio of US $45,017 per quality-adjusted life-year gained, it would remain a cost-effective and commonly acceptable choice for HFrEF patients (Table 7) [93].
Conclusions
LCZ696, a novel combination comprised of neprilysin inhibitor sacubitril and angiotensin receptor blocker valsartan, exerts its pharmaceutical action through simultaneous RAAS blockade and NPS augmentation. It has an advantage over traditional RAAS inhibition (ACEI or ARB) in lowering blood pressure in hypertensive patients (particularly for patients with elderly systolic hypertension and of Asian origin), reducing cardiovascular mortality in patients with HFrEF, as well as improving surrogate endpoints (e.g. NT-proBNP) in patients with HFpEF. Moreover, animal studies suggest that LCZ696 may also be a promising therapeutic agent for chronic renal disease, post-MI and stroke. Ongoing trials including PARAGON-HF and UK HARP-III will provide more information for clinical consideration. Both the FDA and the CHMP have approved its indication in patients with HFrEF, with close surveillance of associated side-effects. Taken together, there are good grounds to believe that LCZ696 may herald a new age in the treatment of cardiovascular disease.
References
Vardeny O, Tacheny T, Solomon SD. First-in-class angiotensin receptor neprilysin inhibitor in heart failure. Clin Pharmacol Ther. 2013;94(4):445–8.
Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49(3):419–26.
Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68.
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett Jr JC. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–93c.
Bayes-Genis A, Barallat J, Galan A, de Antonio M, Domingo M, Zamora E, et al. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65(7):657–65.
Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T. Neutral endopeptidase inhibition: augmented atrial and brain natriuretic peptide, haemodynamic and natriuretic responses in ovine heart failure. Clin Sci (Lond). 1996;91(3):283–91.
Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension. 2004;44(6):913–8.
Wilkinson IB, McEniery CM, Bongaerts KH, MacCallum H, Webb DJ, Cockcroft JR. Adrenomedullin (ADM) in the human forearm vascular bed: effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP). Br J Clin Pharmacol. 2001;52(2):159–64.
Erdos EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3(2):145–51.
Abassi Z, Golomb E, Keiser HR. Neutral endopeptidase inhibition increases the urinary excretion and plasma levels of endothelin. Metabolism. 1992;41(7):683–5.
McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8(1):79–84.
Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, et al. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992;10(7):607–13.
Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26(6 Pt 2):1160–6.
Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97(23):2323–30.
Trippodo NC, Fox M, Monticello TM, Panchal BC, Asaad MM. Vasopeptidase inhibition with omapatrilat improves cardiac geometry and survival in cardiomyopathic hamsters more than does ACE inhibition with captopril. J Cardiovasc Pharmacol. 1999;34(6):782–90.
Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356(9230):615–20.
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–11.
Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE)[J]. Circulation. 2002;106(8):920–6.
Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356(9230):608–9.
Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, et al. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153(5):947–55.
Hegde LG, Yu C, Renner T, Thibodeaux H, Armstrong SR, Park T, et al. Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol. 2011;57(4):495–504.
Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53(3):275–6.
Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99(2):71–5.
Gan L, Langenickel T, Petruck J, Kode K, Rajman I, Chandra P, et al. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56(1):78–86.
Han Y, Ayalasomayajula S, Pan W, Yang F, Yuan Y, Langenickel T, et al. Pharmacokinetics, safety and tolerability of sacubitril/valsartan (LCZ696) after single-dose Administration in Healthy Chinese Subjects. Eur J Drug Metab Pharmacokinet. 2016;1-8
Akahori M, Ayalasomayajula S. Pharmacokinetics after single ascending dose, food effect, and safety of sacubitril/valsartan (LCZ696), an angiotensin receptor and neprilysin inhibitor, in healthy Japanese subjects. Eur J Drug Metab Pharmacokinet. 2016;1–10.
Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11(4):404–15.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–60.
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More 'malignant' than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–22.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Colvin MM, et al. ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68(13):1476–88.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61.
Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–7.
Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, et al. Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289–90.
Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68(3):241–8.
Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF) trial. Circ Heart Fail. 2016;9(3):e002744.
Vardeny O, Claggett B, McMurray JJV, Packer M, Rouleau J, Teerlink JR, et al. Efficacy of LCZ696 persists at lower than target doses in the PARADIGM-HF trial. J Card Fail. 2016;21(8):S9–S10.
Jhund PS, Fu M, Bayram E, Chen CH, Negrusz-Kawecka M, Rosenthal A, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–84.
Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9(1):e002560.
Simpson J, Jhund PS, Cardoso JS, Martinez F, Mosterd A, Ramires F, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores. J Am Coll Cardiol. 2015;66(19):2059–71.
McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, et al. A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J. 2015;36(7):434–9.
Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41(9):1510–8.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95.
Jhund PS, Claggett B, Packer M, Zile MR, Voors AA, Pieske B, et al. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: an analysis of the PARAMOUNT trial. Eur J Heart Fail. 2014;16(6):671–7.
Jhund PS, Claggett BL, Voors AA, Zile MR, Packer M, Pieske BM, et al. Elevation in high-sensitivity troponin T in heart failure and preserved ejection fraction and influence of treatment with the angiotensin receptor neprilysin inhibitor LCZ696. Circ Heart Fail. 2014;7(6):953–9.
Novartis Pharmaceuticals. Efficacy and safety of LCZ696 compared to valsartan, on morbidity and mortality in heart failure patients with preserved ejection fraction (PARAGON-HF). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2013 Aug 8- [cited 2016 Jul 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT01920711.
Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–66.
Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705.
Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–6.
Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61(1):112–9.
Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58(5):839–46.
Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62(3):542–9.
Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60(6):1451–7.
Pu Q, Brassard P, Javeshghani DM, Iglarz M, Webb RL, Amiri F, et al. Effects of combined AT1 receptor antagonist/NEP inhibitor on vascular remodeling and cardiac fibrosis in SHRSP. J Hypertens. 2008;26(2):322–33.
Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, et al. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens. 2015;28(12):1409–17.
Williams B, Cockcroft JR, Kario K, et al. Principal results of the prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin receptor blocker measuring arterial stiffness in the elderly (PARAMETER) study. European Society of Cardiology 2015 Congress:London, UK. Abstract 4143. August 31, 2015
Kario K, Tamaki Y, Okino N, Gotou H, Zhu M, Zhang J. LCZ696, a first-in-class angiotensin receptor-neprilysin inhibitor: the first clinical experience in patients with severe hypertension. J Clin Hypertens (Greenwich). 2016;18(4):308–14.
Lazzeri C, Franchi F, Porciani C, Fronzaroli C, Casini Raggi V, De Feo ML, et al. Systemic hemodynamics and renal function during brain natriuretic peptide infusion in patients with essential hypertension. Am J Hypertens. 1995;8(8):799–807.
Franco-Saenz R, Somani P, Mulrow PJ. Effect of atrial natriuretic peptide (8-33-Met ANP) in patients with hypertension. Am J Hypertens. 1992;5(5 Pt 1):266–75.
Predel HG, Schulte-Vels O, Glanzer K, Meyer-Lehnert H, Kramer HJ. Atrial natriuretic peptide in patients with essential hypertension. Hemodynamic, renal, and hormonal responses. Am J Hypertens. 1991;4(11):871–9.
Singer DR, Markandu ND, Buckley MG, Miller MA, Sugden AL, Sagnella GA, et al. Prolonged decrease in blood pressure after atrial natriuretic peptide infusion in essential hypertension: a new anti-pressor mechanism? Clin Sci (Lond). 1989;77(3):253–8.
Cataliotti A, Costello-Boerrigter LC, Chen HH, Textor SC, Burnett Jr JC. Sustained blood pressure-lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin Proc. 2012;87(4):413–5.
Patel P, Chen HH. Natriuretic peptides as a novel target in resistant hypertension. Curr Hypertens Rep. 2015;17(3):18.
Benigni A, Zoja C, Zatelli C, Corna D, Longaretti L, Rottoli D, et al. Vasopeptidase inhibitor restores the balance of vasoactive hormones in progressive nephropathy. Kidney Int. 2004;66(5):1959–65.
Cao Z, Burrell LM, Tikkanen I, Bonnet F, Cooper ME, Gilbert RE. Vasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized rats. Kidney Int. 2001;60(2):715–21.
Seymour AA, Asaad MM, Lanoce VM, Langenbacher KM, Fennell SA, Rogers WL. Systemic hemodynamics, renal function and hormonal levels during inhibition of neutral endopeptidase 3.4.24.11 and angiotensin-converting enzyme in conscious dogs with pacing-induced heart failure. J Pharmacol Exp Ther. 1993;266(2):872–83.
Cataliotti A, Boerrigter G, Chen HH, Jougasaki M, Costello LC, Tsuruda T, et al. Differential actions of vasopeptidase inhibition versus angiotensin-converting enzyme inhibition on diuretic therapy in experimental congestive heart failure. Circulation. 2002;105(5):639–44.
Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17(5):510–7.
Wang BH, von Lueder TG, Kompa AR, Huang L, Webb R, Jordaan P, et al. Combined angiotensin receptor blockade and neprilysin inhibition attenuates angiotensin-II mediated renal cellular collagen synthesis. Int J Cardiol. 2015;186:104–5.
Bodey F, Hopper I, Krum H. Neprilysin inhibitors preserve renal function in heart failure. Int J Cardiol. 2015;179:329–30.
ISRCTN Registry. London: current control trials, c/o BioMed Centrol. 2010. UK Heart and Renal Protection (UK HARP-III); 2014 Jan 20[cited 2016 Jul 21]; Available from: http://www.isrctn.com/ISRCTN11958993.
von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8(1):71–8.
Marshall JM, Chamberlin KW. New drug review: FDA approves sacubitril/valsartan to treat heart failure. Drug Topics. 2015;159(11)
European Medicines Agency. New medicine to treat heart failure recommended for approval. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/09/WC500194185.pdf. Accessed Sep 25, 2015.
Moe GW, Ezekowitz JA, O'Meara E, Lepage S, Howlett JG, Fremes S, et al. The 2014 Canadian cardiovascular society heart failure management guidelines focus update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol. 2015;31(1):3–16.
Bayes-Genis A. Neprilysin in heart failure: from oblivion to center stage. JACC Heart Failure. 2015;3(8):637–40.
Vodovar N, Seronde MF, Laribi S, Gayat E, Lassus J, Januzzi Jr JL, et al. Elevated plasma B-type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. JACC Heart Failure. 2015;3(8):629–36.
Dec GW. LCZ696 (sacubitril/valsartan): can We predict who will benefit? J Am Coll Cardiol. 2015;66(19):2072–4.
Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18(8):1–10.
Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. (2016)
Filippatos G, Farmakis D, Parissis J, Lekakis J. Drug therapy for patients with systolic heart failure after the PARADIGM-HF trial: in need of a new paradigm of LCZ696 implementation in clinical practice. BMC Med. 2015;1(13):1–3.
Desai AS, Solomon S, Claggett B, McMurray JJ, Rouleau J, Swedberg K, et al. Factors associated with Noncompletion during the run-in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM-HF trial. Circ Heart Fail. 2016;9(6):e002735.
Ayalasomayajula S. Jordaan, Pierre, Parasar, Albrecht, et al. abstract 448: assessment of drug interaction potential between LCZ696 and warfarin. Hypertension. 2013;62(Suppl 1):A448–8.
Ayalasomayajula S, Jordaan P, Pal P, Albrecht D, Langenickel T, Sunkara G, et al. Abstract 449: assessment of pharmacokinetic drug interaction between LCZ696 and digoxin. Hypertension. 2013;62(Suppl 1):A449–9.
Ayalasomayajula S, Pan W, Han Y, Yang F, Langenickel T, Pal P, et al. Assessment of drug-drug interaction potential between atorvastatin and LCZ696, a novel angiotensin receptor neprilysin inhibitor, in healthy Chinese male subjects. Eur J Drug Metab Pharmacokinet. 2016;1-10
Hsiao HL, Langenickel TH, Greeley M, Roberts J, Zhou W, Pal P, et al. Pharmacokinetic drug-drug interaction assessment between LCZ696, an angiotensin receptor neprilysin inhibitor, and hydrochlorothiazide, amlodipine, or carvedilol. Clin Pharmacol Drug Dev. 2015;4(6):407–17.
Gan L, Jiang X, Mendonza A, Swan T, Reynolds C, Nguyen J, et al. Pharmacokinetic drug-drug interaction assessment of LCZ696 (an angiotensin receptor neprilysin inhibitor) with omeprazole, metformin or levonorgestrel-ethinyl estradiol in healthy subjects. Clin Pharmacol Drug Dev. 2016;5(1):27–39.
King JB, Bress AP, Reese AD, Munger MA. Neprilysin inhibition in heart failure with reduced ejection fraction: a clinical review. Pharmacotherapy. 2015;35(9):823–37.
Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol. 2016;1(6):1–4.
Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, et al. Cost-effectiveness analysis of Sacubitril/Valsartan vs Enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
We confirm that there is no funding support in this manuscript and that we did not receive any support from the sponsor Novartis. We declare that we are the sole authors of the manuscript and the article was not written by any agency or bureau with any external financial support.
Conflict of Interest
The authors declare that they do not have any conflict of interest with respect to this manuscript.
Ethical Approval
This article does not contain studies with human participants or animals performed by any of the authors.
Additional information
L. M. Lin and M. F. Wu contributed equally to this article.
Rights and permissions
About this article
Cite this article
Lin, L.M., Wu, Y., Wu, M.F. et al. Focus on the Novel Cardiovascular Drug LZC696: from Evidence to Clinical Consideration. Cardiovasc Drugs Ther 30, 623–633 (2016). https://doi.org/10.1007/s10557-016-6699-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6699-5