Abstract
Surgery/anesthetic technique-stimulated immunosuppression in the perioperative period might cause an increase in cancer-related mortality. Whether anesthetic technique can affect the outcomes of cancer patients remains inconclusive. This review discusses data from the available literature on anesthetic techniques applied in oncologic surgery, the long-term outcomes of anesthetic technique, and their relation to survival and cancer recurrence. Searches of the PubMed database up to June 30, 2016, were conducted to identify publications with the terms “anesthetic technique and cancer recurrence,” “regional anesthesia and cancer recurrence,” “local anesthesia and cancer recurrence,” “anesthetic technique and immunosuppression,” and “anesthetic technique and oncologic surgery.” Surgery/anesthesia-stimulated activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) provides immunosuppression through several soluble factors. Volatile anesthetics and opioids suppress cell-mediated immunity (CMI) and promote the proliferation of cancer cells and angiogenesis, whereas propofol does not suppress CMI and inhibits tumor angiogenesis. Regional anesthesia (RA) protects CMI and diminishes the surgical neuroendocrine stress response by blocking afferent neural transmission that stimulates the HPA axis and SNS, decreasing the requirement for opioids and volatile anesthetics and thereby decreasing cancer recurrence. Preclinical and retrospective studies highlight a potential benefit of anesthetic technique in reducing cancer-related mortality and recurrence by attenuating immunosuppression following surgical treatment in patients with specific types of cancer. Several well-planned, prospective, randomized controlled trials (RCTs) are underway that may provide more conclusive and definitive results regarding the benefits of anesthetic technique on survival in oncologic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a major cause of morbidity and mortality worldwide. Despite the use of surgery in an attempt to cure the majority of solid tumors, metastasis from residual cancer cells still remains a major cause of morbidity and mortality [1]. As is the case with most cancers, loco-regional recurrence and distant metastases are all too common, even after successful surgical treatment and adjuvant therapy. Cancer metastasis is a complex process in which cancer cells evade the immune system. Cancer cells gain the ability to proliferate, migrate, and invade adjacent tissues, and together with angiogenesis, these capabilities facilitate the successful metastasis of cancer [2].
General anesthesia (GA) and surgical stress during surgery suppress the immune response by directly affecting the immune system or by activating the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) [3]. Along with surgical stress, blood transfusion, hypothermia, and postoperative pain, anesthetics per se are associated with immunosuppression during perioperative periods because anesthetics/analgesics have direct suppressive effects on cellular and humoral immunity [3, 4]. Surgery/anesthetic-stimulated immunosuppression, such as the decreased activity of natural killer (NK) cells and lymphocytes, may induce growth and metastasis of residual cancer cells, thereby leading to a worse prognosis [5]. Given that volatile anesthetics have demonstrated a predominantly protumor effect, while propofol and nonsteroidal anti-inflammatory agents have mostly antitumor effects, the anti-inflammatory effects of anesthetics may be beneficial in distinct situations during the progression of cancer cells [6]. In surgery, a number of perioperative factors may influence the development of cancer metastases. For example, the anesthetic technique used during the perioperative period may affect cancer metastasis after surgery [7–10].
Regional anesthesia (RA) has been proposed to reduce the incidence of cancer recurrence by attenuating the neuroendocrine stress response during surgery and reducing opioid requirements, thereby diminishing their immunosuppressive effects, and by providing antitumor and anti-inflammatory effects directly through systemic local anesthetic action [11]. Increasing numbers of laboratory and animal studies suggest that analgesics affect the cellular components of cancer as well as noncancer cells and may influence cancer outcomes by directly stimulating tumor growth and inhibiting immune surveillance [12, 13]. Opioids cause immunosuppression and stimulate cancer cells in vitro; even adjunct analgesics may additionally promote tumor cell growth [9]. These results lead to the hypothesis that regional analgesic techniques may provide survival advantages compared with systemic analgesics [14, 15]. Although many retrospective and meta-analyses highlight the potential benefit of RA, current available data examining the relationship between regional anesthesia/analgesia and decreasing cancer recurrence do not provide any definitive answers.
In this review, a description of the perioperative period, current knowledge and evidence for anesthetic technique used on patient outcome after cancer surgery, and proposed hypotheses from the available literature on the effects of anesthetic technique on cancer recurrence are presented. To try to unravel controversial findings on exactly how surgery/anesthetic technique-induced immunosuppression leads to an increase in cancer-related mortality, the potential role for RA and propofol in reducing cancer recurrence during the perioperative period is discussed comprehensively. This discussion provides a hypothesis that may help integrate the significance of anesthetic technique in oncologic surgery.
2 Perioperative period influencing immune function
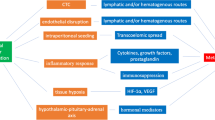
In cancer patients, various tumor-derived soluble factors have established an immunosuppressive tumor microenvironment to facilitate tumor progression and metastasis by helping tumors to evade immune surveillance from cell-mediated immunity (CMI) [16]. Under these conditions, the perioperative period is critical in influencing long-term outcomes from primary surgery for cancer patients. Various factors influence whether residual cancer cells or preexisting micrometastases become able to initiate new metastases or are eliminated by the immune system [17–19]. In the perioperative period, factors influencing immune function and tumor metastasis are represented by the surgery per se and the anesthetic/analgesic technique and/or agents used [19]. Both surgery and anesthetic/analgesic agents stimulate the HPA axis and SNS during the perioperative period. This neuroendocrine paracrine response leads to systemic increase in several immunosuppressive soluble factors, including catecholamines, prostaglandins, glucocorticoids, and opioids. These, in turn, lead to the suppression of NK cells and cytotoxic T lymphocytes (CTLs), in association with a decrease in interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), thereby shifting the cytokine balance of T-helper 1 (Th1)/T-helper 2 (Th2) toward anti-CMI Th2 dominance [20]. Catecholamines induce increases in angiogenic factors, such as vascular endothelial growth factor (VEGF) and matrix metalloproteinase 2 and 9 (MMP 2/9) [21, 22], and proinflammatory cytokines, such as IL-6 and IL-8 [23, 24]. Anti-inflammatory cytokines, such as IL-10, IL-4, and transforming growth factor-β (TGF-β), are also induced in response to a dysregulated balance of the proinflammatory/anti-inflammatory and Th1/Th2 cytokines [25]. Th2 cytokines lead to the accumulation of arginase-1-expressing myeloid-derived suppressor cells (MDSCs) in lymphoid tissue that cause an arginine-deficient environment, resulting in impairment of lymphocyte function in response to the synergistic effect of prostaglandin E2 (PGE2) [26]. Tumor-derived PGE2 induces MDSCs and tumor-associated macrophages (TAM, M2 phenotype), which leads to an immunosuppressive environment and a proinflammatory response that contributes to tumor angiogenesis [27, 28]. In fact, tumor-derived MDSCs inhibit NK cell activity through the production of TGF-β, suggesting that PGE2 plays an important role in inducing MDSCs [29]. Thus, surgery/anesthesia-stimulated stress and immunosuppression are involved in tumor progression and the metastatic process (Fig. 1). The suppression of the immune system occurs within few hours during surgery and continues for several days, which could be in proportion to the extent of surgical treatment. Since the immune system protects from cancer as well as from infection, a surgical stress response impairs the antimetastatic CMI, thereby increasing the opportunity of dissemination and metastasis of cancer cells in cancer surgery. Perioperative periods may provide the best chance for residual cancer cells to spread through the immunosuppressive effect of anesthetic agents and so affect long-term recurrence rates. Although immune perturbations may be induced by a preoperative psychological stress response following a cancer diagnosis, the actual perioperative period can be divided into three parts: a preoperative period made up of a few preoperative hours, an intraoperative period, and a postoperative period consisting of several postoperative days after surgical treatment.
A potential cascade showing how surgery/anesthesia-stimulated immunosuppression drives tumor progression and metastasis during the perioperative period. In the presence of tumor-derived soluble factors, surgery/anesthesia-stimulated immunosuppression through the HPA axis and SNS can increase various soluble factors that lead to tumor progression and metastasis. An increase in various soluble factors is exploited for proinflammatory and anti-inflammatory responses in tumor angiogenesis and immunosuppressive microenvironments to escape tumor immune surveillance. In these processes, two key molecules, catecholamine and PGE2, may play crucial roles in immunosuppression and angiogenesis, which was mediated via the induction of TAMs, TGF-β, VEGF, MDSCs, and Tregs. HPA hypothalamic-pituitary-adrenal, SNS sympathetic nervous system, CMI cell-mediated immunity, NK natural killer, CTLs cytotoxic T lymphocytes, MDSCs myeloid-derived suppressor cells, TAMs tumor-associated macrophages, Tregs regulatory T cells, PGE 2 prostaglandin E2, VEGF vascular endothelial growth factor, IL interleukin, TGF-β transforming growth factor-β, Th T-helper, TNF-α tumor necrosis factor-α, IFN-γ interferon-γ, MMP matrix metalloproteinase
3 The angiogenic and metastatic cascades in tumor progression
Given that most anesthetic agents suppress immune function, which might be associated with tumor growth and an increase of metastasis through angiogenesis, and that surgical manipulation per se might stimulate tumor growth and metastasis, a potential mechanism by which perioperative factors induce tumor angiogenesis has been described. Metastasis is characterized by the colonization of cancer cells from the primary site to distant sites. Tumor invasion occurs as part of the tumor-host interaction, where tumor cells and stromal cells, such as cancer-associated fibroblasts (CAFs) and TAMs, exchange cytokines that remodel the local extracellular matrix, stimulate migration, and promote proliferation and survival (Fig. 2). In the process of metastasis, primary tumor cells survive through several steps of the metastatic cascade [30]. Firstly, cancer cells escape from an antitumor immune response mediated by killer cells, such as NK cells and CTLs, and produce systemic factors that establish an environment that promotes metastasis at the proceeding metastatic site (premetastatic niche). Secondly, tumor cells also change the microenvironment of the primary site to increase blood vessel density (angiogenesis). This process enhances tumor cell leakage from the primary site to the surrounding stroma by invasion, resulting in penetration (intravasation) into blood and/or lymphatic vessels, which allows them to circulate and spread. Thirdly, circulating tumor cells (CTCs) and arrested tumor cells escape from the blood vessel or lymphatic circulation (extravasation). Finally, formation of colonization and angiogenesis in a metastatic tumor proceeds. Once metastatic tumor cells reach the target organ, angiogenesis is required for growing the metastatic tumor. Angiogenesis occurs in several steps, including the release of angiogenic factors and proteolytic enzymes to degrade the basement membrane of the capillary vessels, endothelial proliferation and migration, formation of microvessels, and their differentiation [31].
A schematic representation of the metastatic process from the primary lesion to distant metastatic sites in the tumor microenvironment. Tumor invasion occurs within a tumor-host immune balance in the tumor microenvironment, which leads to the formation of microvessels and lymphatics through the interaction of tumor cells and stromal cells, such as CAFs. This process is mediated by exchanged cytokines and chemokines that remodel the local extracellular matrix, stimulate migration, and promote proliferation and survival. Subsequently, invading tumor cells are released as CTCs to regional lymph nodes or blood vessels to grow as metastases. Some metastatic tumors remain as dormant metastases or as preexisting micrometastases. In this process, most CTCs are killed by immune effector cells, and only those that evade immune surveillance undergo clonal expansion and form metastatic lesions. CD8+ T cells restrict the metastatic outgrowth of cancer cells disseminated from the primary tumor. The innate immune system, in the form of NK cells, is thought to be important in immune surveillance, protecting from metastasis during intravascular tumor seeding that occurs during surgery. Under these conditions, various tumor-derived soluble factors, such as PGE2 and VEGF, are induced by surgery/anesthesia through the activation of the HPA axis and SNS, thereby establishing an immunosuppressive tumor microenvironment to facilitate growing metastatic lesions. CTCs circulating tumor cells, NK natural killer, CAFs cancer-associated fibroblasts, RLN regional lymph node, HPA hypothalamic-pituitary-adrenal, SNS sympathetic nervous system, CMI cell-mediated immunity, MDSCs myeloid-derived suppressor cells, TAMs tumor-associated macrophages, Tregs regulatory T cells, PGE 2 prostaglandin E2, VEGF vascular endothelial growth factor
In the clinic, cancer metastasis can be categorized as the following three types. Firstly, despite metastasis not being detected at the initial diagnosis, it develops within few or several years after the surgical resection. Secondly, metastasis is detected simultaneously with the primary lesion at diagnosis. Thirdly, only the metastatic lesion is detected at diagnosis. In most clinical situations experienced in cancer patients, metastasis occurring for several years after surgery is considered as dormant metastasis, which is a poorly understandable phenomenon with a quiescent state. In turn, the clinically asymptomatic disease remains in an undetectable state for years, only to come out as relapse. The dormant state of the metastatic tumor can be explained in two possible ways. One is a lack of angiogenic ability in dormant tumors. The other is immunological equilibrium between the tumor and the host immune response, which prevents growth of tumors in the microenvironment. Since neuroendocrine mediators regulate the biology of tumor progression, which may lead to reactivation of dormant tumor cells through stimulation of angiogenesis by endogenous mediators, the neuroendocrine dynamics of the HPA axis and SNS may be involved in the loss of tumor dormancy [32]. Since microscopic metastases can remain in a dormant state for a long time, reactivation of dormant cells to promote tumor growth depends on sequential steps, such as switching to the angiogenic phenotype, which is required for the initial recruitment of new vessel formation. To grow in size and gain metastatic potential, tumors need to make a switch to angiogenesis through disrupting the balance of proangiogenic and antiangiogenic factors in the tumor microenvironment [33].
4 Retrospective analyses of the effect of regional anesthesia/analgesia in surgery on cancer recurrence
4.1 Breast and ovarian cancer
A number of laboratory studies have shown that immunosuppression induced by several perioperative factors may influence the clinical outcomes of patients having cancer surgery. Paravertebral anesthesia/block (PVA/PVB) may attenuate perioperative factors that promote tumor growth and metastasis. Five retrospective analyses described the effect of RA in breast surgery on cancer recurrence (Table 1). The first study noted in 2006 that PVA reduced the risk of cancer recurrence or metastasis by approximately one fourth during the follow-up period compared with opioid analgesia and that cancer recurrence after breast cancer surgery was lower with the use of RA than with GA/opioid analgesia [34]. Although one study indicated that the use of RA had a potential benefit in the reduction of breast cancer recurrence [35], another three studies showed no association between anesthesia type and cancer recurrence [36–38], although the overall rate of recurrence in one study was very small [37]. In the case of ovarian cancer and the effect of RA on cancer recurrence, five studies have been reported, including two with positive results, two showing a potential survival benefit of supplemented epidural anesthesia (EP), and one negative result (Table 1). The first study, performed in 2011, showed that EP/analgesia for ovarian cancer surgery may increase in the 3- and 5-year overall survival (OS) [39]. Subsequently, another study indicated that an intraoperative epidural significantly reduced the risk of cancer recurrence, although no effect was found when it was used only postoperatively or without EP [40]. Intraoperative use of EP significantly reduced the tumor recurrence risk after surgery, possibly due to the preservation of the immune system in ovarian cancer patients. Further, a potential benefit for the epidural group was reported in two studies: One study noted no significant difference in OS between the epidural and no epidural groups, but an EP was favored for disease-free survival (DFS) [41]. The other study showed a limited additional benefit on DFS in patients who received postoperative epidural analgesia for more than 48 h [42]. Another study using a prospective clinical registry noted that after propensity scoring matching and weighting, there was no clinical benefit in OS or time to recurrence in patients who received EP and/or analgesia in ovarian cancer surgery [43].
4.2 Digestive cancer
Based on the hypothesis that long-term outcomes would be improved by supplemented EP/analgesia during surgery, which may attenuate the immunosuppressive effect of surgery and enhance tumor immune surveillance, eight studies of colorectal cancer have been reported (Table 2). The first study in colon cancer, in 2008, noted a significantly improved survival in the supplemented EP/GA group compared with those receiving GA/opioid anesthesia in an early follow-up period [44]. Similarly, two studies reported a survival benefit from EP/analgesia supplementation [45, 46]. A study of patients with liver metastasis showed a potential survival benefit in supplemented perioperative EP [47]. Three studies also showed the potential survival benefit of EP/analgesia during surgery [48–50]. A potential survival benefit in older patients was reported in one study [50]. In contrast, another study noted negative results for EP/analgesia supplementation in laparoscopic colorectal resection, using a prospective database [51]. Another large cohort study reported the potential survival benefit of epidural supplementation in open colectomy. However, adjusting for covariates, including blood transfusion, the supplemented EP did not reduce cancer recurrence [49]. Regarding other intestinal cancers, three studies have reported positive results, while three studies show negative results for a survival benefit in epidural supplementation. A study on larynx or hypopharynx cancers reported that supplementation of perioperative cervical EP compared with postoperative morphine increased cancer-free survival and OS in a single-center study [52]. Similarly, supplementation of perioperative EP also improved survival in pancreatic cancer [53]. In addition, the use of postoperative epidural analgesia increased the time to cancer recurrence and OS in gastro-esophageal cancer [54]. However, postoperative EP with morphine increased cancer recurrence and death in comparison with postoperative intravenous analgesia with fentanyl in patients undergoing surgical resection of hepatocellular carcinoma, suggesting an unfavorable effect of morphine on cancer recurrence, even via the epidural route [55]. In addition, supplemented perioperative EP and/or analgesia for gastric cancer and epidural analgesia for esophageal cancer did not affect recurrence or survival [56, 57].
4.3 Prostate, bladder, and other cancers
In the case of prostate cancer, 11 studies have reported on the effects of EP/analgesia supplementation during surgical resection on long-term postoperative survival, including two positive results, one potential benefit of supplemented EP, and eight negative results (Table 3). The first study noted a positive result in 2008, showing that the GA/EP group had a reduced risk of cancer recurrence in comparison with the GA/opioid group [58]. A similar potential association between EP and cancer recurrence was reported using propensity matching for EP vs. GA, suggesting that open prostatectomy with GA accompanied by epidural analgesia with postoperative opioids was associated with a substantial reduction in the risk of clinical cancer progression [59]. However, several subsequent studies showed contradictory results [60–68]. Among these, one study noted that intraoperative administration of sufentanil was associated with an increase in the risk of cancer recurrence after radical prostatectomy [61]. In the case of bladder cancer, one study reported a negative result, while another showed the potential benefit of supplemented RA in reducing the risk of cancer recurrence [69, 70].
In other cancers, one study after nonsmall cell lung cancer (NSCLC) surgery noted that the type of postoperative analgesia, including intravenous, patient-controlled anesthesia vs. patient-controlled EP vs. a combination of these after surgery, was not associated with better recurrence-free survival (RFS) or OS rates [71]. Of note, however, in a retrospective analysis regarding whether opioid-avoiding anesthetic techniques might be associated with increase in cancer RFS, a higher recurrence rate of NSCLC within 5 years was reported in patients who were administered increased doses of opioids for the first 96 h after surgery [72]. In the case of abdominal cancer, one study reported a negative result and another a trend toward a survival benefit for EP. The first study comparing the recurrence and survival of patients undergoing major abdominal surgery for cancer noted that supplemented EP was not associated with reduced cancer-free survival [73]. This was a prospective RCT trial with a long-term follow-up study in which patients were randomly allocated to have GA with or without EP for at least three postoperative days. The other study was a retrospective analysis using a prospective randomized study comparing two postoperative techniques of analgesia: GA with bupivacaine thoracic EP and GA with fentanyl followed by continuous subcutaneous (SC) morphine [74]. Although the analgesia type was not a significant predictive factor for RFS after 5 years, the anesthesia effect changed moderately over the follow-up period. The hazard ratio for OS (EP/SC morphine) reached statistical significance after 5, 6, and 8 years, suggesting that the duration of follow-up may impact the analgesia’s effect on survival [74]. In the case of malignant melanoma, two studies were noted, including a positive result and a potential benefit of local anesthesia. One study in 2000 reported that GA for the primary excision of cutaneous melanoma was associated with a decrease in the survival rate compared with local anesthesia, indicating an increased risk of death for melanoma patients treated with GA [75]. A potential benefit of local anesthesia was also reported when spinal anesthesia (SA) or GA for inguinal lymph node dissection after primary malignant melanoma was compared; a trend toward a better cumulative survival rate for patients who underwent SA was observed and confirmed by further analysis comparing the use of SA with a patient subgroup treated with balanced, volatile GA [76].
5 Meta-analyses of the effect of anesthetic technique in surgery on cancer recurrence
In order to clarify the hypothesis that patients undergoing cancer surgery with RA would achieve a better outcome for RFS and OS compared with those who received GA, seven meta-analyses have been reported comparing GA/RA and GA (Table 4). Six of seven studies indicated a potential survival benefit of supplemented RA in patients who underwent surgery, while one study reported negative results. A meta-analysis of 14 studies, including 18 substudies, of human cancers undergoing surgery noted a benefit in OS in favor of EP compared with GA alone, and a significant positive association between EP and increased OS was found in colorectal cancer [77]. However, a significant relationship between EP and RFS was not found, suggesting that EP and/or analgesia might be associated with improved OS in operable cancer patients having surgery, in particular in colorectal cancer, but is not associated with cancer control. The second report from four secondary data analyses of prospective RCTs of patients undergoing resection of their primary abdominal cancer tumors, including prostate and colon cancers, noted no advantage for either GA/EP or GA in terms of OS and progression-free survival [78]. In another analysis of 10 studies, despite the overall results showing no significant difference between the GA/EP and GA groups in postoperative recurrence and metastasis rates, overall results from four studies of prostate cancer patients suggested that GA/EP was associated with an obvious decrease in recurrence or metastasis of prostate cancer compared with GA alone [79]. In addition, in an investigation of the short-term effect of anesthesia on survival, pooled data demonstrated that GA/EP significantly reduced cancer recurrence or metastasis over a less than 2-year follow-up compared with GA alone [79]. These results suggested that combined GA/EP may be associated with an improved prognosis of cancer patients undergoing surgical treatment, even with a cautious interpretation of the heterogeneous data used for the analysis. Another recent analysis of 20 studies evaluating the effects of supplemented RA on cancer recurrence and survival after cancer surgery noted that, while the use of perioperative RA was not associated with reduced cancer recurrence, it was associated with increased OS, suggesting that RA may improve OS after cancer surgery [80]. Similarly, another report from six studies of colorectal cancer noted that supplemented EP was associated with a significantly longer OS compared with no EP, but not with prolonged RFS, suggesting a survival benefit of improved OS and reduced all-cause mortality [81]. Another report analyzing 10 studies of prostate cancer testing whether the use of a neuraxial anesthetic technique is associated with better long-term outcome after surgical resection noted that the anesthetic technique did not increase biochemical recurrence-free survival; however, the use of regional analgesia appeared to improve OS [82]. A very recent report including 21 studies in a comparison of neuraxial anesthesia, with or without GA, vs. GA only noted a potential association between neuraxial anesthesia and improved OS and RFS compared with GA in cancer surgery, specifically in colorectal cancer, supporting a potential association between neuraxial anesthetic technique and a risk reduction of cancer recurrence [83].
6 Retrospective analyses of propofol or volatile anesthesia and cancer recurrence in surgery
Inhalational anesthetic agents such as sevoflurane have a proinflammatory effect, while the intravenous anesthetic agent, propofol, which is hypnotic, has an anti-inflammatory and antioxidative effect. Three studies reported a potential relationship between patient survival and the use of volatile or propofol anesthesia after cancer surgery, including two showing a potential benefit and one a positive result for propofol (Table 4). A previous study noted that overall 1- and 5-year survival rates for breast, colon, or rectal cancers, combined, were in favor of propofol compared with sevoflurane, suggesting that propofol anesthesia may be superior to volatile anesthesia for some types of cancer in surgical treatment [84]. Another study examining the potential association between propofol anesthesia and cancer recurrence or OS in patients receiving modified radical mastectomy noted that, despite the administration of opioid analgesia during the perioperative period, the propofol group had a reduced cancer recurrence compared with the sevoflurane group [85]. This study suggested that propofol anesthesia may reduce the risk of cancer recurrence during the first 5 years after mastectomy. Further, a large retrospective analysis investigating the potential relationship between anesthetic technique and long-term outcome in patients receiving surgical treatment in solid tumors noted that mortality was approximately 50% greater with volatile than with propofol anesthesia, demonstrating a relationship between the type of anesthetic technique and patient survival in cancer surgery [86]. Of interest, in a multivariate analysis according to surgical specialty, survival for patients undergoing gastrointestinal surgery with volatile inhalational anesthesia was significantly worse than that for the propofol group [86]. These studies suggest that the use of volatile inhalational agents in anesthesia may augment cancer cell growth, whereas propofol may have a protective effect from cancer cell growth after oncologic surgery.
7 Limited interpretation for retrospective analysis of regional anesthesia/analgesia and cancer recurrence in surgery
The anesthetic technique might influence patient outcome in cancer surgery due to the diversity of effects on immunosuppression, angiogenesis, and dissemination of residual tumor cells. Although supplemented RA may reduce immunosuppression and thereby improve survival, contradictory results of retrospective analyses, including meta-analyses, of various types of cancer have been published since the year 2000. In fact, the potential survival benefit of supplemented RA to improve OS and cancer recurrence still remains to be proven. While several positive results and potential benefits for the use of supplemented RA have been reported, the evidence is limited and requires cautious interpretation, since some of these data are indirect and inconsistent and may risk bias within or across studies in the analyses. Differential results of previous clinical studies may be due to several confounding variables, such as the use of a different histological grading, presence or absence of radiotherapy and chemotherapy, a different radical surgery grade, and the presence of perioperative anemia, a blood transfusion, or hypothermia. In addition, heterogeneous populations in the analyses undertook different anesthetic techniques, including the use of perioperative, intraoperative, and/or postoperative RA, with or without GA, and with or without opioids or the use of different opioids or different volatile anesthetics, and so on. Thus, the benefit of supplemented RA on survival and cancer recurrence may be masked by the influence of other confounding factors in comparisons between studies. Furthermore, there are methodological problems, such as a small sample size for a potential positive effect, including patient heterogeneity, surgical procedures in a series of studies, and the difficulty of isolating an influence from the multifactorial perioperative environment. In addition, epidural limitations may exist due to the lack of strong evidence for the supplementation of RA to reduce cancer recurrence and/or risk of metastasis, the limited availability of RCTs, and epidural utilization and effectiveness that is difficult to assess in the intraoperative period. Based on laboratory and animal studies on the differential effect of anesthetic technique/agent on immunosuppression and tumor development, the influence of anesthetics needs to be assessed by a direct effect in the analysis, even in the presence of diverse effects for an anesthetic agent or a differential effect dependent on cancer type. Nevertheless, a retrospective analysis would yield a limited interpretation of a direct effect due to several biases; rather, it is a beneficial tool for exploring which factor tended to be associated with survival benefit. Since the benefits of RA in reducing cancer recurrence in certain cancers may have a sound theoretical basis, prospective RCTs are needed to evaluate any effect of anesthetic technique on metastasis in various types of cancer.
8 Ongoing prospective randomized controlled trials of anesthetic technique and cancer recurrence
Currently, seven prospective RCTs on anesthetic technique in the recurrence of breast, colorectal, and lung cancers and of malignant melanoma are ongoing (Table 5). The first trial in breast cancer surgery was designed and commenced in January 2007, based on the finding that PVA reduced the risk of cancer recurrence or metastasis in breast cancer surgery [87]. In this multicenter trial, stage I–III breast cancer patients undergoing mastectomy or lumpectomy with axillary node dissection are randomly allocated to EP or PVA/analgesia, or GA/morphine analgesia. Patients will be followed for up to 10 years after surgery to evaluate cancer recurrence and metastasis. Despite the fact that the first trial assessing the relationship between anesthetic technique and cancer recurrence commenced in 2007, the results have not been published, and recruitment to the trial continues. The main reason for the delay in trial completion and publication is that the number of patients needed for trials with time-to-event outcomes depends on the number of outcome events, recurrences in this case, rather than enrollment. There have been fewer recurrences than anticipated, and enrollment thus continues (personal communication, Daniel I. Sessler). Subsequently, another trial for colorectal cancer was designed and commenced in December 2007, comparing recurrence rates in patients who are randomly allocated to GA/EP/analgesia or GA/opioid analgesia. The patients will be followed for up to 5 years after surgery to evaluate whether local recurrence/metastatic cancer after open and laparoscopic resection of colon cancer is lower in patients randomized to EP/analgesia than to sevoflurane GA/postoperative opioid analgesia. Another trial for lung cancer surgery commenced in August 2010 and was designed to determine the effect of GA/EP compared with GA on cancer recurrence. The patients will be followed for up to 5 years after surgery, with the primary outcome of DFS. In addition, as a secondary endpoint, NK cell activity and markers of immunological function, such as cytokines and cortisol, will be measured at repeated perioperative time points and up to 3 years after surgery. The above three trials were mainly organized by the Cleveland Clinic, Cleveland, in the USA. Another trial for colorectal cancer surgery was designed and commenced as a multicenter study in Sweden in March 2011. Patients are randomized to one of two groups with either epidural analgesia or patient-controlled analgesia. The patients will be followed for up to 5 years to record cancer-specific as well as all-cause mortality, to determine whether epidural analgesia can reduce cancer-related mortality after surgical treatment. In this trial, inflammatory and immunological markers, including VEGF, HIF-1α, microRNA mi21, CTCs, IFN-γ, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, TNF-α, and PGE2, will be measured pre surgery and post surgery. Another trial for malignant melanoma in patients who undergo radical inguinal lymph node dissection was designed in Germany in March 2012 to compare RA and GA, consisting of SA/bupivacaine, GA/sufentanil, and propofol/rocuronium/sevoflurane, respectively. The primary endpoint is OS up to 5 years, and the secondary endpoint is the measurement of changes of the total number of immune cells, including T lymphocytes, B lymphocytes, and NK cells, and their activity, changes in TGF-β, and the activation status of platelets from baseline until 15 min prior to the end of surgery and from baseline until 24 h and 5 days postoperatively. If RA prevents perioperative immunosuppression and reduces postoperative metastatic cancer dissemination, optimized anesthetic management might improve long-term outcomes after cancer surgery. Another trial was designed and initiated for patients with either breast or colorectal cancer after radical surgery as a multicenter study in Sweden in November 2013, to compare propofol with sevoflurane anesthesia in terms of OS. The patients will be followed for up to 5 years to evaluate whether 1- and 5-year survival rates following propofol anesthesia are better than those following sevoflurane anesthesia. Similarly, another trial to test the effects of propofol anesthesia and inhalational anesthesia on cancer cell cytotoxicity, micrometastasis, and cancer recurrence in patients having breast cancer resection was designed in the Republic of Korea in February 2014. The primary outcome measurement is the number of NK cells 24 h after surgery. If the immunosuppression of total intravenous anesthesia with propofol and remifentanil is lower than that of inhalational anesthesia with sevoflurane and remifentanil, cancer recurrence may be decreased.
9 Potential rationale and background for anesthetic technique improving cancer-related death rates
Despite the fact that surgery is the most effective treatment for cancer, many dormant tumor cells may already exist in sites distant from the primary lesion. Surgical treatment may also release tumor cells from the primary lesion into the circulatory system through the lymphatic system and bloodstream due to surgical manipulation that may lead to residual disease in the formation of micrometastases by scattering tumor cells. The immune system plays a crucial role in eradicating cancer cells, and immune competence is required to prevent further disease progression of residual cancer cells in the perioperative period. Thus, perioperative immune function is important for the development of cancer recurrence. The perioperative period, including the preoperative, intraoperative, and postoperative periods, is a critical point for eradicating residual cancer cells, which eventually influence long-term outcome. The perioperative period can be characterized by immunosuppression, angiogenesis, and an increased load of CTCs. Whether residual cancer cells result in the appearance of clinical metastases is mostly affected by the balance between antimetastatic immune responses of the host’s defense systems and by the tumor’s ability to grow in the metastatic site. In clinical practice, the host immune system often fails to eradicate residual cancer cells, permitting loco-regional recurrence and distant metastasis to develop after surgical treatment.
At present, three perioperative factors are considered to shift the balance to the initiation and progression of residual cancer cells. The first is surgery per se, which scatters cancer cells into the systemic circulation, suppresses CMI, including the functions of CTLs and NK cells, reduces antiangiogenic factors such as angiostatin and endostatin, increases proangiogenic factors such as VEGF, and releases several growth factors that promote the growth of cancer cells [88, 89]. Secondly, general anesthetics per se, except propofol, also impair various immune functions for macrophages, dendritic cells, T lymphocytes, and NK cells [90]. Thirdly, opioid analgesia inhibits immune function, which leads to increased angiogenesis and promotes tumor growth [91]. Therefore, opioid-sparing analgesia may help to keep the function of NK cells and reduce metastatic spread of cancer [92]. Regional anesthesia/analgesia may prevent or attenuate surgery-stimulated adverse effects by inhibiting the neuroendocrine stress response, which blocks not only the afferent neural transmissions from reaching to the central nervous system but also the descending efferent activation of the SNS. Regional analgesia reduces the release of endogenous opioids; thereby, opioid-induced immune suppression may be reduced [93]. With the combination of regional and general anesthesia/analgesia, the proportion of general anesthetics required is reduced and also probably their induced immunosuppression, as well as the need for GA, minimizing the opioid requirement for postoperative pain relief.
Regarding the effect of opioids, whether they act as modulators of either cell proliferation or cell death in affecting tumor progression is controversial [94–96]. Evidence for opioids suppressing the immune response includes the fact that various immune-competent cells express opioid receptors and undergo apoptosis when treated with opioids. Opioid-induced cell proliferation and cell death appear to be dependent on the concentration or duration of treatment. Growth-promoting effects occur at low concentrations or with single doses of opioids, while growth-inhibitory effects occur with chronic treatment or at relatively high concentrations of opioid [97]. The μ-opioid receptor (MOR) is overexpressed in several human cancers, leading to the promotion of tumor growth and metastasis [98]. Morphine stimulated proliferation of microvascular endothelial cells and angiogenesis at the concentrations observed in patients. Morphine at clinically relevant doses enhanced tumor neovascularization and caused an increase in tumor progression of breast cancer cells in vitro and in vivo [94, 99]. Given that laboratory and clinical studies suggest that differences in the recurrence of certain types of cancer are dependent on whether patients received RA/GA or GA/opioid analgesia, the differences in cancer recurrence may be due to immunosuppression and the direct effects of opioids on tumor growth of angiogenesis-dependent cancers. On the other hand, the preoperative and postoperative administration of morphine attenuated the tumor-promoting effects of surgery, and morphine treatment also attenuated the surgery-induced stress response in animal studies [100, 101]. These findings suggest that the preoperative administration of morphine may play a key role in modulating surgery-induced increases in metastasis. Further, fentanyl had antitumor effects, with reduction of cell clone formation and inhibition of cell migration and invasion of colorectal cancer cells in vitro [102]. Despite the benefits of opioid-sparing RA techniques on patient outcome, as has been suggested in clinical trials of surgical treatment, it is uncertain whether the benefit comes from directly the lack of opioids or from supplemented RA. Nevertheless, research has shown that in specific types of cancer, morphine may be beneficial and that the MOR plays a role in tumor progression. Opioids may play a role in the development of cancer metastasis and recurrence, which appears to differ depending on the type of cancer cell [103].
Using clinical samples to support the hypotheses that anesthetic techniques affect cancer-related mortality and cancer recurrence via immune function and tumor growth-related factors in cancer patients, a recent study on colon cancer patients having fast-track surgery during the perioperative period noted significant increases in lymphocytes and Th1 cells, and decreases in Th2 and regulatory T cells were found in patients who received EP/GA post surgery in comparison with those who received GA only [104]. A previous study on colon cancer patients randomized to propofol-epidural anesthesia (PEA) or GA noted that patients receiving PEA showed that the levels of VEGF-C, TGF-β, and IL-6 were decreased, whereas the level of IL-10 was increased after surgery compared with those receiving GA [105]. Further, a recent study on colon cancer surgery in patients randomly allocated to receive PEA or sevoflurane/opioid noted that serum from patients having PEA better inhibited proliferation and invasion and induced apoptosis in colon cancer cells in vitro, compared with that from patients having sevoflurane/opioid [106]. A controlled randomized study comparing PVA with sevoflurane/opioid noted that PVA attenuated the cytokine responses of IL-6, IL-10, IL-12, and IFN-γ after breast cancer surgery [107]. Interestingly, the surgical specimens from breast cancer patients recruited in an ongoing RCT (NCT00418457) were immunohistochemically stained to highlight immune cell infiltration. Propofol-paravertebral anesthesia increased the infiltration of NK and T-helper cells into breast cancer tissues compared with sevoflurane/opioid, in contrast to suppressor T cells or macrophages [108]. A recent pilot study from an ongoing prospective RCT (NCT00418457) showed that serum from patients administered PVA for breast cancer surgery induced apoptosis to a greater extent in ER-negative breast cancer cells compared with that from patients administered sevoflurane/opioid anesthesia [109]. Further, another pilot study using blood sample from breast cancer patients before surgery and 24 h after surgery in an ongoing prospective RCT (NCT00418457) noted that blood sample from patients administered PVA had greater NK cell cytotoxicity in vitro compared with that from patients administered sevoflurane/opioid [110]. These findings suggest that anesthetic technique may alter cytokines and serum factors associated with cancer cell function and immune function in metastasis, thereby providing an explanation for how anesthetic technique may affect cancer recurrence.
10 Conclusions
In the past decade, despite a focus on the relationship between anesthetic technique and cancer recurrence that has been the most interesting topic in oncologic surgery, currently available data do not provide any definitive answers to the hypothesis that the use of RA rather than GA can reduce surgical stress, the use of volatile anesthetics, and opioid consumption, thereby reducing perioperative immunosuppression, angiogenesis, and eventually, cancer recurrence to prolong patient survival. The lack of definitive answers is due, in part, to the heterogeneous and limited methodologic nature of previous studies. Although supplemented loco-regional anesthesia or propofol-based anesthesia appears to reduce cancer recurrence after oncologic surgery, there is no evidence showing that simple changes of anesthesia in clinical practice can provide a survival benefit after surgical treatment in cancer patients. In addition, how anesthetic agents affect the immune system in association with patient outcomes remains to be elucidated. Thus, the puzzle of the relationship between anesthetic technique and cancer recurrence has not yet been unraveled. Nevertheless, from preclinical and retrospective studies, a potential effect of anesthetic techniques on cancer recurrence and survival of minimal residual cancer through immunological and nonimmunological mechanisms still exists. Since a minimal requirement of volatile anesthetics and opioids contributes to better outcomes in oncologic surgery, an approach to avoid immunosuppressive anesthetic agent needs to be at least considered in the clinical practice of cancer treatment. The effect of anesthetic technique on cancer outcome must be highlighted as not only an issue for anesthesiologists but also an important issue for surgeons working together to cure cancer patients in oncologic surgery. Several well-planned prospective RCTs are ongoing that will provide more promising results to verify the benefits of anesthetic technique on reducing cancer recurrence in oncologic surgery.
References
Heaney, A., & Buggy, D. J. (2012). Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? British Journal of Anaesthesiology, 109(Suppl 1), i17–i28. doi:10.1093/bja/aes421.
Talmadge, J. E., & Fidler, I. J. (2010). AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Research, 70(14), 5649–5669. doi:10.1158/0008-5472.CAN-10-1040.
Alsina, E., Matute, E., Ruiz-Huerta, A. D., & Gilsanz, F. (2014). The effects of sevoflurane or remifentanil on the stress response to surgical stimulus. Current Pharmaceutical Design, 20(34), 5449–5468.
O’Dwyer, M. J., Owen, H. C., & Torrance, H. D. (2015). The perioperative immune response. Current Opinion in Critical Care, 21(4), 336–342. doi:10.1097/MCC.0000000000000213.
Bar-Yosef, S., Melamed, R., Page, G. G., Shakhar, G., Shakhar, K., & Ben-Eliyahu, S. (2001). Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology, 94(6), 1066–1073.
Melamed, R., Bar-Yosef, S., Shakhar, G., Shakhar, K., & Ben-Eliyahu, S. (2003). Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesthesiaa & Analgesia, 97(5), 1331–1339.
Snyder, G. L., & Greenberg, S. (2010). Effect of anaesthetic technique and other perioperative factors on cancer recurrence. British Journal Anaesthesiology, 105(2), 106–115. doi:10.1093/bja/aeq164.
Green, J. S., & Tsui, B. C. (2013). Impact of anesthesia for cancer surgery: continuing professional development. Canadian Journal Anaesthesia, 60(12), 1248–1269. doi:10.1007/s12630-013-0037-1.
Ash, S. A., & Buggy, D. J. (2013). Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery? An update of available evidence. Best Practice & Research Clinical Anaesthesiology, 27(4), 441–456. doi:10.1016/j.bpa.2013.10.005.
Cassinello, F., Prieto, I., del Olmo, M., Rivas, S., & Strichartz, G. R. (2015). Cancer surgery: how may anesthesia influence outcome? Journal of Clinical Anesthesia, 27(3), 262–272. doi:10.1016/j.jclinane.2015.02.007.
Votta-Velis, E. G., Piegeler, T., Minshall, R. D., Aguirre, J., Beck-Schimmer, B., Schwartz, D. E., et al. (2013). Regional anaesthesia and cancer metastases: the implication of local anaesthetics. Acta Anaesthesiologica Scandinavica, 57(10), 1211–1229. doi:10.1111/aas.12210.
Byrne, K., Levins, K. J., & Buggy, D. J. (2016). Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Canadian Journal Anaesthesia, 63(2), 184–192.
Meserve, J. R., Kaye, A. D., Prabhakar, A., & Urman, R. D. (2014). The role of analgesics in cancer propagation. Best Practice & Research Clinical Anaesthesiology, 28(2), 139–151. doi:10.1016/j.bpa.2014.04.004.
Bajwa, S. J., Anand, S., & Kaur, G. (2015). Anesthesia and cancer recurrences: the current knowledge and evidence. Journal Cancer Research and Therapeutics, 11(3), 528–534. doi:10.4103/0973-1482.157321.
Xuan, W., Hankin, J., Zhao, H., Yao, S., & Ma, D. (2015). The potential benefits of the use of regional anesthesia in cancer patients. International Journal of Cancer, 137(12), 2774–2784. doi:10.1002/ijc.29306.
Kim, R., Emi, M., Tanabe, K., & Arihiro, K. (2006). Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Research, 66(11), 5527–5536.
Kavanagh, T., & Buggy, D. J. (2012). Can anaesthetic technique effect postoperative outcome? Current Opinion in Anaesthesiology, 25(2), 185–198. doi:10.1097/ACO.0b013e32834f6c4c.
Kurosawa, S., & Kato, M. (2008). Anesthetics, immune cells, and immune responses. Journal of Anesthesia, 22(3), 263–277. doi:10.1007/s00540-008-0626-2.
Lee, B. M., & Cata, J. P. (2015). Impact of anesthesia on cancer recurrence. Revista Española de Anestesiología y Reanimación, 62(10), 570–575. doi:10.1016/j.redar.2015.04.003.
Greenfeld, K., Avraham, R., Benish, M., Goldfarb, Y., Rosenne, E., Shapira, Y., et al. (2007). Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain, Behavior, and Immunity, 21(4), 503–513.
Sood, A. K., Bhatty, R., Kamat, A. A., Landen, C. N., Han, L., Thaker, P. H., et al. (2006). Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Research, 12(2), 369–375.
Wong, H. P., Ho, J. W., Koo, M. W., Yu, L., Wu, W. K., Lam, E. K., et al. (2011). Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sciences, 88(25–26), 1108–1112. doi:10.1016/j.lfs.2011.04.007.
Bernabé, D. G., Tamae, A. C., Biasoli, É. R., & Oliveira, S. H. (2011). Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain, Behavior, and Immunity, 25(3), 574–583. doi:10.1016/j.bbi.2010.12.012.
Yang, E. V., Kim, S. J., Donovan, E. L., Chen, M., Gross, A. C., Webster Marketon, J. I., et al. (2009). Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain, Behavior, and Immunity, 23(2), 267–275. doi:10.1016/j.bbi.2008.10.005.
Calcagni, E., & Elenkov, I. (2006). Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences, 1069, 62–76.
Marik, P. E., & Flemmer, M. (2012). Immunonutrition in the surgical patient. See comment in PubMed Commons below Minerva Anestesiologica, 78(3), 336–342.
Sica, A., Schioppa, T., Mantovani, A., & Allavena, P. (2006). Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eurlopian Journal of Cancer, 42(6), 717–727.
Obermajer, N., Wong, J. L., Edwards, R. P., Odunsi, K., Moysich, K., & Kalinski, P. (2012). PGE2-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunological Investigations, 41(6–7), 635–657. doi:10.3109/08820139.2012.695417.
Mao, Y., Sarhan, D., Steven, A., Seliger, B., Kiessling, R., & Lundqvist, A. (2014). Inhibition of tumor-derived prostaglandin-E2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clinical Cancer Research, 20(15), 4096–4106. doi:10.1158/1078-0432.CCR-14-0635.
Kitamura, T., Qian, B. Z., & Pollard, J. W. (2015). Immune cell promotion of metastasis. Nature Reviews Immunology, 15(2), 73–86. doi:10.1038/nri3789.
John, A., & Tuszynski, G. (2001). The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathology & Oncology Research, 7(1), 14–23.
Zappalà, G., McDonald, P. G., & Cole, S. W. (2013). Tumor dormancy and the neuroendocrine system: an undisclosed connection? Cancer and Metastasis Reviews, 32(1–2), 189–200. doi:10.1007/s10555-012-9400-x.
Folkman, J. (2002). Role of angiogenesis in tumor growth and metastasis. Seminars in Oncology, 29(6 Suppl 16), 15–18.
Exadaktylos, A. K., Buggy, D. J., Moriarty, D. C., Mascha, E., & Sessler, D. I. (2006). Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology, 105(4), 660–664.
Koonce, S. L., Mclaughlin, S. A., Eck, D. L., Porter, S., Bagaria, S., Clendenen, S. R., et al. (2014). Breast cancer recurrence in patients receiving epidural and paravertebral anesthesia: a retrospective, case-control study. Middle East Journal of Anesthesiology, 22(6), 567–571.
Starnes-Ott, K., Goravanchi, F., & Meininger, J. C. (2015). Anesthetic choices and breast cancer recurrence: a retrospective pilot study of patient, disease, and treatment factors. Critical Care Nursing Quarterly, 38(2), 200–210. doi:10.1097/CNQ.0000000000000062.
Tsigonis, A. M., Al-Hamadani, M., Linebarger, J. H., Vang, C. A., Krause, F. J., Johnson, J. M., et al. (2016). Are cure rates for breast cancer improved by local and regional anesthesia? Regional Anesthesia and Pain Medicine, 41(3), 339–347. doi:10.1097/AAP.0000000000000379.
Kairaluoma, P., Mattson, J., Heikkilä, P., Pere, P., & Leidenius, M. (2016). Perioperative paravertebral regional anaesthesia and breast cancer recurrence. Anticancer Research, 36(1), 415–418.
Lin, L., Liu, C., Tan, H., Ouyang, H., Zhang, Y., & Zeng, W. (2011). Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. British Journal of Anaesthesia, 106, 814–822. doi:10.1093/bja/aer055.
de Oliveira Jr., G. S., Ahmad, S., Schink, J. C., Singh, D. K., Fitzgerald, P. C., & McCarthy, R. J. (2011). Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Regional Anesthesia and Pain Medicine, 36, 271–277. doi:10.1097/AAP.0b013e318217aada.
Capmas, P., Billard, V., Gouy, S., Lhommé, C., Pautier, P., Morice, P., et al. (2012). Impact of epidural analgesia on survival in patients undergoing complete cytoreductive surgery for ovarian cancer. Anticancer Research, 32(4), 1537–1542.
Elias, K. M., Kang, S., Liu, X., Horowitz, N. S., Berkowitz, R. S., & Frendl, G. (2015). Anesthetic selection and disease-free survival following optimal primary cytoreductive surgery for stage III epithelial ovarian cancer. Annals of Surgical Oncology, 22(4), 1341–1348. doi:10.1245/s10434-014-4112-9.
Lacassie, H. J., Cartagena, J., Brañes, J., Assel, M., & Echevarría, G. C. (2013). The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population. Anesthesia & Analgesia, 117(3), 653–660. doi:10.1213/ANE.0b013e3182a07046.
Christopherson, R., James, K. E., Tableman, M., Marshall, P., & Johnson, F. E. (2008). Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesthesia & Analgesia, 107(1), 325–332. doi:10.1213/ane.0b013e3181770f55.
Holler, J. P., Ahlbrandt, J., Burkhardt, E., Gruss, M., Röhrig, R., Knapheide, J., et al. (2013). Peridural analgesia may affect long-term survival in patients with colorectal cancer after surgery (PACO-RAS-Study): an analysis of a cancer registry. Annals of Surgery, 258(6), 989–993. doi:10.1097/SLA.0b013e3182915f61.
Vogelaar, F. J., Abegg, R., van der Linden, J. C., Cornelisse, H. G., van Dorsten, F. R., Lemmens, V. E., et al. (2015). Epidural analgesia associated with better survival in colon cancer. International Journal of Colorectal Disease, 30(8), 1103–1107. doi:10.1007/s00384-015-2224-8.
Zimmitti, G., Soliz, J., Aloia, T. A., Gottumukkala, V., Cata, J. P., Tzeng, C. W., et al. (2016). Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Annals of Surgical Oncology, 23(3), 1003–1011. doi:10.1245/s10434-015-4933-1.
Gupta, A., Björnsson, A., Fredriksson, M., Hallböök, O., & Eintrei, C. (2011). Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. British Journal of Anaesthesia, 107(2), 164–170. doi:10.1093/bja/aer100.
Cummings 3rd, K. C., Xu, F., Cummings, L. C., & Cooper, G. S. (2012). A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population-based study. Anesthesiology, 116(4), 797–806. doi:10.1097/ALN.0b013e31824674f6.
Gottschalk, A., Ford, J. G., Regelin, C. C., You, J., Mascha, E. J., Sessler, D. I., et al. (2010). Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology, 113(1), 27–34. doi:10.1097/ALN.0b013e3181de6d0d.
Day, A., Smith, R., Jourdan, I., Fawcett, W., Scott, M., & Rockall, T. (2012). Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. British Journal of Anaesthesia, 109(2), 185–190. doi:10.1093/bja/aes106.
Merquiol, F., Montelimard, A. S., Nourissat, A., Molliex, S., & Zufferey, P. J. (2013). Cervical epidural anesthesia is associated with increased cancer-free survival in laryngeal and hypopharyngeal cancer surgery: a retrospective propensity-matched analysis. Regional Anesthesia and Pain Medicine, 38(5), 398–402. doi:10.1097/AAP.0b013e31829cc3fb.
Call, T. R., Pace, N. L., Thorup, D. B., Maxfield, D., Chortkoff, B., Christensen, J., et al. (2015). Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology, 122(2), 317–324. doi:10.1097/ALN.0000000000000489.
Hiller, J. G., Hacking, M. B., Link, E. K., Wessels, K. L., & Riedel, B. J. (2014). Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiologica Scandinavica, 58(3), 281–290. doi:10.1111/aas.12255.
Cao, L., Chang, Y., Lin, W., Zhou, J., Tan, H., Yuan, Y., et al. (2014). Long-term survival after resection of hepatocelluar carcinoma: a potential risk associated with the choice of postoperative analgesia. Anesthesia & Analgesia, 118(6), 1309–1316. doi:10.1213/ANE.0000000000000207.
Cummings 3rd, K. C., Patel, M., Htoo, P. T., Bakaki, P. M., Cummings, L. C., & Koroukian, S. (2014). A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: a population-based study. Regional Anesthesia and Pain Medicine, 39(3), 200–207. doi:10.1097/AAP.0000000000000079.
Heinrich, S., Janitz, K., Merkel, S., Klein, P., & Schmidt, J. (2015). Short- and long term effects of epidural analgesia on morbidity and mortality of esophageal cancer surgery. Langenbeck’s Archives of Surgery, 400(1), 19–26. doi:10.1007/s00423-014-1248-9.
Biki, B., Mascha, E., Moriarty, D. C., Fitzpatrick, J. M., Sessler, D. I., & Buggy, D. J. (2008). Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology, 109, 180–187. doi:10.1097/ALN.0b013e31817f5b73.
Wuethrich, P. Y., Hsu Schmitz, S. F., Kessler, T. M., Thalmann, G. N., Studer, U. E., Stueber, F., et al. (2010). Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology, 113(3), 570–576. doi:10.1097/ALN.0b013e3181e4f6ec.
Tsui, B. C., Rashiq, S., Schopflocher, D., Murtha, A., Broemling, S., Pillay, J., et al. (2010). Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Canadian Journal of Anesthesia, 57(2), 107–112. doi:10.1007/s12630-009-9214-7.
Forget, P., Tombal, B., Scholtès, J. L., Nzimbala, J., Meulders, C., Legrand, C., et al. (2011). Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? European Journal of Anaesthesiology, 28(12), 830–835. doi:10.1097/EJA.0b013e32834b7d9a.
Wuethrich, P. Y., Thalmann, G. N., Studer, U. E., & Burkhard, F. C. (2013). Epidural analgesia during open radical prostatectomy does not improve long-term cancer-related outcome: a retrospective study in patients with advanced prostate cancer. PloS One, 8(8), e72873. doi:10.1371/journal.pone.0072873.
Sprung, J., Scavonetto, F., Yeoh, T. Y., Kramer, J. M., Karnes, R. J., Eisenach, J. H., et al. (2014). Outcomes after radical prostatectomy for cancer: a comparison between general anesthesia and epidural anesthesia with fentanyl analgesia: a matched cohort study. Anesthesia & Analgesia, 119(4), 859–866. doi:10.1213/ANE.0000000000000320.
Roiss, M., Schiffmann, J., Tennstedt, P., Kessler, T., Blanc, I., Goetz, A., et al. (2014). Oncological long-term outcome of 4772 patients with prostate cancer undergoing radical prostatectomy: does the anaesthetic technique matter? European Journal of Surgical Oncology, 40(12), 1686–1692. doi:10.1016/j.ejso.2014.02.223.
Scavonetto, F., Yeoh, T. Y., Umbreit, E. C., Weingarten, T. N., Gettman, M. T., Frank, I., et al. (2014). Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: a large, retrospective matched cohort study. British Journal of Anaesthesia, 113(Suppl 1), i95–102. doi:10.1093/bja/aet467.
Tseng, K. S., Kulkarni, S., Humphreys, E. B., Carter, H. B., Mostwin, J. L., Partin, A. W., et al. (2014). Spinal anesthesia does not impact prostate cancer recurrence in a cohort of men undergoing radical prostatectomy: an observational study. Regional Anesthesia and Pain Medicine, 39(4), 284–288. doi:10.1097/AAP.0000000000000108.
Ehdaie, B., Sjoberg, D. D., Dalecki, P. H., Scardino, P. T., Eastham, J. A., & Amar, D. (2014). Association of anesthesia technique for radical prostatectomy with biochemical recurrence: a retrospective cohort study. Canadian Journal of Anesthesia, 61(12), 1068–1074. doi:10.1007/s12630-014-0221-y.
Kamuf, J., Pospich, M., & Heid, F. (2014). Cancer-free or overall survival rate following radical prostatectomy is not influenced by perioperative pain management. Journal of Anesthesia & Clinical Research, 5, 7. doi:10.4172/2155-6148.1000422.
Jang, D., Lim, C. S., Shin, Y. S., Ko, Y. K., Park, S. I., Song, S. H., et al. (2016). A comparison of regional and general anesthesia effects on 5 year survival and cancer recurrence after transurethral resection of the bladder tumor: a retrospective analysis. BMC Anesthesiology, 16, 16. doi:10.1186/s12871-016-0181-6.
Weingarten, T. N., Taccolini, A. M., Ahle, S. T., Dietz, K. R., Dowd, S. S., Frank, I., et al. (2016). Perioperative management and oncological outcomes following radical cystectomy for bladder cancer: a matched retrospective cohort study. Canadian Journal of Anesthesia, 63(5), 584–595. doi:10.1007/s12630-016-0599-9.
Cata, J. P., Gottumukkala, V., Thakar, D., Keerty, D., Gebhardt, R., & Liu, D. D. (2014). Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. See comment in PubMed Commons below Journal of Clinical Anesthesia, 26(1), 3–17. doi:10.1016/j.jclinane.2013.06.007.
Maher, D. P., Wong, W., White, P. F., McKenna Jr., R., Rosner, H., Shamloo, B., et al. (2014). Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. British Journal of Anaesthesia, 113(Suppl 1), i88–i94. doi:10.1093/bja/aeu192.
Myles, P. S., Peyton, P., Silbert, B., Hunt, J., Rigg, J. R., Sessler, D. I., & ANZCA Trials Group Investigators (2011). Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. British Medical Journal, 342, d1491. doi:10.1136/bmj.d1491.
Binczak, M., Tournay, E., Billard, V., Rey, A., & Jayr, C. (2013). Major abdominal surgery for cancer: does epidural analgesia have a long-term effect on recurrence-free and overall survival? Annales Françaises d’Anesthésie et de Réanimation, 32(5), e81–e88. doi:10.1016/j.annfar.2013.02.027.
Schlagenhauff, B., Ellwanger, U., Breuninger, H., Stroebel, W., Rassner, G., & Garbe, C. (2000). Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Research, 10(2), 165–169.
Gottschalk, A., Brodner, G., Van Aken, H. K., Ellger, B., Althaus, S., & Schulze, H. J. (2012). Can regional anaesthesia for lymph-node dissection improve the prognosis in malignant melanoma? British Journal of Anaesthesia, 109(2), 253–259. doi:10.1093/bja/aes176.
Chen, W. K., & Miao, C. H. (2013). The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PloS One, 8(2), e56540. doi:10.1371/journal.pone.0056540.
Cakmakkaya, O. S., Kolodzie, K., Apfel, C. C., & Pace, N. L. (2014). Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database of Systematic Reviews, 11, CD008877. doi:10.1002/14651858.CD008877.pub2.
Pei, L., Tan, G., Wang, L., Guo, W., Xiao, B., Gao, X., et al. (2014). Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: a meta-analysis of retrospective and prospective studies. PloS One, 9(12), e114667. doi:10.1371/journal.pone.0114667.
Sun, Y., Li, T., & Gan, T. J. (2015). The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: a systematic review and meta-analysis. Regional Anesthesia and Pain Medicine, 40(5), 589–598. doi:10.1097/AAP.0000000000000273.
Sun, X., Yang, C., Li, K., & Ding, S. (2015). The impact of anesthetic techniques on survival for patients with colorectal cancer: evidence based on six studies. Hepato-Gastroenterology, 62(138), 299–302.
Lee, B. M., Singh Ghotra, V., Karam, J. A., Hernandez, M., Pratt, G., & Cata, J. P. (2015). Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Management, 5(5), 387–595. doi:10.2217/pmt.15.30.
Weng, M., Chen, W., Hou, W., Li, L., Ding, M., & Miao, C. (2016). The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget, 7(12), 15262–15273. doi:10.18632/oncotarget.7683.
Enlund, M., Berglund, A., Andreasson, K., Cicek, C., Enlund, A., & Bergkvist, L. (2014). The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Upsala Journal of Medical Sciences, 119(3), 251–261. doi:10.3109/03009734.2014.922649.
Lee, J. H., Kang, S. H., Kim, Y., Kim, H. A., & Kim, B. S. (2016). Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean Journal of Anesthesiology, 69(2), 126–132. doi:10.4097/kjae.2016.69.2.126.
Wigmore, T. J., Mohammed, K., & Jhanji, S. (2016). Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology, 124(1), 69–79. doi:10.1097/ALN.0000000000000936.
Sessler, D. I., Ben-Eliyahu, S., Mascha, E. J., Parat, M. O., & Buggy, D. J. (2008). Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemporary Clinical Trials, 29(4), 517–526. doi:10.1016/j.cct.2008.01.002.
Zetter, B. R. (1998). Angiogenesis and tumor metastasis. Annual Review of Medicine, 49, 407–424.
Antoni, M. H., Lutgendorf, S. K., Cole, S. W., Dhabhar, F. S., Sephton, S. E., McDonald, P. G., et al. (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer, 6(3), 240–248.
Kurosawa, S. (2012). Anesthesia in patients with cancer disorders. Current Opinion in Anesthesiology, 25(3), 376–384. doi:10.1097/ACO.0b013e328352b4a8.
Sacerdote, P., Bianchi, M., Gaspani, L., Manfredi, B., Maucione, A., Terno, G., et al. (2000). The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesthesia & Analgesia, 90(6), 1411–1414.
Ben-Eliyahu, S., Page, G. G., Yirmiya, R., & Shakhar, G. (1999). Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. International Journal of Cancer, 80(6), 880–888.
Chae, B. K., Lee, H. W., Sun, K., Choi, Y. H., & Kim, H. M. (1998). The effect of combined epidural and light general anesthesia on stress hormones in open heart surgery patients. Surgery Today, 28(7), 727–731.
Gupta, K., Kshirsagar, S., Chang, L., Schwartz, R., Law, P. Y., Yee, D., et al. (2002). Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Research, 62(15), 4491–4498.
Singhal, P. C., Sharma, P., Kapasi, A. A., Reddy, K., Franki, N., & Gibbons, N. (1998). Morphine enhances macrophage apoptosis. Journal of Immunology, 160(4), 1886–1893.
Hatzoglou, A., Bakogeorgou, E., & Castanas, E. (1996). The antiproliferative effect of opioid receptor agonists on the T47D human breast cancer cell line, is partially mediated through opioid receptors. European Journal of Pharmacology, 296(2), 199–207.
Lin, X., Wang, Y. J., Li, Q., Hou, Y. Y., Hong, M. H., Cao, Y. L., et al. (2009). Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. Federation of European Biochemical Societies Journal, 276(7), 2022–2036. doi:10.1111/j.1742-4658.2009.06938.x.
Singleton, P. A., Mirzapoiazova, T., Hasina, R., Salgia, R., & Moss, J. (2014). Increased μ-opioid receptor expression in metastatic lung cancer. British Journal of Anaesthesia, 113(Suppl 1), i103–i108. doi:10.1093/bja/aeu165.
Bimonte, S., Barbieri, A., Rea, D., Palma, G., Luciano, A., Cuomo, A., et al. (2015). Morphine promotes tumor angiogenesis and increases breast cancer progression. BioMed Research International, 2015, 161508. doi:10.1155/2015/161508.
Page, G. G., Ben-Eliyahu, S., Yirmiya, R., & Liebeskind, J. C. (1993). Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain, 54(1), 21–28.
Page, G. G., McDonald, J. S., & Ben-Eliyahu, S. (1998). Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. British Journal of Anaesthesia, 81(2), 216–223.
Li, A. X., Xin, W. Q., & Ma, C. G. (2015). Fentanyl inhibits the invasion and migration of colorectal cancer cells via inhibiting the negative regulation of Ets-1 on BANCR. Biochemical and Biophysical Research Communications, 465(3), 594–600. doi:10.1016/j.bbrc.2015.08.068.
Juneja, R. (2014). Opioids and cancer recurrence. Current Opinion in Supportive and Palliative Care, 8(2), 91–101. doi:10.1097/SPC.0000000000000056.
Chen, W. K., Ren, L., Wei, Y., Zhu, D. X., Miao, C. H., & Xu, J. M. (2015). General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. International Journal of Colorectal Disease, 30(4), 475–481. doi:10.1007/s00384-014-2098-1.
Xu, Y. J., Chen, W. K., Zhu, Y., Wang, S. L., & Miao, C. H. (2014). Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. British Journal of Anaesthesia, 113(Suppl 1), i49–i55. doi:10.1093/bja/aeu148.
Xu, Y. J., Li, S. Y., Cheng, Q., Chen, W. K., Wang, S. L., Ren, Y., et al. (2016). Effects of anaesthesia on proliferation, invasion and apoptosis of LoVo colon cancer cells in vitro. Anaesthesia, 71(2), 147–154. doi:10.1111/anae.13331.
Sultan, S. S. (2013). Paravertebral block can attenuate cytokine response when it replaces general anesthesia for cancer breast surgeries. Saudi Journal of Anaesthesia, 7(4), 373–377. doi:10.4103/1658-354X.121043.
Desmond, F., McCormack, J., Mulligan, N., Stokes, M., & Buggy, D. J. (2015). Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Research, 35(3), 1311–1319.
Jaura, A. I., Flood, G., Gallagher, H. C., & Buggy, D. J. (2014). Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. British Journal of Anaesthesia, 113(Suppl 1), i63–i67. doi:10.1093/bja/aet581.
Buckley, A., McQuaid, S., Johnson, P., & Buggy, D. J. (2014). Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. See comment in PubMed Commons below British Journal of Anaesthesia, 113(Suppl 1), i56–i62. doi:10.1093/bja/aeu200.
Acknowledgements
I would like to thank Prof. Daniel I. Sessler, Department of Outcomes Research, Cleveland Clinic, for providing fruitful comments regarding the ongoing trial of NCT00418457. I also thank Accixx Biomedical Consulting (www.Accixx.com) for the scientific editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding support
There was no funding support for this manuscript.
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev 36, 159–177 (2017). https://doi.org/10.1007/s10555-016-9647-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9647-8