Abstract
Purpose of Review
The purpose of this article is to provide a brief review of current literature examining the potential oncologic protective role local anesthetics may provide in the perioperative setting during cancer surgery.
Recent Findings
Paradoxically, curative surgery for cancer appears to favor the development of recurrences and distant metastasis. The accumulated knowledge about cancer biology and our understanding of the pathophysiological events occurring during the perioperative period have opened several theories on potential mechanisms for this century old observation. In such, cell-mediated immune response, the first line attack against cancer is suppressed during the perioperative period possibly providing circulating tumor cells with a fruitful environment allowing them to escape the immune system to form local recurrence and metastasis. In parallel, surgical stress, acute pain, and inflammatory cytokines released during surgery all directly or indirectly contribute to the immunosuppression state. Current data indicates that surgery itself cannot be regarded as the sole culprit for this paradox, and other factors during the perioperative period such as anesthetic drugs or anesthesia techniques are conceivably involved as well. As an example, volatile anesthetic seem to have negative effects while propofol appears to have protective effects. Opioids were also found to be immunosuppressive and therefore may not represent an ideal choice. Of the drugs used in anesthesia, local anesthetics seem to be the most attractive with regard to cancer surgery.
Summary
Depending on their mode of administration, local anesthetics have been found in several reports to directly or indirectly blunt the systemic response to surgery, preserve immune function, alter cytokine release, and interfere with cancer cell signaling pathways. They are also analgesic and have anti-inflammatory properties. The former and the latter are sought effects in the perioperative period. However, their impact on recurrence, overall survival and metastasis remains controversial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer remains one of the leading causes of mortality worldwide and incidence of new cancer cases per year is estimated to increase to 23.6 million by 2030 globally [1]. In the United States (US), an estimated 1.7 million new cases of cancer will be diagnosed in 2018 and more than 600, 000 people will die from their disease [2]. Fortunately, the US cancer mortality rate has decreased since the early 1990s demonstrating an overall trend of progress in the field of treatment. Despite this progress, factors increasing cancer incidence, including aging population and obesity are also on the rise [3]. These factors, not surprisingly, are projected to dramatically increase the number of patients undergoing cancer treatment [4]. Surgery continues to be a mainstay in the treatment of most solid tumors [5]. Unfortunately, most cancer deaths in patients previously treated surgically are caused by recurrence at the site and metastatic disease [6]. In the last decade, much has been discussed about the effects of anesthesia and analgesia techniques on cancer recurrence and metastasis [7–8]. Several reports suggest certain type of anesthetic techniques may offer better overall survival compared to others however, there is no general consensus [9]. The focus of this article is to review current literature and determine what if any protective role against cancer proliferation and recurrence exists for local anesthetics in clinical practice.
Impact of Perioperative Period on Cancer Recurrencee
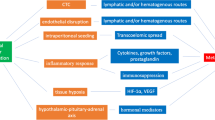
The phenomenon of cell progression and metastasis following surgical removal of tumor still remain a major cause of recurrence and metastasis [6]. Several theories have been advanced to elucidate the pathological mechanisms underpinning cell transformation after curative cyto-reductive surgery for all type of cancer [10]. There are multiple reports suggesting certain predominant factors occurring during the perioperative period could negatively impact cancer survival. These factors can be divided into mechanical and humoral factors. Mechanistically, minimal residual disease at the site of the tumor and the release of circulating tumor cells (CTCs) into the circulation at the time of tumor resection are believed to be important contributors to cancer recurrence during the perioperative period [11,12,13,14] Prooncogenic inflammatory cytokines released in the circulation and the suppression of cancer-mediated immunity in response to surgical stress represent the humoral component that may be implicated in cancer relapse after surgery [11,12,13,14,15, 16]. Theoretically, minimal residual disease and circulating cancer cells are both directly or indirectly influenced in the postoperative period by the abundant presence of prooncogenic cytokines and by the suppression of cell-mediated immunity potentially affecting short- and long-term recurrences [12,13,14,15,16,17]. While the intent of surgery is curative in most cases, the perioperative period therefore can paradoxically act as a potential trigger for recurrences. Reciprocally, the perioperative period can be regarded as an ideal venue for therapeutic intervention. The steady increase in primary cancer diagnosis and surgical treatment paradoxically leading to metastatic recurrence has generated considerable interest in the perioperative period and other factors that may also influence cancer recurrence. Anesthetics and analgesics are important perioperative considerations. In vivo and in vitro studies of volatile anesthetics and opioids have shown an increase in tumor proliferation while anti-inflammatory drugs, regional anesthesia, and local anesthetics (LA) may inhibit cell proliferation [18–19] [20].
Cancer Cell Progression
Genetic and epigenetic modifications in cell organization commonly lead to an evasion from normal regulatory cellular functions resulting in cell transformation [21]. Following acquisition of new genetic material, a given cell must undergo several steps of cellular replications and genetic modifications before achieving full malignant potential [22]. During the transformative journey a cancer cell undertakes, it surrounds itself with an architectural structure called tumor microenvironment. Within this tissular structure lays the fundamental signaling pathways and machinery responsible for cancer cell survival and progression. It is also the lieu where cancer cells gain invasiveness and metastatic potential [23–24]. Recent understandings in cancer microbiology unveiled tangible explanation of the mechanisms underlying cancer cell progression. The concept of cancer stem cells and cancer stem cell niches eloquently illustrates cancer cell perpetuation. It stands on the premise; cancer stem cells bear the properties of self-renewal, escape cell death, evade the immune system and can remain dormant for extended period of time. For a detailed review about cancer stem cells and cancer cell stem niches refer to the article by Plaks V et al. [25]. Cancer cell signaling pathways are implicated in cell proliferation, invasion, and motility. The Src [cellular src gene] and FAK [Focal adhesion kinase] are involved in cell invasion [26–27]. Likewise, the Ras/MAP kinase pathway activation is associated with anti-apoptotic features and hence cell survival. The Pl-3 kinases regulate cell growth, motility, and survival [28]. Signaling pathway inhibitors can target these pathways. Local anesthetics have been found to inhibit these signaling pathways as well [29]. Inflammation plays an important part in cancer cell progression. Pro-inflammatory cytokines, catecholamines, and prostaglandins released in response to surgery are all known to be directly or indirectly involved in cancer cell progression through effectors such as IL-6, TNFα, STAT3, VEGEF, and TGFβ among others [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Local Anesthetics
Local anesthetics (LAs) have been used as anesthetics and analgesics for more than a century. LAs are weak bases, which at a pH equal to the pKA of the ionized base, will exist in equilibrium in ionized and unionized form. The unionized (uncharged) form will cross the cellular membrane and, once in the cytosol, will again equilibrate with the ionized form. This ionized form binds the intracellular portion of the voltage-gated sodium channel in a dose-dependent manner, inhibiting sodium influx and preventing action potential generation and conduction. LAs structures consist of hydrophilic (typically a tertiary amine) and hydrophobic (typically an aromatic moiety) domains and are classified by their intermediate amide or ester linkage. The two classes of LA differ in both mechanism and site of metabolism. Amino esters are metabolized rapidly in plasma via pseudocholinesterase-mediated ester hydrolysis and their water-soluble metabolites are excreted in the urine. The rate of ester hydrolysis is dependent on the type and location of substitution on the aromatic ring such that as an example, chloroprocaine is hydrolyzed many times faster than tetracaine. Amide-linked LAs are metabolized by dealkalization reactions in the liver where an ethyl group is cleaved from the tertiary amine and renal clearance plays only a minor role. In clinical practice, LAs can be administered via several routes—(Fig. 1) local infiltration, peripherally, neuraxially, and intravenously. IV administration of amide LA lidocaine at low concentration is regularly used in multimodal analgesia in the perioperative period. To date, most of the optimistic studies exploring the effects of anesthesia and analgesia on cancer recurrence are dominated by the role of loco-regional anesthesia [7–8,9,10]. Whether the effects observed are inherent to the direct action of local anesthetics on cancer cells or an indirect action such as blunting of the surgical stress response or both is not completely determined.
Direct Action of Local Anesthetics on Cancer Cells
LAs exert most of their electrical inhibitory action on nerve fiber through binding to voltage-gated sodium channel (VGSC) resulting in a complete blockade of action potential transmission. These VGSC are expressed in both excitable and non-excitable cells including cancer cells [33]. In cancer cells, VGSC activity has been linked to increased metastatic activity and cellular invasion. Expression of these channels has been found in various types of cancers including prostate, breast, cervical, colon, lung, skin, and ovarian cancers [34]. Hypothetically, binding of LAs to VGSC expressed in the circulating cancer cells released at the time of surgery may affect recurrence by decreasing metastatic potential and hence an improvement in overall survival. The beneficial impact of local application of LAs on cancer recurrence was observed in patients undergoing melanoma excision. Schlagenhauff et al. found an increase recurrence-free interval in patients who had local anesthesia infiltration as their primary anesthetic and a slight decrease in survival in patients who received general anesthesia [35]. Whether this is due to LAs infiltration in the vicinity of the tumor and binding with VGSC is biologically possible but uncertain. Earlier studies, however, failed to show this benefit. More recently, Koffler et al. showed that patient who received tumescent local anesthesia for melanoma surgery when compared to general anesthesia had a better distant metastasis-free survival; however, overall survival was not different between groups [36]. Cell signaling pathways are important in normal cell development, progression, survival, apoptosis, and normal regulatory functions. Multiple genetic alterations of these pathways during early stages of cancer veer natural functions from normality to abnormality. Such pathway, the Src tyrosine protein kinase involved in cell survival, angiogenesis, proliferation, and invasion pathways has been found to be overly expressed in colon, breast, and prostate cancer. Interestingly, the LAs lidocaine and ropivacaine have been found to inhibit Src tyrosine kinase pathway in an in vitro lung cancer cell model [29]. Likewise, Zheng et al. studied the effect of ropivacaine on proliferation and survival pathways of chronic myeloid leukemia cell lines. They found, ropivacaine to inhibit proliferation of chronic myeloid leukemia cell lines via arresting cell at G2/M stage in a dose and time dependent manner [37]. Additionally, ropivacaine was found to inhibit phosphorylation of substances involved on PI3K/Akt/mTOR signaling pathways. Moreover, the combination of ropivacaine with specific tyrosine kinase inhibitors resulted in synergistic effect in targeting chronic myeloid leukemia cell lines [37]. Importantly, this synergistic effect of LAs anesthetic when added to chemotherapeutic drugs was previously described in a human Hepatocellular carcinoma cell line (HepG2) cells. Similarly to ropivacaine, lidocaine inhibited the growth of HepG2 cell in a dose-dependent and time manner in vitro and in a Xenograft model in vivo. In this experiment, lidocaine arrested cell progression in the G0/G1. Lidocaine exerted its action through an increase in Bax protein and activated capsase3 and a reduction in Bcl-2 protein through the ERK (1/2) and p38 pathways. This was also observed in a thyroid cancer cell model. K1 thyroid cancer cells were incubated with lidocaine and bupivacaine for 24 and 48 h. Both lidocaine and bupivacaine inhibited thyroid cancer cell growth. In this model as well, both drugs caused an elevation of Bax protein and reduction in Bcl-2 protein resulting in higher ratio of pro-apoptotic to anti-apoptic actions [38]. Several other effects of LAs action on cancer cell progression, proliferation, invasion, and metastasis have been described for various cancers.
Indirect Actions of Local Anesthetics on Cancer Cells
Action on Natural Killer Cell Activity
Natural killer cell, an effector of the innate immune system, acts as the first line of defense against cancer. Together with elements of the adaptive immune system, they detect, attack, and destroy cancer cells from the circulation in a process called immunoediting [39–40]. This process described five decades ago delineates a stepwise process of the immune system dealings with cancer cells. In the first step (elimination phase), natural killer (NK) cells first recognize, destroy, and eliminate tumor cells from the circulation. However, genetic and epigenetic instability within the tumor microenvironment renders the immune system capacity to recognize and destroy cancer cells challenging. In this instance, tumor cells and the immune system enter a “truce period” otherwise known as the equilibrium phase. In this phase, tumor cells enter a period of dormancy and are controlled by the immune system. Tumor dormancy refers to tumors present in patients for long period of time with potential for reactivation, multiplication, progression, and overt development. The latter coincides with the last phase of immunoediting: evasion from immune system [39,40,41]. As described above, mechanical and humoral factor during the perioperative period may play an important role in dictating local and distant recurrence following surgery highlighting the importance of a competent immune system. Unfortunately, the impact of the surgical stress response has been shown to induce a state of immunosuppression overall and especially natural killer cell activity [42–43]. The activation of the hypothalamus-pituitary axis, stimulation of the sympathetic nervous system, the secretion of pro-inflammatory cytokines during the perioperative all contribute to a certain amount to mitigating the immune response. Regional and neuraxial anesthesia and/or analgesia can be considered as the prototype model for the indirect actions of local anesthetic. When given peripherally or neuraxially, LAs mitigate sympathetic nervous system activation, blunt the surgical stress, results in a better inflammatory cytokines profile, and hence preservation of the immune system, in return better outcomes will be achieved with regard to cancer recurrence [44–45]. Unfortunately, literature reports and meta-analyses published over the last decade assessing the impact of regional anesthesia and/or analgesia on cancer recurrence have demonstrated mixed results and no consensus has been obtained regarding the best anesthetic approach for these patients stressing the heterogeneity and the complexity of tumor cells and cancer treatment in general. In vivo and in vitro studies have found that the NK cell cytotoxic activity is preserved and sometimes enhanced by the action of local anesthetics [20]. Cata et al. isolated NK cells from sera of healthy volunteers and from patients who had undergone surgery for cancer followed by an incubation with lidocaine at clinically relevant concentrations. They found lidocaine to stimulate NK activity, NK cytolytic activity, and increased expression of NKG2D receptors. These latter receptors are often unregulated in cancer cells making them susceptible to NK cells cytolytic activity [46–47]. However, previous study found a negative association between NK cells cytotoxicity at three different concentrations of amide local anesthetics bupivacaine, ropivacaine and lidocaine [48].
Action on the Inflammatory System
The relationship between cancer, the inflammatory system, and the immune system is interlinked [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. This relation has been at center of many reviews and studies and is beyond the scope of this article. The anti-inflammatory effects of local anesthetics could be regarded as a potential contributor in limiting the inflammatory response to surgery [50]. When given intravenously during surgery, lidocaine has been found to ameliorate various outcomes associated with the inflammatory derangements seen in the perioperative period [20]. Whether this observation can translate to cancer surgery is undetermined. However, there are some reasons to believe, the anti-inflammatory actions of local anesthetics could be crucial. The inflammatory process induced at the time of surgery, is in part responsible for an increase in vascular permeability leading to a disruption in endothelial barrier hence allowing for circulating tumor cell to escape the vasculature and potentially begin the metastatic process [14,15,16,17,18,19,20,21,22,23,24]. Pro-inflammatory cytokines IL6 is known to correlate with the magnitude of tissue injury, which in itself correlates with the degree of immunosuppression. Early reports found epidural analgesia reduced IL6 production in patient undergoing cervical cancer surgery. Similarly, the anesthetic technique has been shown to impact the release of cytokine in postoperatively. For example, a propofol/paravertebral-based anesthesia technique was found to alter several inflammatory cytokines involved in regulating perioperative immunity compared to a sevoflurane/opioid anesthesia-based anesthesia in patient undergoing breast surgery [17].
Action on the Sympathetic Nervous System
The physiological response to surgical stress is invariably associated with the release of neurotransmitters such as epinephrine and norepinephrine leading to an activation of the sympathetic and the neuroendocrine system. Similarly, the hypothalamic pituitary adrenal axis is also stimulated. In turn, the activation of these systems is known to induce perioperative immunosuppression [51–52]. Additionally, the catecholamine norepinephrine has been implicated in the activation of the signal transducer of activation and transcription (STAT3) via interaction with β1 and β2 adrenergic receptors in ovarian cancer cells [30]. Regional anesthesia has been shown to abrogate the stress response and hence positively impacting the immune response to surgery. As an example, in patients undergoing hip and knee surgery under spinal anesthesia had less postoperative infection compared to patients receiving general anesthesia [53]. This effect is believed to be related to the blunting of ascending neural pathways responsible for the activation of the sympathetic and neuroendocrine system. Several studies suggest a close relationship between cancer and activation of adrenergic receptors. It appears also, the β adrenergic system is more linked than α adrenergic system in promoting disease progression [42]. However, a recent meta-analysis suggests that beta-blockers do not have protective effect on cancer recurrence [54].
Conclusion
Local anesthetics appear to have properties that potentially prevent cell growth through modulation of the immune function and their anti-inflammatory actions. They also seem to have the capacity to disrupt cell cycle by interacting with key element of cell function. Although, clinical reports from human subjects do not seem to universally invite their utilization to prevent cancer recurrence or the formation of distant metastasis, in vitro and in vivo studies seem to suggest otherwise. Currently, it is unknown if the use of LA’s during the perioperative period can help prevent recurrence and improve overall survival. Clinical randomized trials are needed to assess the effects of local anesthetics at clinically relevant concentrations and modes of administration on cancer cell progression and the preservation or enhancement of cancer immunity.
References
The Website of the National Cancer Institute (http://www.cacner.gov). Retrieved 07/21/2018.
The website of the National Cancer Institute: cancer trends progress report (hhtp://progressreport.cancer.gov/after/economic).
The website of the National Institute of Cancer SEER cancer statistics review (http://seer.cancer.gov/csr/1975-2015).
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–52.
Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I. Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers (Basel). 2010;2(2):305–37.
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–4.
Forget P, Tombal B, Scholtes JL, Nzimbala J, Meulders C, Legrand C, et al. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur J Anaesthesiol. 2011;28(12):830–5.
Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies.PLoS One. 2013;8(2):e56540.
Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110(6):1636–43.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
O’Leary DP, O’Leary E, Foley N, Cotter TG, Wang JH, Redmond HP. Effects of surgery on the cancer stem cell niche. Eur J Surg Oncol. 2016;42(3):319–25.
Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114.
Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra392.
Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, et al. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72(10):771–6.
Gutman H, Risin D, Pollock RE, Pellis NR. Effect of surgery on peripheral blood lymphocyte locomotion through type I collagen. Cancer. 1993;71(9):2833–7.
Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35(6):490–5.
Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The Effect of Perioperative Intravenous Lidocaine on Postoperative Pain and Immune Function. Anesthesia and Analgesia. 2009;109(5):1464–9.
Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i63–7.
Wang HL, Yan HD, Liu YY, Sun BZ, Huang R, Wang XS, et al. Intraoperative intravenous lidocaine exerts a protective effect on cell-mediated immunity in patients undergoing radical hysterectomy. Mol Med Rep. 2015;12(5):7039–44.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation.Cell. 2011;144(5):646–74.
Lee G, Hall RR, 3rd, Ahmed AU: Cancer stem cells: cellular plasticity, niche, and its clinical relevance. J Stem Cell Res Ther 2016, 6(10).
Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63(11):1115–28.
Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206.
Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–38.
Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37(1):9–15.
Gu MM, Gao D, Yao PA, Yu L, Yang XD, Chun-Gen X, Zhou J, Shang ZF, Li M: The p53-inducible gene 3 promotes cell migration and invasion via activating the FAK/Src pathway in lung adenocarcinoma. Cancer Sci 2018.
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW, et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol. 2015;8:22.
Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, et al. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117(3):548–59.
Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12(2):369–75.
Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23(2):267–75.
Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer. 2013;132(12):2721–9.
Fraser SP, Foo I, Djamgoz MB. Local anaesthetic use in cancer surgery and disease recurrence: role of voltage-gated sodium channels? Br J Anaesth. 2014;113(6):899–902.
Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell TM, Coombes RC, et al. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1638):20130105.
Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res. 2000;10(2):165–9.
Kofler L, Breuninger H, Hafner HM, Schweinzer K, Schnabl SM, Eigentler TK, et al. Lymph node dissection for melanoma using tumescence local anaesthesia: an observational study. Eur J Dermatol. 2018;28(2):177–85.
Zheng Q, Peng X, Yu H. Local anesthetic drug inhibits growth and survival in chronic myeloid leukemia through suppressing PI3K/Akt/mTOR.Am J Med Sci. 2018;355(3):266–73.
Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9(2):e89563.
Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures.Immunity. 2013;39(1):11–26.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48.
Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19(4):419–28.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–9.
Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, et al. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg. 1996;171(1):68–72.
Li JM, Shao JL, Zeng WJ, Liang RB. General/epidural anesthesia in combination preserves NK cell activity and affects cytokine response in cervical carcinoma patients undergoing radical resection: a cohort prospective study.Eur J Gynaecol Oncol. 2015;36(6):703–7.
Cata JP, Ramirez MF, Velasquez JF, Di AI, Popat KU, Gottumukkala V, et al. Lidocaine stimulates the function of natural killer cells in different experimental settings. Anticancer Res. 2017;37(9):4727–32.
Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity.Reg Anesth Pain Med. 2015;40(1):43–8.
Krog J, Hokland M, Ahlburg P, Parner E, Tonnesen E. Lipid solubility- and concentration-dependent attenuation of in vitro natural killer cell cytotoxicity by local anesthetics. Acta Anaesthesiol Scand. 2002;46(7):875–81.
Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–79.
Swanton BJ, Shorten GD. Anti-inflammatory effects of local anesthetic agents. Int Anesthesiol Clin. 2003;41(1):1–19.
Pollock RE, Lotzova E. Surgical-stress-related suppression of natural killer cell activity: a possible role in tumor metastasis. Nat Immun Cell Growth Regul. 1987;6(6):269–78.
Pollock RE, Lotzova E, Stanford SD, Romsdahl MM. Effect of surgical stress on murine natural killer cell cytotoxicity. J Immunol. 1987;138(1):171–8.
Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279–84.
Yap A, Lopez-Olivo MA, Dubowitz J, Pratt G, Hiller J, Gottumukkala V, et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth. 2018;121(1):45–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aric Elmer declares that he has no conflict of interest. Mohamed Tiouririne has stock options with Adial Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cancer Anesthesia
Rights and permissions
About this article
Cite this article
Elmer, D.A., Tiouririne, M. Local Anesthetics (LAs) and Cancer Cell Progression: Is There an Oncologic Protective Role for LAs in Clinical Practice?. Curr Anesthesiol Rep 8, 393–398 (2018). https://doi.org/10.1007/s40140-018-0297-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-018-0297-y