Abstract
Heart failure affects over five million Americans each year and contributes to morbidity, mortality, and high health care costs. Despite the benefits of RAAS and SNS blockers, 5-year survival rates in patients with heart failure remain low, necessitating continued research and new drug targets. LCZ696 (sacubitril/valsartan) is an angiotensin-receptor neprilysin inhibitor recently approved for HFrEF, with dual actions that result in enhancement of natriuretic peptide levels and blockade of angiotensin II activities. This drug shows promise in further improving clinical outcomes in HFrEF and is being studied in patients with HFpEF. In the PARADIGM-HF study, LCZ696 (sacubitril/valsartan) was shown to reduce the composite of cardiovascular mortality and heart failure hospitalizations compared with enalapril in patients with HFrEF taking guideline-directed medical therapies and resulted in prolonged survival. In trials, hypotension occurred more frequently with LCZ696 (sacubitril/valsartan) compared to an ACE inhibitor, warranting careful dose titration. Further clinical experience with LCZ696 (sacubitril/valsartan) will provide additional information on tolerability in a broad range of patients of various demographics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 5.1 million Americans over 20 years are diagnosed with heart failure. That number is expected to rise by 25 % by the year 2030. At age 40, the risk of developing heart failure is 20 % for both men and women and the lifetime risk is even greater for those who also have hypertension [1]. The incidence of heart failure is highest among African American males and lowest among Caucasian females [2–4]. Heart failure with reserved ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) each make up about half of the overall heart failure disease burden [5]. Heart failure is responsible for over one million hospitalizations every year [1], and nearly one in four of those patients will be readmitted to the hospital within 30 days of discharge [6]. The incidence of heart failure increases with age and the majority of the disease burden is seen in patients age 65 and older [1]. It is estimated that 50 % of people with heart failure will die due to complications of the disease within 5 years [7, 8]. Health care costs are also expected to rise by over 120 % from the 2013 estimate of $32 billion to nearly $70 billion by the year 2030 [9].
The pathophysiology of HFpEF varies greatly from HFrEF. As such, while there are well-defined treatment strategies for HFrEF that are known to reduce morbidity and mortality of this syndrome, optimal treatment of HFpEF remains unclear. Neurohormonal blockers have not demonstrated mortality benefits in HFpEF but some have reduced hospitalizations [10]. Consequently, HFpEF is significantly more challenging to treat, and additional studies are required to continue to uncover strategies for treating these patients.
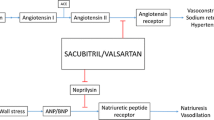
The current approach for reducing the morbidity and mortality in the setting of HFrEF has focused on blocking the renin-angiotensin-aldosterone-system (RAAS) and the sympathetic nervous system (SNS) with the use of ACE inhibitors (or angiotensin receptor blockers or ARBs if ACE inhibitors are not tolerated), beta blockers, and mineralocorticoid receptor antagonists. However, even with optimal use of these medications, the 5-year mortality rate in patients with heart failure remains close to 50 % [7, 8]. New therapies are needed to further improve clinical outcomes for this devastating syndrome. LCZ696 (sacubitril/valsartan) is an angiotensin receptor-neprilysin inhibitor (ARNi) with a novel mechanism which targets the natriuretic peptide system.
Neprilysin Inhibition and the Natriuretic Peptide System and Its Role in Heart Failure
Neprilysin is a neutral endopeptidase responsible for cleaving, among other things, natriuretic peptides. Natriuretic peptides are responsible for inducing natriuresis via urinary sodium excretion. The three natriuretic peptides, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and c-type natriuretic peptide (CNP), are naturally occurring endogenous peptides that are released under different conditions. ANP and BNP are released after stretch of the atria and ventricles, respectively, in the setting of myocardial damage and/or overload, ventricular dysfunction, and heart failure. ANP and BNP activate downstream receptors which ultimately lead to vasodilation, natriuresis, and diuresis [11]. CNP is released primarily from endothelial cells and does not appear to have a significant impact on sodium or water excretion, but rather exhibits a paracrine or autocrine role as a vasodilator and acts to stimulate the growth of long bones [12–14]. The clearance of natriuretic peptides is mediated through the natriuretic peptide clearance receptor, or C-type receptor, and enzyme degradation, via the actions of neprilysin. Therefore, inhibition of neprilysin leads to reduced breakdown and increased concentrations of endogenous natriuretic peptides.
Neprilysin is also responsible for the breakdown of other vasodilating peptides, namely adrenomedullin and bradykinin, and others which act as vasoconstrictors, such as angiotensin I, angiotensin II, and endothelin-1, in addition to the peptide amyloid beta protein [15, 16]. As such, neprilysin inhibition leads not only to raised natriuretic peptide concentrations but also increased levels of the vasoconstricting neurohormone angiotensin II.
Attempts at Lone Neprilysin Inhibition
Candoxatril was the first neprilysin inhibitor tested in humans, in patients with essential hypertension. As predicted, levels of natriuretic peptides, angiotensin II, and endothelin-I were increased. This resulted in increased natriuresis, evidenced by a three- to sixfold increase in urinary sodium excretion. Effects on blood pressure were inconsistent, and in one study, participants exhibited increased systemic vascular resistance [17, 18]. In patients with chronic heart failure, intravenous candoxatril increased plasma ANP, showing improved diuresis, natriuresis, reduced right atrial pressure, and reduced pulmonary capillary wedge pressure (PCWP) 1 to 2 h after administration, with no significant changes in systolic blood pressure [18]. In summary, the net effect of neprilysin inhibition is increased concentrations of both vasoconstrictor and vasodilator peptides, which when administered alone shows little effect on blood pressure and only modest benefits in heart failure [17, 19]. These studies reinforced the notion of dual inhibition of both neprilysin and RAAS.
The Development of Dual-Acting Neprilysin/RAAS Inhibitors
Omapatrilat was the first combined neprilysin and ACE inhibitor, also termed a vasopeptidase inhibitor, which showed initial promise in patients with heart failure. However, angioedema was observed more frequently with omapatrilat in clinical trials compared to active controls [20, 21]. Angioedema was thought to occur due to omapatrilat’s inhibition of three enzymes responsible for bradykinin breakdown: ACE, neprilysin, and aminopeptidase P [22–24]. Further research and development of vasopeptidase inhibitors was stopped. Focus shifted to combining a neprilysin inhibitor with an angiotensin receptor blocker (ARB), as the risk for angioedema is significantly reduced compared to ACE inhibitors [24]. This led to the development of LCZ696 (sacubitril/valsartan), a first-in-class angiotensin receptor-neprilysin inhibitor (ARNi).
LCZ696 (Sacubitril/Valsartan) Pharmacology
In LCZ696, the ARB valsartan and the neprilysin inhibitor are fused into a single molecular complex. LCZ696 dissociates into valsartan and AHU377 (sacubitril) shortly after ingestion. Sacubitril is subsequently converted by esterases into its active component LBQ657. The mean half-lives for valsartan, sacubitril, and LBQ657 are 8.9 to 16.6, 1.1 to 3.6, and 9.9 to 11.1 h, respectively [24]. Other pharmacokinetic parameters are summarized in Table 1. The 97/103 mg dose of sacubitril/valsartan administered twice daily is bioequivalent to valsartan 160 mg twice daily [24].
LCZ696 (sacubitril/valsartan) is a weak inhibitor of CYP2C9. Drug interaction testing with warfarin did not yield significant pharmacokinetic and pharmacodynamics effects with co-administration [25]. Digoxin did not alter the pharmacokinetics of LCZ696 [26]. The exposure of metformin decreased by 23 % in the presence of LCZ696 [27], and the maximum concentration of the valsartan component of LCZ696 was decreased by 12 % when co-administered with carvedilol [28]. In vitro, sacubitril was shown to inhibit OATP1B1 and OATP1B3 transporters. OATP transporters are found in the hepatocytes and are responsible for the uptake of drugs from the blood into the liver. Inhibition of OATP transporters may lead to increased plasma concentrations of substrates, including repaglinide and commonly used statin medications [29].
Clinical Trials
HFrEF
PARADIGM-HF was a multi-national, double-blind, randomized, active controlled trial comparing the effects of enalapril 10 mg twice daily versus LCZ696 200 mg twice daily in patients with symptomatic heart failure with reduced ejection fraction. After the trial began, the entry criterion for left ventricular ejection fraction (LVEF) was changed from ≤40 % to an LVEF ≤35 %. All eligible patients participated in two back-to-back single-blind, active run in phases to assess tolerability to both study medications. Participants were titrated over a period of 2 weeks to enalapril 10 mg twice daily, followed by a second run in phase with LCZ696 titrated to 200 mg twice daily. Patients who could not tolerate these doses did not proceed to randomization. After the active run in phase, patients were randomized in a double-blind fashion to receive enalapril 10 mg twice daily or LCZ696 200 mg twice daily. PARADIGM-HF was stopped early by the Data Monitoring Committee due to overwhelming efficacy of LCZ696 compared to enalapril in reducing the composite endpoint of cardiovascular mortality and heart failure hospitalizations (hazard ratio [HR] 0.80, 95 % CI 0.73–0.87). Each component of the composite endpoint was also significantly reduced with LCZ696 compared to enalapril. LCZ696 (sacubitril/valsartan) was also shown to reduce death from any cause (HR 0.84, 95 % CI 0.76–0.93). LCZ696 was well tolerated overall; hypotension was the most common adverse effect during the trial and occurred more frequently among those taking LCZ696 (P < 0.001). More patients stopped the trial due to renal impairment in the enalapril group. Angioedema occurred in 19 patients in the LCZ696 group and 10 patients in the enalapril group; however, no severe cases of angioedema, causing airway compromise, occurred during the trial [30].
Additional Analyses from PARADIGM-HF
A subsequent analysis from PARADIGM-HF examined the clinical progression of surviving patients taking LCZ696 (sacubitril/valsartan) or enalapril. Investigators assessed NYHA functional class, Kansas City Cardiomyopathy Questionnaire (KCCQ) score, worsening heart failure requiring an increase in diuretic dose for longer than 1 month, intravenous therapy, or addition of a new drug, worsening heart failure leading to emergency room visits or hospitalizations, use of interventions for worsening heart failure, and changes in specific biomarkers in heart failure including, NTproBNP, troponin T, plasma BNP, and cGMP. Participants taking LCZ696 (sacubitril/valsartan) had less worsening of NYHA functional class compared to the enalapril group at 8 and 12 months of follow-up (P = 0.001 and 0.03). Additionally, LCZ696 (sacubitril/valsartan) led to an improved score on the KCCQ survey. Participants taking LCZ696 (sacubitril/valsartan) has reduced requirements for diuretic dose changes, intravenous therapy or use of an additional therapy in worsening heart failure. Those in the LCZ696 (sacubitril/valsartan) group had less emergency department visits for heart failure compared with the enalapril group and had less hospitalizations (all-cause, heart failure, and cardiovascular). Less patients taking LCZ696 (sacubitril/valsartan) required intensive care during a hospitalization, and fewer required IV ionotropic drugs during a hospitalization. The number of patients requiring interventions for worsening heart failure, including cardiac resynchronization, ventricular assist device placement, or cardiac transplant, was not significantly different between groups. Concentrations of NTproBNP and troponin T were both significantly decreased in the LCZ696 (sacubitril/valsartan) group compared to enalapril (P < 0.0001 for both) at 4 weeks and 8 months. Additionally, cGMP was significantly increased with LCZ696 (sacubitril/valsartan) compared to enalapril (P < 0.0001) at 4 weeks and 8 months [31].
Another post hoc analysis examined the effects of LCZ696 (sacubitril/valsartan) and enalapril on mode of death during the PARADIGM-HF trial [32]. The majority of deaths (80.9 %) in PARADIGM-HF were cardiovascular, with only 14.8 % of deaths from non-cardiovascular causes. The number of deaths due to non-cardiovascular causes between LCZ696 (sacubitril/valsartan) and enalapril was similar (P = 0.59). The remaining deaths, 4.3 %, could not be classified as either cardiovascular or non-cardiovascular cause, and were equally divided between both treatment groups (P = 0.97). LCZ696 (sacubitril/valsartan) resulted in a 20 % reduced risk for sudden cardiac death and a 21 % reduced risk for death due to worsening heart failure compared with enalapril.
The effect of age on study outcomes and tolerability in PARADIGM-HF were also examined [33]. Participants were analyzed by four age categories: <55, 55–64, 65–74, and ≥75 years. Older patients were more likely to have a worse NYHA class, had more comorbidities, a worse KCCQ scores compared to younger age groups at baseline. Average tolerated enalapril dose was 19.0, 19.0, 18.9, and 18.5 mg for age groups <55, 55–64, 65–74, and ≥75 years, respectively (P < 0.001). Average tolerated LCZ696 (sacubitril/valsartan) dose was 377, 381, 371, and 367 mg, respectively, for the same age groups. The occurrence of primary endpoint of cardiovascular death or heart failure hospitalization was reduced in the LCZ696 (sacubitril/valsartan) group compared to enalapril for the <55, 55–64, and 65–74 age groups, but not the ≥75 age group. For cardiovascular deaths, LCZ696 (sacubitril/valsartan) significantly lowered the risk in the 55–64 and 65–74 age groups, but not among the <55 or ≥75 age groups. Heart failure hospitalizations were lower with LCZ696 (sacubitril/valsartan) among those aged <55 and 55–64 years but were not significantly lower in patients aged 65–74 and ≥75 years. Of those taking LCZ696 (sacubitril/valsartan), all-cause mortality was significantly reduced among those aged 65–74 years (HR 0.81, 95 % CI 0.68–0.97) but not in other age groups. Generally, adverse effects increased in frequency with age; hypotension was the most common adverse effect in both treatment groups.
HFpEF
PARAMOUNT was a multicenter, randomized, active-controlled, double-blind trial examining the efficacy of LCZ696 (sacubitril/valsartan) 200 mg twice daily compared with valsartan 160 mg twice daily in patients with heart failure with preserved ejection fraction (HFpEF) [34]. Patients with NYHA functional class II-III, LVEF of 45 % or greater, and NT-proBNP greater than 400 pg/mL were eligible to participate. Participants were studied for 12 weeks and followed for an additional 24 weeks. The primary endpoint for the study was change in NT-proBNP at 12 weeks. Secondary endpoints were quality of life, measured by the KCCQ, change in NYHA class, changes in blood pressure, and changes in echocardiographic measures. Those randomized to LCZ696 (sacubitril/valsartan) showed significant reduction in NT-proBNP at 12 weeks. Significant reductions in blood pressure were noted at 12 and 36 weeks. Additionally, at 36 weeks, patients showed reductions in left atrial size (P = 0.003) and left atrial dimension (P = 0.034) and improvements in New York Heart Association (NYHA) class (P = 0.05) [34]. As a proof-of-concept study, PARAMOUNT paved the path for a larger phase III trial in patients with HFpEF. The PARAGON study will be powered to measure differences in the composite endpoint of cardiovascular death and heart failure hospitalizations.
Sacubitril/Valsartan Dosing Considerations
LCZ696 (sacubitril/valsartan) was approved in the USA in July 7th, 2015, under the trade name Entresto. LCZ696 (sacubitril/valsartan) is available in three doses, 24/26, 49/51, and 97/103 mg, and is indicated for use in adults with NYHA class II-IV chronic heart failure with reduced left ventricular ejection fraction [35]. LCZ696 (sacubitril/valsartan) should be administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB, and without regard to meals. LCZ696 (sacubitril/valsartan) can be dosed at 24/26 mg twice daily in patients with renal dysfunction (eGFR <30 mL/min/1.73 m2) or moderate hepatic impairment (Child-Pugh class B). Additionally, clinicians may initiate LCZ696 (sacubitril/valsartan) at a lower dose in patients who are naïve to ACE inhibitor or ARB therapy or who were on low doses of these RAAS blockers. Age-related declines in renal and hepatic function result in increased drug concentrations of LCZ696 (sacubitril/valsartan) among older adults, but dose adjustment is not recommended based on age [36]. Suggested starting doses of LCZ696 (sacubitril/valsartan) based on previous doses of RAAS blockers are listed in Table 2.
Remaining Questions
There are limited data and experience with initiation of LCZ696 (sacubitril/valsartan) in patients hospitalized with acutely decompensated heart failure. While PARADIGM-HF included individuals with NYHA functional class IV and the labeling includes such patients, the majority studied had mild to moderate heart failure symptoms, limiting experience in those with severe heart failure. More widespread experience with LCZ696 (sacubitril/valsartan) will further inform questions regarding tolerability, specifically with respect to risk for angioedema. RAAS blockade has been associated with an increased prevalence of angioedema, especially among African Americans [38, 39], as such providers should monitor these patients closely. The possibility of LCZ696 (sacubitril/valsartan) replacing ACE inhibitors as first-line therapy in patients newly diagnosed with heart failure will be determined following clinical experience and additional research with this new drug.
Conclusions
Natriuretic peptides offer benefit in heart failure through vasodilation and natriuresis. Neprilysin is an enzyme responsible for the breakdown of natriuretic peptides. LCZ696 (sacubitril/valsartan) is an angiotensin receptor neprilysin inhibitor with the potential to positively affect clinical outcomes patients with heart failure beyond current standard of care. Its dual mechanism of augmentation of natriuretic peptides and RAAS blockade has proven superiority over enalapril in a large clinical trial and improves survival. Additional studies are needed to examine exact mechanisms responsible for potential ancillary benefits of neprilysin inhibition. Results from ongoing trials will delineate the role of neprilysin inhibition in the setting of HFpEF and cardiovascular disease. Further clinical experience with LCZ696 (sacubitril/valsartan) will provide information on tolerability in a broad range of patients of various ethnicities.
References
Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245.
Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atheroscleroris. Arch Intern Med. 2008;168(19):2138–45.
Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168(4):418–24.
Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation. 2002;106(24):3068–72.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9.
Ranasinghe I, Wang Y, Dharmarajan K, Hsieh AF, Bernheim SM, Krumholz HM. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: a retrospective observational cohort study. PLoS Med. 2014;11(9), e1001737.
Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–50.
Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–402.
Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Levin ER, Gardner DG, Sampson WK. Natriuretic peptides. N Engl J Med. 1998;339(5):321–8.
Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99(2):76–82.
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett Jr JC. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–893c.
Stingo AJ, Clavell AL, Aarhus LL, Burnett Jr JC. Cardiovascular and renal actions of C-type natriuretic peptide. Am J Physiol. 1992;262(1 Pt 2):H308–312.
Lisy OJ, Jougasaki M, Schirger JA, Chen HH, Barclay PT, Burnett Jr JC. Neutral endopeptidase inhibition potentiates the natriuretic actions of adrenomedullin. Am J Physiol. 1998;275(3 Pt 2):F410–4.
Liu Y, Studzinski C, Beckett T, Murphy MP, Klein RL, Hersh LB. Circulating neprilysin clears brain amyloid. Mol Cell Neurosci. 2010;45(2):101–7.
Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26(6 Pt 2):1160–6.
McDowell G, Nicholls DP. The therapeutic potential of candoxatril, a neutral endopeptidase inhibitor, in humans. Cardiovasc Drug Rev. 2000;18(4):259–70.
Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The international ecadotril multi-centre dose-ranging study investigators. Lancet. 1998;35(9116):1657–8.
Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002;106(8):920–6.
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–11.
Blumberg AL, Denny SE, Marshall GR, Needleman P. Blood vessel-hormone interactions: angiotensin, bradykinin, and prostaglandins. Am J Physiol. 1977;232(3):H305–310.
Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension. 2004;44(6):913–8.
Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor. (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Ayalasomayajula S, Jordaan P, Pal P, et al. Assessment of drug interaction potential between LCZ696 and warfarin. Hypertension. 2013;62:A448.
Ayalasomayajula S, Jordaan P, Pal P, et al. Assessment of pharmacokinetic drug interaction between LCZ696 and digoxin. Hypertension. 2013;62:A449.
Mendonza A, Mizuki A, Langenickel T, et al. Assessment of pharmacokinetic drug interaction between LCZ696 and metformin. Hypertension. 2013;62:A456.
Hsiu-Ling H, Greeley M, Pal P, et al. Assessment of pharmacokinetic drug–drug interaction between LCZ696 and carvedilol. Hypertension. 2013;62:A457.
Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693–705.
McMurray J, Packer M, Desai A, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Packer M, McMurray J, Akshay S, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61.
Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015. doi:10.1093/eurheartj/ehv186.
Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015.
Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95.
Product Information: ENTRESTO(TM) oral tablets, sacubitril valsartan oral tablets. Novartis Pharmaceuticals Corporation (per Manufacturer), East Hanover, NJ, 2015.
Gan L, Langenickel T, Petruck J, et al. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2015. doi:10.1002/jcph.571.
McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison and ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Eur J Heart Fail. 2013;15(9):1062–73.
Sondhi D, Lippmann M, Murali G. Airway compromise due to angiotensin-converting enzyme inhibitor-induced angioedema: clinical experience at a large community teaching hospital. Chest. 2004;126(2):400–4.
Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of Afrigin origin. Br J Clin Pharmacol. 1999;48(6):861–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jared Mills declares that he has no competing interests.
Orly Vardeny has received consulting honoraria from Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathophysiology of Myocardial Failure
Rights and permissions
About this article
Cite this article
Mills, J., Vardeny, O. The Role of Neprilysin Inhibitors in Cardiovascular Disease. Curr Heart Fail Rep 12, 389–394 (2015). https://doi.org/10.1007/s11897-015-0270-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-015-0270-8