Abstract
Purpose
Resveratrol has been shown to have vasoprotective effects by upregulating oxidative defense mechanisms in a variety of pathophysiological conditions. However, the effect of resveratrol on diabetic oxidative stress and vascular and metabolic abnormalities is not completely understood. Therefore, this study was designed to evaluate whether long-term resveratrol supplementation has a protective effect on vascular function and integrity in association with metabolic parameters and oxidative stress in insulin-dependent diabetes.
Methods
Diabetes was induced in rabbits with alloxan and maintained for 8 weeks. We used a resveratrol dose of 5 mg/L (10 weeks, starting 14 days before alloxan injection) and 50 mg/L (8 or 10 weeks, starting concomitantly or 14 days before alloxan injection) in the drinking water of rabbits.
Results
Relaxation to acetylcholine was impaired (control 75.6 ± 3.59%, versus diabetic 42.23 ± 2.53%) and contractions to phenylephrine increased (control 136.89 ± 2.27%, versus diabetic 159.37 ± 6.27%) in aortas from diabetic animals. These changes were associated with increased basal or NAD(P)H-induced superoxide production, as well as lipid peroxide and superoxide dismutase (SOD) levels in the aortic samples. The maximal relaxation to acetylcholine improved by 75.74 ± 9.04% in diabetic rabbits treated with resveratrol. The increased contractions to phenylephrine were not restored to control values after resveratrol treatments, but sensitivity to the contractions tended to decrease. Resveratrol increased nitrite/nitrate levels and suppressed basal or NAD(P)H-induced superoxide production and lipid peroxide levels in the aortas. Importantly, resveratrol increased serum insulin levels without affecting blood glucose and the lipid profile in diabetic rabbits. Using electron microscopic examinations, resveratrol was found to markedly protect the endothelial integrity from diabetes.
Conclusion
Overall, there was no noticeable difference between resveratrol treatment groups on the recovery from diabetes. Our results indicate that resveratrol alleviates type 1 diabetes-induced vasculopathy by decreasing vascular oxidative stress and thereby increasing the bioavailability of nitric oxide without changing metabolic abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial nitric oxide is an important vasodilator substance that is capable of modulating vascular tension. Numerous studies have reported that endothelial nitric oxide-mediated relaxation is decreased in experimental type 1 diabetes, including in an alloxan-induced model [1]. Superoxide production, which is derived from NADPH oxidase, may play a critical role in reducing nitric oxide bioavailability in diabetes-induced endothelial and vascular dysfunction. NADPH oxidase activity was reported to increase in association with enhanced expression of gp91phox and p22phox in the aortas from type 1 diabetic rats [2]. In diabetes, overactivation of NADPH oxidase causes consumption of cofactor NADPH, which is required for eNOS activation [3, 4]. Suppression of NADPH oxidase may have beneficial effects on diabetic vascular complications by reducing superoxide and improving nitric oxide.
Previously, resveratrol was shown to decrease the activity of NADPH oxidase in endothelial cells and arterial preparations [5–8]. Resveratrol has vasoprotective effects in several experimental models due to suppression of NADPH oxidase and activation of SIRT1-dependent mechanisms [7, 9, 10]. In healthy rats, resveratrol was found to increase endothelial function by suppressing NAD(P)H-dependent superoxide production [8, 11]. Moreover, resveratrol improves endothelial relaxation to acetylcholine by increasing levels of eNOS mRNA and eNOS phosphorylation in obese and type 2 diabetic mice [9, 12]. On the other hand, treatment with resveratrol increases insulin sensitivity in mice with high-calorie diet-induced obesity [13, 14]. However, resveratrol has controversial effects on insulin secretion and blood glucose concentrations in type 1 and 2 diabetic rat and mouse models [15–18]. The effect of resveratrol on type 1 diabetic vascular dysfunction, oxidative stress and metabolic changes is still unknown. Therefore, this study was performed to investigate whether long-term resveratrol supplementation has a protective effect on endothelial function and integrity in association with a reduction in metabolic and oxidative stress in type 1 diabetic rabbit model. We hypothesized that chronic administration of supplemental resveratrol would prevent diabetic vasculopathy by decreasing vascular oxidative stress.
Methods
Animals and diets
The study protocol was approved by the Ethical Animal Research Committee of Gazi University (G.U.ET-05.047). Male New Zealand rabbits, weighing 1.85–2.05 kg, were housed in temperature-controlled rooms (20–22°C) under a 12-h light: dark cycle and fed with a standard commercial chow diet. The rabbits were randomly divided into five groups: control; diabetic; 5 mg/L resveratrol pretreated diabetic, starting 14 days before alloxan injection; 50 mg/L resveratrol pretreated diabetic, starting 14 days before alloxan injection; 50 mg/L resveratrol treated diabetic, starting concomitantly with the alloxan injection. Resveratrol or its vehicle (0.05% ethanol, control group) was given to rabbits ad libitum in drinking water for 8 or 10 weeks. The concentrations of resveratrol were chosen from our previous in vivo observations [8, 11]. Water intake was recorded periodically. The body weights of the rabbits were recorded before starting and after drinking the resveratrol or the vehicle. The experiments were performed on the day after the last resveratrol or vehicle treatment. The rabbits were euthanized by sodium thiopental overdose (250 mg/kg, i.p.) 8 weeks after the alloxan injection.
Induction of diabetes by alloxan injection
A single dose of alloxan (100 mg/kg) was injected into the lateral ear vein to induce diabetes mellitus. Alloxan (100 mg/ml) was freshly dissolved in sterile saline. Control rabbits received an equivalent amount of sterile saline. The concentrations of glucose in the blood samples obtained from a marginal ear vein were measured 3, 5 and 15 days after alloxan administration and on the day of the experiment using a glucometer (Roche Diagnostics). Concentrations of glucose that exceeded 250 mg/dl were considered to be diabetic, while the vehicle-treated rabbits served as controls.
Measurement of lipids, insulin, estrogen and testosterone in the serum
Cardiac blood samples of non-fasted control and diabetic rabbits who were at the end of the 8 or 10 week resveratrol and vehicle treatment were centrifuged at 4°C and 10.000 g for 10 min. Serum samples were immediately stored at −20°C until the samples were assayed. Serum high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterols and triglycerides were determined by standard enzymatic techniques. Serum insulin (Mercodia AB, Uppsala, Sweden), estrogen and testosterone (ALPCO Diagnostics, NH, USA) were measured using commercially available specific radioimmunoassay (RIA) kits according to the manufacturer’s protocols.
Measurement of SOD, catalase and lipid peroxide in the aorta
The aortae were homogenized with phosphate buffer 1/10 (w/v), pH 7.4, centrifuged at 10,000 g for 20 min and then supernatant was ultracentrifuged at 100,000 g for 30 min. Aortic samples from rabbits were immediately stored at −80°C until the samples were assayed. The levels of SOD, catalase and lipid peroxide in the aorta samples were measured using commercially available RIA kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocols.
Measurement of nitrite/nitrate production in the serum and aorta
The production of nitric oxide in serum and aortic samples obtained from the rabbits was evaluated by measuring of nitrite/nitrate concentrations using a commercially available colorimetric assay kit (Cayman Chemical, Ann Arbor, MI). Serum and aortic samples were prepared as mentioned above. Potassium nitrate was used as a standard.
Measurement of resveratrol levels in the liver
To verify that the rabbits were ingesting resveratrol in drinking water, we measured trans-resveratrol levels in liver samples; resveratrol has been shown to accumulate in the liver [8]. The LC/APCI–MS analyses for the screening and quantification of trans-resveratrol were performed by an Agilent 1100 HPLC system (Waldbronn, Germany), consisting of a binary pump, an auto sampler and a temperature-controlled column oven that coupled to an Agilent 1100 MS detector equipped with an APCI interface. The analytical separation was performed on an ACE 5 C18 4.6 × 250 mm using acetonitrile: 0.01 mM acetic acid in a 0.2% aqueous solution of formic acid (50:50) at a flow rate of 0.5 ml/min. The injection volume was 20 μl and the DAD wavelength was 320 nm. The detection limit of trans-resveratrol was 7 ng/ml.
Preparation of the thoracic aortic rings
The thoracic aortas were isolated and immediately submersed in a cold Krebs solution of the following composition (mM): NaCl 118, KCl 4.73, KH2PO4 1.2, MgSO4.7H2O 1.2, CaCl2 2.5, NaHCO3 25, glucose 11 and EDTA 0.026. After removing fat and connective tissues from the aorta, 3–4 mm rings were mounted in a 5 ml organ bath containing the Krebs solution at 37°C and aerated with 95% O2 and 5% CO2. Care was taken not to disturb the endothelial layer during the preparation of the aortic rings. Changes in isometric force were measured using force displacement transducers (EMKA Technologies, Paris, France). The optimal point between length and tension had been determined previously by repeated exposure to phenylephrine (3 × 10−6 M) at different resting tensions. An initial tension of 2 g was determined to be optimal for maximal phenylephrine responsiveness in rabbit aortae. The rings were allowed to equilibrate for approximately 90 min along with exchange of bathing solution every 15 min. Four to six rings were prepared from each aorta and studied in parallel. Each ring was subjected to only one assay.
Experimental protocol for vascular contraction and relaxations
At the end of 90-min equilibration period, two reproducible contractions in response to KCl (40 mM) and phenylephrine (10−6 M) were obtained in the rabbit aortic rings. The intactness of the endothelium was tested functionally by applying acetylcholine (10−6 M) to phenylephrine (3 × 10−7 M)-contracted aortic rings. The rings from control rabbits exhibiting <70% relaxation in response to acetylcholine were discarded from the experiments.
The cumulative concentration-response curves to phenylephrine (10−9–10−4 M) were obtained in aortic rings with endothelium from control, diabetic and resveratrol-treated diabetic rabbits. To assay the relaxant effects of acetylcholine and sodium nitroprusside, aortic rings were precontracted with 3 × 10−7 M phenylephrine, which corresponded to 74–90% of the maximal contractions in the aortic rings in all groups. Acetylcholine (10−9–10−4 M) and sodium nitroprusside (10−10–10−5 M) were applied at cumulative concentrations to the phenylephrine-contracted rings. In pilot time-match experiments, we determined that the plateau of precontraction induced by phenylephrine is stable enough for the period necessary to construct the cumulative-relaxation curves of acetylcholine and sodium nitroprusside.
Additionally, the contractions in response to L-NOARG (100 μM), a nitric oxide synthase (NOS) inhibitor, were measured in the rabbit aortic rings. Inhibition of nitric oxide production by NOS inhibitors is used to determine the capacity of basal endothelial nitric oxide production in isolated, quiescent vascular preparations. Endothelium-intact aortic rings were allowed to reach a maximum contraction for 40 min.
Expression of eNOS mRNA in the aorta
The relative quantification of eNOS mRNA expression in rabbit aorta was determined by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) method. We chose this method due to the small number of specimens and genes tested in spite of some advantages of real time RT-PCR which is a high sensitive method. The RNAs were purified using RNeasy fibrous tissue kit (Qiagen, Germany). The 1 μg of total RNA were transcribed to cDNA using oligo (dT)12–18 primer and murine leukemia virus (MuLV)-reverse transcriptase (Fermentas) and were scaled up to a final volume of 20 μl. The RT reaction was performed at 42°C for 60 min, followed by heating at 70°C for 10 min. The PCR amplification was performed in a final volume 50 μl consisting of 1× PCR buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 0.2 μM primers, two U HotStart Taq polymerase (Fermentas) and 3 μl cDNA. PCR amplification was performed for 5 min at 95°C, followed by 30 s for denaturation at 95°C, 60 s for annealing at 60°C, and 60 s for extended at 72°C, followed by a final extension for 10 min at 72°C. In all experiments, PCR was performed for 35 cycles. Negative controls for all PCRs were prepared with lacking template DNA. DNA markers (100 bp ladder) and RT-PCR products were loaded on to 2% agarose gel. The bands were visualized with ethidium-bromide under UV light. Densitometric analysis of bands was performed by using BioRad Quantite One 4.6.6. soft-ware program (BioRad Ltd. England). The expression of glyceraldhyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal standard. The sequences of the oligonucleotides were as follows; eNOS sense 5′-GCTGGAGTGGTTCGCGGCC-3′, eNOS antisense 5′-CTCCAGGATGTTGTAGCGGTGA-3′ (GenBank No: NM-001082733; 976–1149), GADPH sense 5′-TCCACCACCCTGTTGCTGTA-3′, GADPH antisense 5′-ACCACGGTGCACGCCATCAC-3′ (GenBank No: NM-001082253; 594–1045).
Measurement of superoxide production in the aorta
The production of superoxide in the aortic segments with and without endothelium from control, diabetic and resveratrol-supplemented diabetic rabbits was measured by using lucigenin-enhanced chemiluminescence at low concentration (5 μΜ) of lucigenin, which is described as a sensitive and reliable sensor for monitoring superoxide production in intact vessel [19]. To obtain the aortic segments without endothelium, the endothelium was removed by gently rubbing the intimal surface of the vessels with a roughed polyethylene tube. The aortic segments (3–4 mm) were placed in Krebs-HEPES buffer and incubated in the dark for 10 min at 37°C. HEPES buffer (200 μl) with lucigenin (5 μmol/l) was added to the wells of the scintillation plates and equilibrated in the dark for 10 min at 37°C. The chemiluminescence signals were recorded every minute for 10 min in a scintillation counter (1450 Microbeta Wallac TRILUX liquid scintillation counter). The amount of superoxide was calculated by comparison with a standard curve using xanthine/xanthine oxidase. Superoxide generation in aortic segments was expressed as counts/min/mg dry tissue weight.
The basal superoxide production was measured in the arterial segments from the all groups. Additionally, superoxide generation that was stimulated with the substrates of NADPH oxidase (100 μM NADH or NADPH, 10 min at 37°C) was detected in the aortic segments. In another group of experiments, NAD(P)H-stimulated signals were determined in the vessels that were preincubated with the flavin-containing enzyme inhibitor DPI (10 μM) for 10 min at 37°C. The chemiluminescence signals were also recorded in the wells containing buffer, lucigenin and the chemicals in the vascular segments to determine the possible interaction between lucigenin and the chemicals.
Electron microscopic examination in the aorta
Aortic samples are immediately immersed in buffered glutaraldehyde (2.5%) and paraformaldehyde (2%) solutions and fixed for at least 24 h. The samples are postfixed with osmium tetroxide (2%) during the first hour and dehydrated with ethanol. Tissues sections of 60–90 nm (fine) and 1–2 mm (semi-fine) were obtained from the blocks embedded in araldite. Semi-fine sections were stained with a 5% toluidine solution, and fine sections were contrasted with uranyl acetate and lead citrate. The samples were examined with a JEOL JEM 1200 electron microscope (Japan Electron Optics Laboratory). Ultrastructures were scored as described in Table 6. Electron micrographs were printed with one or five micrometer bars on the side.
Chemicals
Chemicals for the bioassay and superoxide measurement were purchased from Sigma Chemical Company (St Louis, MO). The other chemicals were obtained from Merck (Rahway, NJ). Trans-resveratrol was purchased from Sigma or Herb-Tech (China). The purity of resveratrol from the two sources was compared by HPLC analysis and there was no difference between them. The drinking water stocks with resveratrol or vehicle were prepared weekly in 0.05% vol/vol ethanol and stored in dark bottles at 2–4°C. Acetylcholine was dissolved in 0.001 N HCl. A stock solution of phenylephrine was prepared in distilled water. All subsequent dilutions were made in Krebs solution and stored in the dark by keeping cold.
Statistical analysis
The results are given as mean±standard error of the mean. Contractile responses to phenylephrine were expressed as a percentage of the 40 mM KCl-induced contraction. The relaxations to acetylcholine were expressed as percent decreases of the precontraction to phenylephrine. The maximal response (Emax) and potency (EC50) of the agents were determined by non-linear curve fitting using the Prism 4.03 GraphPad program. EC50 values are given as –log M. Statistical analyses of the data were performed using the Student’s t-test or ANOVA followed by the Bonferroni post-hoc analysis, as appropriate. Values were considered to be significantly different when the p value was less than 0.05.
Results
Resveratrol and water consumption, body weight, blood glucose, insulin and lipid concentrations
In all the diabetic groups, consumption of water was importantly elevated when compared with untreated controls; however, there was no significant difference in water intake between diabetic control and resveratrol-supplemented diabetic groups. The amounts of resveratrol added in the drinking water were calculated to be approximately 1.5 ± 0.2 and 17 ± 1.3 mg/kg/day for 5 mg/L and 50 mg/L resveratrol supplementation, respectively. The terminal body weights were comparable in all groups (Table 1).
All of the blood parameters including glucose levels were investigated in non-fasted rabbits in order to avoid possible influence of recurrent fasting stress depending on the repeated measurements of blood glucose levels in the testing period to verify diabetes. Alloxan-induced diabetes leaded a drastic increase in blood glucose levels associated with an important decrease in serum insulin levels. Resveratrol supplementation elevated insulin levels, which was particularly prominent in the 50 mg/L resveratrol groups, without significantly changing glucose levels (Table 1).
The serum levels of LDL were not different between the groups. However, decreased HDL and increased triglycerides levels were found in the diabetic control groups. There was a trend toward an increase in HDL levels and a decrease in triglycerides levels in resveratrol-treated diabetic rabbits, but these differences were not statistically significant (Table 1).
Estrogen and testosterone levels in the serum
The serum concentrations of estrogen and testosterone are shown in Table 1. Neither diabetes nor resveratrol treatments altered estrogen or testosterone levels in the serum.
SOD, catalase and lipid peroxide levels in the aorta
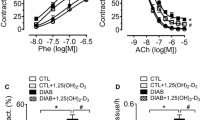
SOD levels were increased in the aortic samples from diabetic rabbits, while catalase levels were unchanged. Resveratrol supplementation did not alter SOD and catalase levels in diabetic animals (Table 2). High lipid peroxide production was measured in the aortas from diabetic animals and determined to be decreased with resveratrol supplementation. In this favorable influence, we observed that resveratrol pretreatments (5 and 50 mg/L, starting 14 days before diabetes induction) appeared more effective than administering resveratrol (50 mg/L) during the onset of diabetes (Table 2).
Contractile response to phenylephrine in the aortic rings
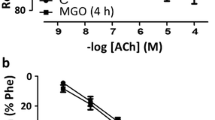
Phenylephrine (10−9–10−4 M) produced concentration-dependent contractions in the aortic rings with a higher potency and maximal response in diabetic rabbits than in the controls. Resveratrol supplementation partially decreased the sensitivity, but not the maximal contractions, to phenylephrine in the endothelium-intact aortae obtained from diabetic rabbits when compared with diabetic controls (Fig. 1, Table 3). Thus, any treatment schedule with resveratrol was not able to completely restore the concentration-response curves to phenylephrine in the diabetic group.
The endothelial relaxations to acetylcholine and the influence of L-NOARG on the aortic rings
Acetylcholine (10−9–10−5 M) produced relaxations in phenylephrine-precontracted aortic rings with endothelium in a concentration-dependent manner from control and diabetic rabbits (Fig. 2). The maximal relaxation in response to acetylcholine was lowered in the aortic rings from diabetic rabbits compared with rabbits in the control group (Emax: 75% vs. 42%, n = 7–11, p < 0.05), but the potencies were similar between the two groups (EC50: 7.38 ± 0.11 vs. 7.33 ± 0.22, Table 4). Resveratrol supplementation significantly increased the maximal relaxation but paradoxically decreased the potency of acetylcholine in endothelium-intact aortic rings from diabetic rabbits. There were no significant differences between the three different resveratrol applications regarding the changes that occurred on the acetylcholine dose-response curves (Fig. 2, Table 4).
Pretreatment with L-NOARG almost completely abolished the acetylcholine-induced relaxations in the rabbit endothelium-intact arteries (Table 5). There were no significant differences between the groups. Furthermore, pretreatment with L-NOARG increased basal contractile tone of the arteries in the control, diabetic and resveratrol-supplemented groups. L-NOARG-induced contractions were not different between the groups (Table 5).
Nitrite/nitrate levels in the aorta and serum
Nitrite/nitrate levels were lower in the aortic and serum samples of diabetic rabbits compared with rabbits in the control groups. Resveratrol supplementation significantly increased nitrite/nitrate levels in the aortas and serum of the diabetic rabbits except for aortic samples from resveratrol administration during onset of diabetes (Fig. 3a, b).
A, B Nitric oxide production was detected as nitrite/nitrate in serum A and aortae B obtained from control, diabetic, 5 or 50 mg/L resveratrol-pretreated diabetic (Res5-Diabetic, Res50-Diabetic) and 50 mg/L resveratrol-treated diabetic (Diabetic-Res50) rabbits. Values are expressed as mean±SEM, n = 6–9. *p < 0.05, significantly different from control; #p < 0.05, significantly different from diabetic rabbits
eNOS mRNA expression in the aorta
eNOS mRNA expression in aortae was comparable between control and diabetic groups. Resveratrol treatment did not change eNOS mRNA levels in the aortae from diabetic rabbits. The findings were confirmed in at least five independent assays (Fig. 4a, b).
A, B eNOS mRNA expression in rabbit aorta. A Representative gel showing products amplified following RT-PCR for eNOS mRNA (173 bp) and GAPDH mRNA (451 bp). Lanes: [M] marker (100 bp ladder), [1] control, [2] diabetic, [3] 5 mg/L resveratrol-pretreated diabetic (Res5-Diabetic), [4] 50 mg/L resveratrol-pretreated diabetic (Res50-Diabetic), [5] 50 mg/L resveratrol-treated diabetic (Diabetic-Res50). B eNOS gene expression levels were normalized according to GAPDH expression levels
Basal and NAD (P) H-induced superoxide production in the aortic segments
The basal superoxide levels were higher in endothelium-intact and denuded aortic segments from diabetic rabbits than those of controls. Resveratrol supplementation significantly suppressed the increased basal superoxide generation in diabetic arteries. NADPH or NADH (100 μM) stimulation produced a higher superoxide production in endothelium-intact aortas than in endothelium-denuded aortas in control, diabetic and resveratrol-supplemented diabetic rabbits (Fig. 5a, b). Moreover, superoxide production in response to NADPH was more abundant in comparison with NADH in aortae from almost all groups. NADPH or NADH stimulation was more pronounced in diabetic arteries than in control arteries. The aortas isolated from resveratrol-supplemented diabetic animals displayed refractoriness to superoxide production in response to NADPH or NADH stimulation. DPI (10 μM) almost completely abolished both NADPH and NADH–stimulated superoxide production in the aortas from all groups (Fig. 5a, b). The difference between the resveratrol-treated groups was not quite significant.
A, B The stimulation of superoxide production with NADPH and NADH in aortae with (+E; A) and without endothelium (−E; B) from control, diabetic, 5 or 50 mg/L resveratrol-pretreated diabetic (Res5-Diabetic, Res50-Diabetic) and 50 mg/L resveratrol-treated diabetic (Diabetic-Res50) rabbits. The inhibitory effect of DPI (10 μM) application on NAD(P)H-stimulated superoxide production was also tested. Values are mean±SEM, n = 7–9. *p < 0.05, significantly different from control; #p < 0.05, significantly different from diabetic rabbits
Histological investigation of the aortic sections
Drastic lesions were identified in the aortas from diabetic rabbits by increased vacuolization, irregular matrix and internal elastic lamina, desquamation of the endothelial cell and swelling of the mitochondria, as shown in the representative photographs (Fig. 6). Subendothelial regeneration and improved endothelial integrity were observed in the groups pretreated with resveratrol. Nucleus, cytoplasm and mitochondria scores are presented in Table 6. Diabetes caused profound changes in the morphology of the nucleus, cytoplasm and mitochondria in the samples tested. Pretreatment with resveratrol at doses of 5 and 50 mg/L markedly restored these abnormalities. The difference between resveratrol applications was not quite significant, except when resveratrol was administered during the onset of diabetes.
In the control group, normal vascular endothelial cell: the basal lamina is thin, there are many free ribosome’s and rough endoplasmic reticulum (RER) and a few mitochondriae (M). In the diabetic group, there is increased vacuolization (V) in the endothelial cytoplasm. The nucleus (A) and mitochondriae are highly damaged. Desquamated and fragmented endothelial cells, degeneration areas and separations in the subendothelium indicated disturbed endothelial integrity. In the 50 mg/L resveratrol-treated diabetic (Diabetic-Res50) group, some endothelial cells are separated from the basal lamina. There is vacuolization in the cytoplasmic detail, mitochondriae are swollen and their cristae are damaged. The cellular membrane and internal elastic lamina are irregular. In the 50 mg/L resveratrol-pretreated diabetic (Res50-Diabetic) group, subendothelial collagen (K) is increased indicating fibrosis. Intact intercellular connections, endothelial integrity and continuity of the cell membrane indicate a healthy ultrastructure. In the 5 mg/L resveratrol-pretreated diabetic (Res5-Diabetic) group it was observed intact endothelial cells but some endothelial cells and mitochondriae are edematous. There are also some attached erythrocytes. In groups Diabetic-Res50 and Res50-Diabetic, there were increased collagen fibers under the basal lamina
Resveratrol levels in the liver
The serum concentrations of resveratrol (trans-resveratrol) were found to be under the quantifiable amount for both doses (5 and 50 mg/L), whereas the concentrations of resveratrol in the liver extracts were measured as 0.40 ± 0.1–2.06 ± 0.1 μg/ml (n = 4), which is probably due to accumulation of resveratrol in the liver [8]. There were no traces of resveratrol in the livers from control rabbits.
Discussion
The health improving effect of dietary factors may open a new therapeutic era in a variety of pathophysiological conditions such as hypertension, diabetes and diet-induced metabolic disorders. In this context, resveratrol was shown to have vasoprotective effects in diseases associated with oxidative stress [20]. In the current study, we evaluated the effect of resveratrol supplementation on vascular function and integrity in an alloxan diabetic rabbit model. Resveratrol treatment alleviated endothelial dysfunction, vascular oxidative stress and endothelial injury in diabetes without affecting blood glucose and the lipid profile, despite an increase in insulin levels. These results show that resveratrol ameliorates diabetes-induced vasculopathy but not metabolic abnormalities.
Diabetes-induced endothelial dysfunction is a well known abnormality leading to cardiovascular disorders [1]. Previously, it was shown that endothelial relaxation is impaired in association with an increase in superoxide generation in aortas from alloxan diabetic rabbits [21–23]. Consistent with these reports, we demonstrated that endothelial maximal relaxation to acetylcholine is impaired, while maximal contraction to phenylephrine is enhanced in the aortas from diabetic rabbits. NADPH oxidase-derived superoxide may play a critical role in endothelial and vascular dysfunction in diabetes because NADPH oxidase activity and/or expression were reported to increase in the aorta of type 1 diabetic rats [2, 24]. In this study, diabetes-induced endothelial and vascular dysfunction was associated with increased superoxide production, decreased nitrite/nitrate levels and abnormal histopathologic appearance of the aortas. Additionally, enhanced aortic lipid peroxide indicated that the vasculature was under oxidative stress. Elevated SOD levels may represent a compensatory effort to scavenge superoxide, which is in agreement with a previous report that studied the aortas from diabetic rabbits [23]. Increased oxidative stress, despite elevated activity of SOD, may reflect an inadequacy in the antioxidant defense system in the diabetic condition. Moreover, high glucose and triglyceride levels with low HDL and insulin levels are indicators of metabolic disturbance.
In a previous study, it was shown that administration of 0.5 mg/kg of resveratrol during the 14-day experimental period improves hyperglycemia and hyperlipidemia in association with a reduction in plasma insulin levels in streptozotocin-induced diabetic rats [15]. It was also demonstrated that administration of resveratrol lowers plasma glucose and elevates insulin levels in nicotinamide-streptozotocin diabetic rats [16, 25]. Treatment with resveratrol at a dose of 3 mg/kg for 1 week results in an increase in GLUT4 expression in the skeletal muscle of diabetic rats [16]. Moreover, resveratrol was reported to have stimulant effects on insulin secretion by blocking KATP and KV channels in pancreatic beta cells [26]. These results demonstrated that resveratrol decreases plasma glucose through insulin-dependent and independent mechanisms. However, in the present study resveratrol supplementation did not significantly change blood glucose levels in diabetic rabbits, which is in agreement with the results from recent studies in streptozotocin diabetic rats [17] or mice [18] and type 2 diabetic mice [12]. Surprisingly, serum insulin levels are increased in resveratrol-treated diabetic rabbits, but this increase in insulin could not lower high glucose levels. The reasons for this are not clear, but insulin signaling may be disrupted during diabetes or prolonged administration of resveratrol. However, it was previously demonstrated that long-term resveratrol treatment improves insulin resistance in high-fat diet-induced obesity in mice [13, 14]. Our findings on lipid profiles also indicate that metabolic abnormalities were still present because the amelioration effect of resveratrol was not sufficient to correct for the reduced HDL and increased triglyceride levels in diabetic rabbits, in accordance with the results of a previous study in type 1 diabetic mice [18]. Further investigations are required to clarify the therapeutic effectiveness of resveratrol on diabetes-associated metabolic abnormalities.
Resveratrol was shown to improve endothelial relaxation to acetylcholine in aortas from streptozotocin diabetic rats and type 2 diabetic mice [12, 27]. Similarly, in this study, the reduction of the maximal endothelial relaxation to acetylcholine in diabetic animals was markedly recovered after all of the resveratrol supplementations. Interestingly, resveratrol decreased the sensitivity of aortas to acetylcholine in diabetic rabbits. Furthermore, resveratrol was unable to restore the increased contraction to phenylephrine but did partly decrease the sensitivity. It has been previously reported that resveratrol shows a biphasic effect on intracellular calcium levels by inhibiting Ca2+ entry through the voltage-dependent L-type channels and increasing Ca2+ release from intracellular stores and store-activated Ca2+ influx in vascular smooth muscle cells [28, 29]. Moreover, low concentrations (0.3–3 μM) of resveratrol have been reported to produce vasoconstriction in porcine coronary arteries [30]. These findings indicate that the effect of resveratrol on intracellular free calcium levels and vascular contractility is somewhat controversial.
Our results with nitrite/nitrate and L-NOARG provide some evidence for the improving effect of resveratrol on endothelial relaxation through activation of nitric oxide because resveratrol treatment increased aorta and blood nitrite/nitrate levels. Furthermore, L-NOARG pretreatment almost completely abolished endothelial relaxation to acetylcholine in all the groups. The contraction after L-NOARG pretreatment shows the capacity of basal endothelial nitric oxide production in isolated, quiescent vascular preparations, and this production may decrease in diabetic arteries [31]. In this study, absolute contractions to L-NOARG were not different between the experimental groups, which demonstrates that there were no changes in basal nitric oxide production after resveratrol application or the induction of diabetes. Furthermore, resveratrol treatment did not upregulate aortic eNOS mRNA expression which demonstrates that resveratrol may increase nitric oxide bioavailability without affecting its genomic production, consistent with results reported by others in type 2 diabetic mice and hypertensive rats [12, 32]. However, it has been previously shown that resveratrol treatment increases eNOS (Ser1177) phosphorylation, eNOS mRNA expression or eNOS activity in the arteries from type 2 diabetic mice, streptozotocin diabetic mice or obese mice and fructose-fed rats [9, 12, 18, 33]. The discrepancies in the effect of resveratrol on the nitric oxide system could be attributed to the differences in experimental conditions such as resveratrol concentrations, strain, and pathophysiological conditions. Collectively, these data suggest that resveratrol treatment may improve nitric oxide bioavailability in the arteries.
NAD(P)H-induced superoxide production is almost completely inhibited by DPI, which indicates that NADPH oxidase is a source of oxidative stress in the arterial segments. Resveratrol supplementation suppressed NAD(P)H-induced superoxide production in the aortas with and without endothelium in control and diabetic animals. This shows that resveratrol not only affects the endothelium but also other vascular compartments. However, endothelium-intact aortic segments had more superoxide generation capacity, which indicates that the endothelium may contribute to the total superoxide. Moreover, the inhibitory effect of resveratrol was more apparent on NADPH-derived superoxide production than that of NADH. An increased level of basal superoxide in diabetic aortas is indicative of sustained high oxidative stress, which is reduced in resveratrol-supplemented animals. The reduction in superoxide generation within the vasculature by resveratrol may increase nitric oxide availability as evidenced by increased nitrite/nitrate levels and endothelial relaxation, which is in accordance with previous results found in type 2 diabetic mice or obese mice [9, 12]. The impaired endothelial relaxation seen in the diabetic rabbits was most likely due to increased superoxide production in the aorta because resveratrol concomitantly restored these two abnormalities. Resveratrol also diminished lipid peroxide production but did not increase SOD and catalase in diabetic conditions. These results revealed that suppression of superoxide and lipid peroxide production with resveratrol may reduce the need for antioxidant enzymes. Alternatively, high glucose may limit an increase of antioxidants in our study conditions.
Our electron microscopy analysis displayed drastic changes in endothelial structure, which were identified by matrix vacuolization, endothelial desquamation and mitochondrial swelling in accordance with previous studies [24, 34]. This altered morphology may be due to increased vascular oxidative stress, which is determined in diabetic animals. Pretreatment with resveratrol noticeably prevented these abnormalities, although the endothelial ultrastructure still had some irregularities. In this context it is noteworthy that chronic resveratrol treatment improves mitochondrial biogenesis in the aortas of type 2 diabetic mice and endothelial cell lines [35]. Also, resveratrol attenuates hyperglycemia-induced mitochondrial reactive oxygen species production in cultured human coronary arterial endothelial cells [36]. The results obtained from in vivo and in vitro studies demonstrate that resveratrol suppresses high-calorie diet-induced apoptosis in aorta or in branches of femoral artery of mice [9, 37] and oxidative stress-induced apoptosis in endothelial cells [38, 39] via activation of antioxidant factors or SIRT-1 and inhibition of prooxidant enzymes. These properties of resveratrol may contribute to the improvement of endothelial function and integrity consequently exerting vasoprotection.
In this study, the bioavailability of resveratrol (5–50 mg/L) given in the drinking water was confirmed by measuring the amount of resveratrol (0.40–2.06 μg/ml) in the liver; however, the plasma concentration of resveratrol was under the quantifiable amount, which could be due to the rapid metabolism of resveratrol and/or the accumulation of resveratrol in the peripheral organs [8]. The doses of resveratrol used in the current study are somewhat higher than that ingested with food like red and purple grapes, wine and nuts in humans. However, previous in vivo observations demonstrated that the biological effects of resveratrol were determined at concentrations ranging from 3–204 mg/kg when administered orally in experimental studies [20]. On the other hand, resveratrol was also reported to have inhibitory activity on aromatase, which catalyzes the final rate-limiting reaction in estrogen biosynthesis [40]. In accordance , we have recently shown that estrogen concentrations were lowered in both male and female rats following a 3-week resveratrol treatment (50 mg/L) [8]. Of interest, blood estrogen and testosterone concentrations were measured herein, however, no change was detected in their levels after resveratrol treatment in diabetic animals.
In conclusion, resveratrol alleviates type 1 diabetes-induced vasculopathy by decreasing vascular oxidative stress and thereby increasing the bioavailability of nitric oxide without changing metabolic abnormalities. Although resveratrol treatment did not shift the overall diabetic pattern toward control conditions, it significantly protected endothelial function and integrity from diabetes-induced vascular oxidative stress.
References
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74.
Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22.
Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–26.
Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovasc Res. 2009;82:9–20.
Orallo F, Alvarez E, Camina M, Leiro JM, Gomez E, Fernandez P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol. 2002;61:294–302.
Coskun B, Soylemez S, Parlar AI, Ulus AT, Katircioglu SF, Akar F. Effect of resveratrol on nitrate tolerance in isolated human internal mammary artery. J Cardiovas Pharmacol. 2006;47:437–45.
Chow S-E, Hshu Y-C, Wang J-S, Chen J-K. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–7.
Soylemez S, Gurdal H, Sepici A, Akar F. The effect of long-term resveratrol treatment on relaxation to estrogen in aortae from male and female rats: role of nitric oxide and superoxide. Vasc Pharmacol. 2008;49:97–105.
Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending lifespan. Cell Metab. 2008;8:157–68.
Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–35.
Soylemez S, Sepici A, Akar F. Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats. Cardiovasc Drugs Ther. 2009;23:449–58.
Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function. Role of TNF and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–71.
Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42.
Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22.
Su H-C, Hung L-M, Chen J-K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–46.
Chi TC, Chen WP, Chi TL, Kuo TF, Lee SS, Cheng JT, et al. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007;80:1713–20.
Schmatz R, Schetinger MR, Spanevello RM, et al. Effects of resveratrol on nucleotide degrading enzymes in streptozotocin-induced diabetic rats. Life Sci. 2009;84:345–50.
Orimo M, Minamino T, Miyauchi H, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–94.
Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254:319–24.
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506.
Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–32.
Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose impairs endothelium-dependent relaxations by activating protein kinase C. J Clin Invest. 1991;87:1643–8.
Zanetti M, Sato J, Katusic ZS, O’Brien T. Gene transfer of superoxide dismutase isoforms reverses endothelial dysfunction in diabetic rabbit aorta. Am J Physiol Heart Circ Physiol. 2001;280:H2516–23.
Shah DI, Singh M. Possible role of Akt to improve vascular endothelial dysfunction in diabetic and hyperhomocysteinemic rats. Mol Cell Biochem. 2007;295:65–74.
Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605.
Chen W-P, Chi T-C, Chuang L-M, Su M-J. Resveratrol enhances insulin secretion by blocking KATP and KV channels of beta cells. Eur J Pharmacol. 2007;568:269–77.
Silan C. The effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2008;31:897–902.
Campos-Toimil M, Elíes J, Orallo F. Trans- and cis-resveratrol increase cytoplasmic calcium levels in A7r5 vascular smooth muscle cells. Mol Nutr Food Res. 2005;49:396–404.
Campos-Toimil M, Elíes J, Alvarez E, Verde I, Orallo F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. Eur J Pharmacol. 2007;577:91–9.
Jager U, Nguyen-Doung H. Relaxant effect of trans-resveratrol on isolated porcine coronary arteries. Arzneim Forsch Drug Res. 1999;49:207–11.
Bolego C, Cignarella A, Zancan V, Pinna C, Zanardo R, Puglisi L. Diabetes abolishes the vascular protective effects of estrogen in female rats. Life Sci. 1999;64:741–9.
Rush JWE, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med. 2007;232:814–22.
Miatello R, Vázquez M, Renna N, Cruzado M, Zumino AP, Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens. 2005;18:864–70.
Schiller NK, Timothy AM, Chen IL, et al. Endothelial cell regrowth and morphology after balloon catheter injury of alloxan-induced diabetic rabbits. Am J Physiol Heart Circ Physiol. 1999;277:H740–8.
Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20.
Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–81.
Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24.
Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–24.
Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–27.
Wang Y, Lee KW, Chan FL, Shiuan CS, Leung LK. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006;92:71–7.
Acknowledgements
This study was supported by a grant from TUBITAK 2008, Project No: 105 S431.
Conflict of interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akar, F., Pektas, M.B., Tufan, C. et al. Resveratrol Shows Vasoprotective Effect Reducing Oxidative Stress Without Affecting Metabolic Disturbances in Insulin-dependent Diabetes of Rabbits. Cardiovasc Drugs Ther 25, 119–131 (2011). https://doi.org/10.1007/s10557-010-6255-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6255-7