Abstract
Purpose
The increase in endothelin-1 (ET-1) and the decrease in endothelial nitric oxide synthase (eNOS) both induce vasoconstriction and lead to molecular changes associated with diabetes mellitus and atherosclerosis. Glucagon-like peptide-1 (GLP-1) activation stimulates insulin secretion and may prevent atherosclerosis by increasing eNOS synthesis. However, there is paucity of information on the effect of GLP-1 activation on ET-1 expression. This study was conducted to address this issue.
Methods and Results
Human umbilical vein endothelial cells (HUVECs) were incubated with different concentrations of liraglutide, a GLP-1 agonist, and the expression of ET-1 and eNOS and activity of NF-κB were measured. Liraglutide, in a concentration-dependent manner, was observed to promote eNOS expression and to inhibit ET-1 expression both at mRNA and protein levels. Liraglutide also inhibited NF-κB phosphorylation and its translocation from cytoplasm to the nucleus. To ascertain the role of NF-κB activation in the altered expression of ET-1 and eNOS, we treated HUVECs with phorbol 12-myristate 13-acetate (PMA). PMA activated NF-κB and reversed the effects of liraglutide on eNOS and ET-1 expression. The effects of PMA on eNOS and ET-1 expression were reproduced in experiments wherein cells were treated with TNF-α. Further, we measured the generation of IL-6, apowerful pro-inflammatory molecule released by endothelial cells, as a measure of cellular function. PMA increased IL-6 generation, and this effect was blocked by liraglutide.
Conclusions
Our observations suggest liraglutide suppresses ET-1 expression by inhibiting the phosphorylation of NF-κB. This mechanism may underlie the potential anti-atherosclerotic effects of GLP-1 agonists. Of note, these effects of liraglutide were seen in an in vitro setting wherein cellular glucose concentrations were elevated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a common complication of diabetes mellitus [1]. Endothelial dysfunction is a very early event in diabetes and atherosclerosis, and is in part characterized by enhanced endothelin-1 (ET-1) and diminished nitric oxide synthase (eNOS) expression [2]. ET-1 produced by endothelial cells is a potent vasoconstrictor whereas eNOS induces intense vasodilatation through NO synthesis [3]. The balance between ET-1 and eNOS expression maintains the normal function of endothelial cells in terms of cellular integrity, and release of pro-inflammatory cytokines like interleukin-6 (IL-6). A disruption in this delicate balance is thought to be an initiating factor in atherogenesis in diabetes [4].
Glucagon-like peptide-1 (GLP-1), a 30-amino acid gut hormone mainly derived from intestinal L cell, stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying and reduces postprandial hyperglycemia [5]. Most GLP-1 agonists are quickly degraded by dipeptidyl peptidase-4 (DPP-4)accounting for their short half-life. The new GLP-1 receptor agonist liraglutide has a long-half-life in plasma [6] and is often used in patients with diabetes. Studies show that liraglutide improves endothelium-dependent vasorelaxation in patients with diabetes mellitus and exerts cardioprotective effects in animal models [7–9]. Its vasodilator and cardioprotective effects are beyond the benefits achieved as an anti-diabetic agent. Most studies have attributed its salutary effects to enhancement of eNOS expression resulting in the formation of large amounts of NO which is vasodilator and anti-inflammatory molecule. However, the effect of GLP-1 agonists on the expression of ET-1, a potent vasoconstrictor and pro-inflammatory molecule synthesized in endothelial cells is not known.
This study was designed to test the hypothesis that GLP-1 receptor agonist liraglutide may negatively regulate ET-1 via an inhibitory effect on phosphorylation of the transcription factor NF-κB.
Materials and Methods
Reagents
Human umbilical vein endothelial cells (HUVECs) (CRL-1730, human) and F-12 K cell culture medium were obtained from ATCC (Manassas, VA); liraglutide and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma Aldrich (St. Louis, MO); TRIZOL reagent (Invitrogen, Grand Island, NY); real-time PCR primers (Integrated DNA technologies, Coralville, Iowa); GoTaq® qPCR Master Mix kit was bought from Promega (Madison, WI); Antibodies were obtained from Abcam (Cambridge, MA) (GLP-1R, IL-6); Santa Cruz Biotechology (Santa Cruz, CA) (ET-1); Cell Signaling Technology (Danvers, MA)(eNOS), and Sigma Aldrich (St. Louis, MO) (TNF-α).
Cell Culture and Drugs Incubation
HUVECs were recovered and sub-cultured in high glucose(25 mmol/L)F-12 K medium containing 10 % fetal bovine serum (FBS) and 1 % endothelial cell growth supplement (ScienCell, San Diego, CA). After incubation in a 95 % air with humidified atmosphere and 5 % CO2 at 37 °C, 3rd–8th passage cells were used in all experiments. When grown to 60 %–85 % confluence, cells were incubated in different concentrations (0, 10, 100, 1,000 ng/mL) of liraglutide and PMA (100 ng/mL) or tumor necrosis factor-alpha (TNF-α) (5 ng/mL)for 6–24 h and then collected for further analysis.
Real-Time Quantitative PCR
TRIZOL reagent was used to extract total RNA. The cDNA (150 ng) from reverse transcription was amplified using 300 nM primers. Primer sequences are shown in Table 1. GoTaq® qPCR Master Mix kit was used to detect each mRNA. Applied Biosystems 7900 real-time PCR system was used for performing real-time PCR as described previously [10]. The standard cycling condition and reaction system followed with supplied protocols. GAPDH gene was used for normalizing the comparative threshold cycles values.
Western Blotting
Total and phosphorylated NF-κB and IκBα, eNOS, ET-1, IL-6 and GLP-1R proteins were quantified by Western blot. Cells were washed with ice-cold PBS, lysed and centrifuged (13,200 × g, 20 min, 4 °C) before supernatants collection. Protein concentrations were measured using a Bradford kit (Sigma Aldrich, St. Louis, MO). Equal amounts of samples (50 μg) were denatured and subjected to 12 % SDS-PAGE. After electro-transferring onto a PVDF membrane, the separated proteins were then blocked with 5 % non-fat milk (in PBS-T) for 1 h and incubated with primary antibodies overnight at 4 °C. The membranes were then washed and incubated with secondary antibody (1:10,000) and visualized by enhanced chemiluminescence (ECL; Santa Cruz Biotechnology). The intensities of the bands were quantified and analyzed with image J software. β-actin was used for normalizing relative expression of proteins.
Immunofluorescence Studies
Immunostaining of the cultured HUVECs was performed as previously described [11] using standard methods. All samples were imaged with fluorescence microscope using LSM510 software.
Statistical Analysis
All experiments were performed at least in quadruplicate. Values were analyzed by using 2-tailed Newman-Keuls-Student t-test or one-way ANOVA (multiple means). Data are presented as means ± SD. A P value < 0.05 was considered significant.
Results
Liraglutide Stimulates GLP-1R Expression
To assess the effect of liraglutide on GLP-1R expression, we used different concentrations of liraglutide (0–1000 ng/mL). As shown in Fig. 1a, b, liraglutide significantly induced GLP-1R(mRNA and protein) expression in HUVECs in a concentration-dependent manner(P < 0.01 vs. control). The effects of high concentration liraglutide were also confirmed by immunofluorescence (Fig. 1c).
Liraglutide increases GLP-1R expression. For mRNA expression assay, cells were treated with different concentrations of liraglutide for 6-hours. For protein assay, cells were treated for 24-hours. a, b Liraglutide increases GLP-1R mRNA and protein expression. c Immunofluorescence staining for GLP-1R. *P < 0.05 vs. Control, **P < 0.01 vs. Control. Abbreviations: GLP-1: Glucagon-like peptide-1; GLP-1R: Glucagon-like peptide-1 receptor;. LRT: liraglutide
Liraglutide Inhibits ET-1 and Induces eNOS Expression
Next, we studied the effect of liraglutide on ET-1 and eNOS expression. As shown in Fig. 2, ET-1 expression decreased in response to liraglutide in a concentration-dependent manner (P < 0.01 vs. control). Of note, eNOS expression increased, consistent with previous observations [12]. The modulation of ET-1 and eNOS was seen at transcriptional level (qPCR) (Fig. 2a) as well as protein levels (Western blotting and immunofluorescence) (Fig. 2b, c).
Liraglutide decreases ET-1 and increases eNOS expression. a, b ET-1 and eNOS mRNA and protein expression after incubating HUVECs with different concentration of liraglutide. c Immunofluorescence staining for ET-1. *P < 0.05 vs. Control, **P < 0.01 vs. Control. Abbreviations; ET-1: Endothelin-1; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide
Liraglutide Inhibits NF-kB Translocation and IκBα Phosphorylation
To determine the intracellular mechanism of liraglutidein inhibiting ET-1 and enhancing eNOS expression, we measured NF-κBand IκBα expression in HUVECs. As shown in Fig. 3a, b, liraglutide had no effect on NF-kB mRNA or protein levels, but it significantly inhibited phosphorylation of NF-κB p65, especially in 100–1000 ng/ml concentrations (P < 0.05 vs. control). Likewise, IκBα phosphorylation was reduced by liraglutide at high concentration (Fig. 3c). The immunofluorescence studies indicated that high glucose concentration induces NF-κB translocation from cytoplasm to nucleus, and this effect is attenuated by liraglutide (Fig. 3d).
Liraglutide inhibits IκBα and NF-κB phosphorylation. a, b NF-κB activation is inhibited by liraglutide while total NF-κB remains unchanged. c Phosphate IκBα is also inhibited by liraglutide. d Reduction in NF-κBp65 migration from cytoplasm to the nucleus in response to liraglutide. *P < 0.05 vs. Control, **P < 0.01 vs. Control. Abbreviations: IκBα: Inhibitor of kappa B alpha; NF-κB: nuclear factor-KappaB
Confirmation of the Role of NF-κB and IκBα Activation
A previous study [13] showed that liraglutide may regulate endothelial function by inhibiting the NF-κB pathway, we chose PMA as a stimulus for NF-κB. To examine the role of NF-κB in the regulation of ET-1 and eNOS in response to liraglutide, we incubated HUVECs with liraglutide (1,000 ng/mL) alone, or with PMA (100 ng/mL), a potent NF-κB activator [14], for 6 h. As expected, the phosphorylation of NF-κB and its translocation from cytoplasm into nucleus decreased in response to liraglutide (P < 0.05 vs. control) (Fig. 4a, b). Treatment of cells with PMA enhanced NF-κB translocation from cytoplasm into the nucleus despite treatment with liraglutide (Fig. 4c). Similarly, the inhibition of IκBα phosphorylation was blocked by PMA treatment (Fig. 4d). Importantly, cells treated with liraglutide expressed less phosphorylated NF-κB and IκBα (both P < 0.05 vs. PMA) (Fig. 5a)
PMA induces IκBα and NF-κB phosphorylation after liraglutide treatment. a, b PMA counters the effect of liraglutide on NF-κB expression (both mRNA and protein) in HUVECs. c NF-κB migrates into the nucleus in PMA-treated HUVECs compared with liraglutide alone-treated cells. d Phosphate IκBα protein expression increases after PMA treatment to normal levels in liraglutide-treated cells. Cells were treated with PMA (100 ng/mL) for 6-hours.*P < 0.05 vs. Control, †P < 0.05 vs. liraglutide alone treatment. Abbreviation; PMA: phorbol 12-myristate 13-acetate
Liraglutide attenuates the effect of PMA on stimulating NF-κB, ET-1 and inhibiting eNOS protein expressions. a Liraglutide inhibits phosphorylation of IκBα and NF-κB. * P < 0.05 vs. control, † P < 0.05 vs. PMA alone treatment. b PMA treatment of cells almost completely blocked the effect of liraglutide on the expression of ET-1 as well as eNOS. c The effects of PMA on ET-1 and eNOS expression were replicated with the use of TNFα. *P < 0.05 vs. Control,† P < 0.05 vs. LRT alone treatment. §P < 0.05 vs. TNF-αalone treatment.LRT: liraglutide; PMA: phorbol 12-myristate 13-acetate; TNF-α:Tumor necrosis factor-alpha
Modulation of ET-1 and eNOS Expression by PMA and TNFα
As shown earlier, liraglutide decreased ET-1 and enhanced eNOS expression (at both mRNA and protein levels) (both P < 0.05 vs. control) (Fig. 2a, b). Treatment of cells with PMA blocked the effect of liraglutide (P < 0.05 vs. liraglutide). ET-1 immunofluorescence confirmed the Western blotting data on protein expression (Fig. 2c). Also, liragultide attenuated the effect of PMA on ET-1 and eNOS expression. (P < 0.05 vs. PMA) (Fig. 5b). To further prove that NF-κB is a key regulator for liraglutide regulating ET-1 and eNOS expression, we used TNFα as a specific NF-κB activator. The effects of TNFα on ET-1 and eNOS expression were similar to effects of PMA (Fig. 5c).
Of note, the effect of liraglutide on eNOS and ET-1 mRNA were evident at 100 ngml concentration and on protein at 1,000 ng/ml concentration.
Functional Relevance of the Effects of Liraglutide on Endothelial Function
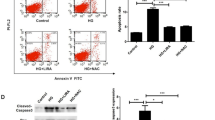
As shown in Fig. 6a, c, the liraglutide effect on ET-1 and eNOS were blocked by PMA treatment, both in transcriptional and translational levels. We also measured IL-6 as a key biomarker of endothelial function [15]. As shown in Fig. 6d, PMA markedly enhanced IL-6 protein levels (P < 0.05 vs. control), and liraglutide blocked this effect of PMA on IL-6 protein expression(P < 0.05 vs. PMA alone).
PMA induces ET-1 and inhibits eNOS after liraglutide treatment and liraglutide improves endothelial dysfunction. a, b ET-1 and eNOS levels return to normal level after PMA treatment in liraglutide-treated cells, suggesting that PMA counters the effects of liraglutide. c Immunofluorescence staining for ET-1. *P < 0.05 vs. Control, † P < 0.05 vs. Liraglutide alone treatment. d Liraglutide inhibits IL-6 protein expression, and PMA counters the effect of liraglutide. *P < 0.05 vs. Control, † P < 0.05 vs. LRT alone treatment. §P < 0.05 vs. PMA alone treatment. LRT: liraglutide; PMA: phorbol 12-myristate 13-acetate. IL-6: interleukin-6
Discussion
In this study, our first important novel observation was that liraglutide, a potent GLP-1R agonist, in modest concentrations decreased ET-1 expression in HUVECs cultured in high glucose medium, both at transcriptional and translational levels, particularly at high concentrations. Although the effect of 10–100 ng/mL liraglutide on ET-1 protein expression was not considered as significant, there is still a tendency of reduction on it. Our second major observation was that liraglutide decreased endothelial IL-6 expression.
To determine the mechanistic basis of ET-1 reduction, we focused onthe effect of liraglutide on the transcription factor NF-κB p65. NF-κB has been recognized as a key nucleus transcriptional factor which regulates the expression of a number of genes. We observed that liraglutide inhibited the activation of NF-kB and reduced its translocation to the nucleus in HUVECs. NF-kB expression is regulated by IκBα (discussed below in detail), and we noted that liraglutide also modulated the phosphorylation of IκBα. It is of note that the protein levels of NF-kB or IκBα were not affected by liraglutide.
We postulated that NF-kB phosphorylation may play a key role in the reduction in ET-1 expression as well as in the increase in eNOS expression by liraglutide. TNF-α has been reported to activate cGMP/PKC pathway and subsequently NF-kB phosphorylation and inhibit eNOS expression in endothelial cells. Also, since liraglutide has been shown to block this signaling pathway [13], we used PMA to prove our proposition it is also a stimulus of NF-kB [16]. As shown in Figs. 5c and 6, PMA treatment of cells almost completely blocked the effect of liraglutideon, the expression of ET-1 as well as eNOS. The effects of PMA on ET-1 and eNOS expression were replicated with the use of TNFα (Fig. 5c).
Obviously, the increase in eNOS coupled with a decrease in ET-1 would be expected to have a salutary effect on endothelial function. While eNOS is a vasodilator and inhibits an inflammatory reaction on the activated endothelium, ET-1 is a powerful vasoconstrictor and has been shown to exert pro-inflammatory effects in vascular tissues [17]. Atherosclerotic tissues, especially from diabetic animals, display a reduction in eNOS expression/activity and an increase in ET-1 expression in concert with endothelial dysfunction/activation and a pro-inflammatory state [18]. The pro-inflammatory state is characterized by excessive NF-kB activity and elevated generation of IL-6 and other cytokines. In the context of vascular inflammation that is commonly observed in diabetes and atherosclerosis, the effects of liraglutide on ET-1 expression and IL-6 formation in endothelial cells described in this study may relate to the potentially beneficial effects of liraglutide in clinical and experimental studies [7–9, 19]. Of note, the effect of liraglutide on eNOS and ET-1 mRNA were evident at 100 ngml concentration and on protein at 1,000 ng/ml concentration. This discrepant effect is not uncommonly seen in in vitro studies and may reflect delayed translation of the gene into protein.
Hattori et al. [4] in an in vitro study suggested that NF-κB is a dominant regulator for eNOS expression in the presence of liraglutide. Gaspari et al. [20] observed that liraglutide could induce eNOS expression and thereby improve endothelial function, as measured by the expression of PAI-1 and vascular adhesion molecules, in an ApoE null mouse model of atherosclerosis.
Several investigators have examined the mechanism of ET-1 activity, and found its expression to be regulated by NF-κB [21–23]. High concentration of glucose has been reported to induce NF-κB activation in different types of cells, including the HUVECs [24, 25]. As a transcriptional regulator of NF-κB, IκB kinase binds to and stabilizes NF-κB. After being phosphorylated, IκB kinase is degraded resulting in the stimulation of NF-κB phosphorylation. Soon thereafter, the activated NF-κB p65 translocates from the cytosol to the nucleus of the cell [26]. This pathway is consistent with our data on the inhibition of phosphorylation of IκBα and NF-κB by liraglutide treatment of cells.
There is also evidence that eNOS is a direct target of NF-κB. Grumbach et al. [27] reported a negative feedback between NF-κB activation and eNOS expression in endothelial cells. Others have also shown that NO can repress the activation of NF-κB through degradation of IκBα [28–31]. We observed in our study that the NF-κB activator PMA negatively influenced eNOS expression in HUVECs further lending credibility to the concept of feedback loop between IκBα, NF-kB and eNOS. It is of note that we also observed that PMA blocked the effect of liraglutide on ET-1 expression, suggesting that there is a feedback loop between IκBα, NF-κB and ET-1 (Fig. 7).
Mechanism of GLP-1-inducedimprovement in endothelial dysfunction and diabetic atherosclerosis. Liraglutide stimulates GLP-1R expression which subsequently inhibits IκBα and NF-κBcomplex and suppresses ET-1 expression and enhances eNOS expression. The modulation of ET-1 and eNOS would be expected to lead to vasodilatation and reduction in IL-6 release from endothelial cells, and perhaps reduction in inflammation and atherosclerosis
Since eNOS is an anti-inflammatory and ET-1 a pro-inflammatory molecule, and both seem to require NF-κB activation, NF-κB may be the key pro-inflammatory transcription factor in this process. Romeo et al. [32], and subsequently Ho et al. [33], reported that the high concentration of glucose activates NF-κB. We provide further support for this suggestion, and advance this concept by showing the modulation of ET-1 and eNOS expression by liraglutide in HUVECs cultured in high glucose concentration which may mimic the diabetic state. Importantly, results of our study with PMA provide definitive information on the role of NF-kB in this process.
We chose IL-6 measurement as a marker of endothelial dysfunction to assess the biological effect of liraglutide. IL-6 is a potent pro-inflammatory molecule released from endothelial cells. Its expression is elevated in diabetes as well as in atherosclerotic blood vessels [34]. IL-6 is also a potent stimulus for the formation of C-reactive protein formed in the liver; the plasma levels of C-reactive protein are well known to be elevated in diabetic patients and others with inflammatory vascular disease [35]. The inhibition of IL-6 by liraglutide seen in this study appears to be an effect of combination of ET-1 decrease and eNOS increase.
Collectively, our observations suggest that the GLP-1R agonist liraglutide is an effective inhibitor of ET-1 and an inducer of eNOS expression. Our study also shows that the upregulation of GLP-1R by liraglutide improves endothelial function (i.e. decrease in the pro-inflammatory IL-6). The effect of liraglutide on ET-1 may provide a novel mechanism which contributes to the present knowledge on improvement of atherosclerosis by GLP-1R agonists. It is likely that the observations described in this study underlie to some extent in the potential vasoprotective and cardioprotective effects of GLP-1R agonists [36].
Abbreviations
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1R:
-
Glucagon-like peptide-1 receptor
- ET-1:
-
Endothelin-1
- eNOS:
-
Endothelial nitric oxide synthase
- NF-κB:
-
Nuclear factor-KappaB
- HUVECs:
-
Human umbilical vein endothelial cells
- PMA:
-
Phorbol 12-myristate 13-acetate
- DPP4:
-
Dipeptidyl peptidase-4
- IκBα:
-
Inhibitor of kappa B alpha
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-alpha
References
Gibbons GW, Shaw PM. Diabetic vascular disease: characteristics of vascular disease unique to the diabetic patient. Semin Vasc Surg. 2012;25:89–92.
Madden JA. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology. 2012;79:S58–62.
Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462:779–94.
Hattori Y, Jojima T, Tomizawa A, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia. 2010;53:2256–63.
Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–69.
Montanya E. A comparison of currently available GLP-1 receptor agonists for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2012;13:1451–67.
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50.
Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15.
Noyan-Ashraf MH, Shikatani EA, SchuikiI, et al. A glucagon-like Peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85.
Wang X, Khaidakov M, Ding Z, et al. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) and cardiac fibroblast growth. Hypertension. 2012;60:1437–42.
Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–33.
Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35.
Shiraki A, Oyama J, Komoda H, et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–82.
Chang MS, Chen BC, Yu MT, Sheu JR, Chen TF, Lin CH. Phorbol 12-myristate 13-acetate upregulates cyclooxygenase-2 expression in human pulmonary epithelial cells via Ras, Raf-1, ERK, and NF-kappaB, but not p38 MAPK, pathways. Cell Signal. 2005;17:299–310.
Kampoli AM, Tousoulis D, Briasoulis A, Latsios G, Papageorgiou N, Stefanadis C. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des. 2011;17:4147–58.
Oh YC, Kang OH, Kim SB, et al. Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 Cells. Eur Rev Med Pharmacol Sci. 2012;16:1184–91.
Davidson KW, Burg M, Shimbo D. Endothelin-1 release and stimulation of the inflammatory cascade: is acute coronary syndrome triggered by watching spectator sports? J Am Coll Cardiol. 2010;55:643–4.
Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001;22:36–52.
Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83.
Gaspari T, Liu H, Welungoda I, et al. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE−/− mouse model. Diab Vasc Dis Res. 2011;8:117–24.
Quehenberger P, Bierhaus A, Fasching P, et al. Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes. 2000;49:1561–70.
Rajapurohitam V, Kilic A, Javadov S, Karmazyn M. Role of NF-κB and p38 MAPK activation in mediating angiotensin II and endothelin-1-induced stimulation in leptin production and cardiomyocyte hypertrophy. Mol Cell Biochem. 2012;366:287–97.
Piechota A, Goraca A. Influence of nuclear factor-κB inhibition on endothelin-1 induced lung edema and oxidative stress in rats. J Physiol Pharmacol. 2011;62:183–8.
Hattori Y, Hattori S, Sato N, Kasai K. High-glucose-induced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–97.
Yang WS, Seo JW, Han NJ, et al. High glucose-induced NF-kappaB activation occurs via tyrosine phosphorylation of IkappaBalpha in human glomerular endothelial cells: involvement of Syk tyrosine kinase. Am J Physiol Renal Physiol. 2008;294:F1065–75.
Suzuki J, Ogawa M, Muto S, et al. Novel IkB kinase inhibitors for treatment of nuclear factor-kB-related diseases. Expert Opin Investig Drugs. 2011;20:395–405.
Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol. 2005;39:595–603.
Katsuyama K, Shichiri M, Marumo F, Hirata Y. NO inhibits cytokine-induced iNOS expression and NF-kappaB activation by interfering with phosphorylation and degradation of IkappaB-alpha. Arterioscler Thromb Vasc Biol. 1998;18:1796–802.
Mohan S, Hamuro M, Sorescu GP, et al. IkappaBalpha-dependent regulation of low-shear flow-induced NF-kappa B activity: role of nitric oxide. Am J Physiol Cell Physiol. 2003;284:C1039–47.
Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008;82:884–91.
Bonavida B, Baritaki S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide. 2011;24:1–7.
Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–8.
Ho FM, Lin WW, Chen BC, et al. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391–9.
Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–21.
Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111:1530–6.
Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–7.
Acknowledgments
This study was supported by funds from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, Washington, DC. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dai, Y., Mehta, J.L. & Chen, M. Glucagon-like Peptide-1 Receptor Agonist Liraglutide Inhibits Endothelin-1 in Endothelial Cell by Repressing Nuclear Factor-Kappa B Activation. Cardiovasc Drugs Ther 27, 371–380 (2013). https://doi.org/10.1007/s10557-013-6463-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-013-6463-z